Abstract
Purpose: The incidence of esophageal adenocarcinoma (EAC) has increased by 700% in Western countries over the last 30 years. Although clinical guidelines call for endoscopic surveillance for EAC among high-risk populations, fewer than 5% of new EAC patients are under surveillance at the time of diagnosis. We studied the accuracy of combined cytopathology and MUC2 immunohistochemistry (IHC) for screening of Intestinal Metaplasia (IM), dysplasia and EAC, using specimens collected from the EsophaCap swallowable encapsulated cytology sponge from Canada and United States.
Patients and methods: By comparing the EsophaCap cytological diagnosis with concurrent endoscopic biopsies performed on the same patients in 28 cases, we first built up the cytology diagnostic categories and criteria. Based on these criteria, 136 cases were evaluated by both cytology and MUC2 IHC with blinded to patient biopsy diagnosis.
Results: We first set up categories and criteria for cytological diagnosis of EscophaCap samples. Based on these, we divided our evaluated cytological samples into two groups: non-IM group and IM or dysplasia or adenocarcinoma group. Using the biopsy as our gold standard to screen IM, dysplasia and EAC by combined cytology and MUC2 IHC, the sensitivity and specificity were 68% and 91%, respectively, which is in the range of clinically useful cytological screening tests such as the cervical Pap smear.
Conclusions: Combined EsophaCap cytology and MUC2 IHC could be a good screening test for IM and Beyond.
Introduction
The incidence of esophageal adenocarcinoma (EAC) is rising rapidly in Western countries, having increased 6–7 folds in North America since the 1980s.Citation1 EAC is a devastating disease that carries an overall 5-year survival of only 18–22%.Citation2 Part of the reason for EAC’s poor prognosis is that most patients present at an advanced stage when they experience obstructive symptoms such as discomfort on swallowing. The single major risk factor for the development of EAC is Barrett’s esophagus (BE), a metaplastic condition in which intestinal-like glandular epithelium replaces the normal squamous mucosa of the esophagus. BE is a consequence of chronic gastroesophageal reflux disease (GERD).Citation3,Citation4 Roughly 15–40% of the adults in western countries have GERD, while 8–20% of the GERD adults develop BE.Citation5,Citation6 Patients with BE have between a 0.12% and 0.5% annual rate of progression to EAC, and an 11.3–40-fold increase in their lifetime risk of developing EAC in comparison to the non-BE population.Citation7–Citation10
In most western countries, clinical guidelines recommend that patients with chronic GERD obtain a baseline endoscopy to diagnose BE.Citation11,Citation12 Patients with BE are then recommended to undergo periodic endoscopic surveillance with biopsy to detect progression to dysplasia and EAC.Citation11,Citation12 However, in practice endoscopic surveillance of patients with BE requires loss of a partial work day for the patient, involves sedation and has a low but real complication rate.Citation13–Citation15 For these reasons, many patients and their providers are reluctant to commit to endoscopy. Furthermore, endoscopy is expensive in comparison to other screening technologies such as mammography, and its cost-effectiveness is in doubt.Citation14 Consequently, just 5% population that is thought to have BE is under surveillance.Citation7,Citation16–Citation19 Therefore, there is an urgent need to develop new methods to screen patients with GERD for BE and to surveil BE patients for progression.
Esophageal balloon cytology has been used in China to screen for esophageal squamous cell carcinomaCitation19–Citation22 and in the USA to detect esophageal carcinoma.Citation23,Citation24 Non-endoscopic screening methods based on swallowable encapsulated sponges have been developed in the United Kingdom,Citation25–Citation27 SwitzerlandCitation28 and in the USA. These tests are administered in the form of a sponge-containing gelatin capsule attached to a string or tether that the patient swallows like a pill. The sponge expands after swallowing and is then retrieved with the tether. During retrieval, the sponge scrapes the esophageal mucosa, collecting epithelial cells for further cytological, immunohistochemical or genetic analysis. In several large studies, swallowable encapsulated sponges were well accepted by patients enrolled in BE surveillance programs.Citation27,Citation29,Citation30 Fitzgerald’s group in the UK has reported IM detection using trefoil factor 3 (TFF3) immunohistochemistry on cell blocks obtained via the Cytosponge™ sampling. The overall sensitivity of the test was 79.9%, increasing to 87.2% for patients with ≥3 cm of circumferential intestinal metaplasia (IM).Citation25 However, the accuracy of swallowable encapsulated sponges for the detection of dysplasia and/or cancer by combined cytopathology and immunohistochemistry has not been reported.
In the current study, we used EsophaCap™ sampling to obtain esophageal cytology specimens at two North American sites (Canada and the USA) from 169 patients with known GERD, BE or dysplasia undergoing routine endoscopic examination. The sponge cytology specimens were examined cytologically alone and in combination with MUC2 immunohistochemistry (IHC). The sensitivity, specificity and accuracy of these methods for detecting intestinal metaplasia, dysplasia and EAC were analyzed.
Materials and methods
Patient recruitment and demographics
We recruited adult (18+) patients with previously documented gastroesophageal reflux disease, BE, or low- or high-grade dysplasia undergoing routine surveillance, diagnostic and/or therapeutic endoscopy at either the St. Michael’s Hospital Endoscopy Unit in Toronto, Canada (159 patients), or the Boston University in Boston, MA, USA (10 patients). We excluded subjects for whom esophageal biopsy or sponge sampling would be contraindicated, such as those with known esophageal strictures. Patients gave written informed consent to participate in the study, and this study was conducted in accordance with the Declaration of Helsinki. The research ethics committees approved the study at all clinical and research sites.
As of September 2016, 250 patients were recruited at St. Michael’s hospital and Boston University Medical Centre. Six patients elected not to attempt to swallow (2.4%). Seventy-five patients tried but were not able to swallow (30%). One hundred and sixty-nine esophageal cytology specimens were collected. The major complaint was gagging, reported by 65% of the patients able to swallow.
The patient cohort included 80% males and 20% females with a mean age of 65.8 years. The vast majority of the studied patients were white (96%) and had a history of current or past cigarette smoking (72%).
Esophageal cytology collection
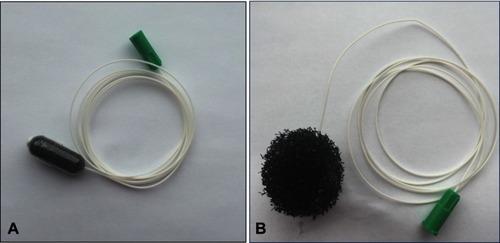
The EsophaCap(™) is a gelatin capsule containing a compressed sponge attached to a tether (). We purchased EsophaCaps(™) from Capnostics LLC (Doylestown PA). Esophageal cytology samples were collected by a registered nurse prior to sedation for endoscopy. The patient swallowed the capsule with a drink of water while holding the tether loosely. In 5 mins after allowing the capsule to dissolve in the proximal stomach, it released a spherical polyurethane sponge in two sizes, 2.5 cm and 3.0 cm in diameter. The back of the throat was then sprayed with 1% lidocaine (lignocaine) and the expanded sponge was then withdrawn by pulling on the tether. After sponge retrieval, the tether was cut and the sponge containing the cytological specimen was placed in preservative fluid (CytoLyt) and kept refrigerated prior to transport to the University of Rochester Pathology department for cytology processing.
Endoscopy and biopsy
Subsequent to sponge cytology collection, typically within 1 hr, patients underwent routine endoscopic examination with biopsy sampling. The biopsies were processed and examined at the Surgical Pathology units at St. Michael’s Hospital and Boston University for routine diagnosis. The first 28 biopsy slides were then transmitted to the University of Rochester for review by the study GI pathologist (ZZ). The rest of the biopsy cases were diagnosed by local GI pathologists.
Cytopathology sample preparation
All specimens were preserved in CytoLyt solution and processed on the Thin Prep Processor (ThinPrep 2000, HOLOGIC, MA) using the standard Non-Gyn protocol. Cell blocks were prepared as follows: the specimen was centrifuged to produce a concentrated cell button (5 min/349 g) that was resuspended in 5 ml buffered formalin and centrifuged again (5 min/349 g) to produce a fixed cell button. The cell button was then paraffin embedded and sectioned. Two unstained slides were cut for each sample. One slide was stained with hematoxylin and eosin, and the other was sent for MUC2 IHC. All ThinPrep slides and cell blocks were submitted for cytopathology (ZZ) and senior cytotechnologist (D. Russell) review.
Cytopathology diagnostic criteria and categories
Based on our review of the first 28 paired cases and previous cytological classification systems for the esophagus,Citation31,Citation32 we developed criteria and categories to classify the glandular cells observed in the ThinPrep and cell block samples (). Based on the morphological criteria, 136 cases were evaluated by one cytopathologist and one senior cytotechnologist, both blinded to patient information and the paired biopsy diagnosis. The number of all glandular cells and squamous cells were counted.
Table 1 Diagnostic categories of esophageal glandular cells in EsophaCap cytology sample
Histologic study of FFPE biopsy specimens
All biopsies were reviewed by gastrointestinal pathologists in St. Michael hospital and Boston Medical Center. The new definition for BE by American College of Gastroenterology is “BE should be diagnosed when there is an extension of salmon-colored mucosa into the tubular esophagus extending ≥1 cm proximal to the gastroesophageal junction (GEJ) with biopsy confirmation of intestinal metaplasia. (IM)”Citation11 Since the length of mucosa is required for the diagnosis of BE. The pathology cannot directly diagnose the BE instead of intestinal metaplasia (IM). The histologic diagnoses were categorized into six categories (see ) including normal squamous epithelium (SE), columnar cell metaplasia (CM), intestinal metaplasia (IM), indefinite dysplasia (ID), low-grade dysplasia (LGD), high-grade dysplasia (HGD) and EAC. Based on ACG guideline for BE, BE should be diagnosed when there is extension of salmon-colored mucosa into the tubular esophagus extending ≥1 cm proximal to the gastroesophageal junction with biopsy confirmation of IM.Citation11 Therefore, our diagnosis from pathology biopsy has to be changed to IM instead of BE. The histological diagnosis was executed independently from the cytological diagnosis. Five cytology cases did not have matched biopsy diagnosis, which were excluded from the statistics.
Construction of tissue microarray
MUC2 immunostain was evaluated by tissue microarrays (TMA) that included 33 cases of BE, 64 cases of CM, 95 cases of SE, 31 cases of LGD, 8 cases of HGD and 109 cases of EAC were constructed from the representative areas of FFPE specimens collected between 1997 and 2005 at the Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, New York. Five-micron sections were cut from the tissue microarrays and were stained with H&E to confirm the presence of the expected tissue histology within each tissue core. Additional sections were cut for MUC2 IHC.
MUC2 IHC on cell blocks and TMA
Tissue sections from the cell block and esophageal TMA were deparaffinized, rehydrated through graded alcohols, and washed with phosphate buffered saline. Antigen retrieval was performed by heating sections in 99°C water bath for 30–40 mins. After endogenous peroxidase activity was quenched and nonspecific binding was blocked, antibody for MUC2 (DAKO, CA) was incubated at room temperature for 30 mins. The secondary antibody (Flex HRP, DAKO, CA) was allowed to incubate for 30 mins. After washing, sections were incubated with Flex DAB Chromogen for 10 mins and counterstained with Flex Hematoxylin for 5 mins. A previously diagnosed BE case served as a positive control. Negative control was performed by replacing antibodies with normal serum.
Data analysis
Summary data are expressed as the means (SDs). All statistical tests are two-sided unless otherwise noted. P-values of less than 0.05 are considered statistically significant.
Results
Criteria and categories for cytological diagnosis
All 169 cytology specimens had adequate numbers of epithelial cells to render a diagnosis. The first 28 cases with matched surgical biopsy were used to set up the diagnostic criteria; the remaining 141 cases were diagnosed blinded to patient information and surgical biopsy diagnosis. Based on the morphology from our 28 cases with matched cytology and biopsy, and previous esophageal cytology classification systems,Citation31,Citation32 we developed a set of diagnostic categories for glandular cells obtained by cytology ().
Analysis of cytological diagnosis
compares the histopathological biopsy diagnosis to the cytological diagnosis for the 141 cases that were diagnosed blindly. Five of 141 cases without matched surgical biopsy diagnosis were excluded from the analysis. Of 136 cases, 34 cases were diagnosed as no columnar cell (NCC). These Thin-Pap smears contained normal squamous cells and no or rare glandular clusters (≤3 clusters) (). Fifteen columnar cell with no-goblet cell (CCNGC) cases contained predominant squamous cells and some large, flat cohesive sheets of glands with honeycomb pattern, smooth, sharp edge and small round or oval nuclei () and columnar cell glands without goblet cells in cell block () as well as negative MUC2 IHC ().
Figure 2 Intestinal metaplasia with no high-grade dysplasia (IMNHGD) and columnar cell with no goblet cells (CCNGC) of EsophaCap samples. (A) CCNGC: The specimen consists of multiple squamous cells and one sheet of glandular cells. The glandular cells are well organized. No goblet cells are identified. (B) IMNHGD: The specimen consists of multiple sheets of glandular cells. The glandular cells are well organized. Focal goblet cells are present (arrowhead). (C and E) CCNGC: One columnar cell gland is present in cell block. The columnar cells are negative for MUC2 immunostain; (D and F) IMNHGD: Goblet cells are present in cell block. The goblet cells and adjacent columnar cells are positive for MUC2 immunostain.
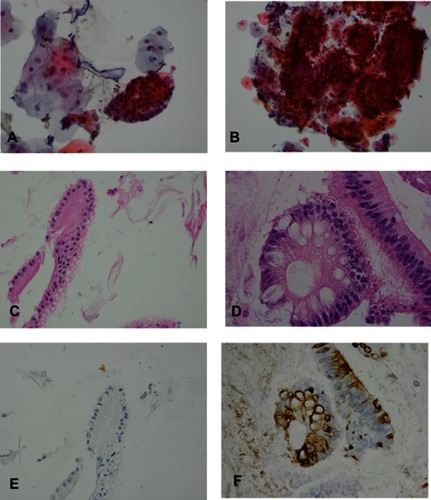
Table 2 Number of surgical biopsy and cytology cases with each category
Thirty-nine intestinal metaplasia with no high-grade dysplasia (IMNHGD) cases consisted of large flat sheets of cells with focal goblet cells and smooth, sharply defined edges. The cytoplasm of goblet cells showed slightly basophilic mucin. The nuclei were small, and linearly arranged in the basal layer (), which could be compatible with surgical biopsy IM cases. Some nuclei showed focal nuclear stratification and hyperchromasia in a honeycomb pattern, which could be compatible with low-grade dysplasia (LGD) cases. However, the differentiation of IM and LGD is thought not to be reliable in esophageal cytology.Citation31 Therefore, we combined these into a single IMNHGD category. Goblet cells were not easily identified in the smears but were readily identified in corresponding cell blocks () and by MUC2 IHC ().
Atypical glandular cells (AGC) consisted of small three-dimensional clusters of atypical glandular cells with nuclear enlargement, overlapping and hyperchromasia, prominent nucleoli and smooth nuclear membrane ( and ). Cytoplasm showed decreased cytoplasmic mucin.
Figure 3 Atypical glandular cells (AGC), suspicious for esophageal adenocarcinoma (SFEAC) and esophageal adenocarcinoma (EAC). (A and B) AGC: The specimen consists of a sheet of glandular cells with increased nuclear size and prominent nucleoli (see arrow). (C and D) SFEAC: The specimen consists of rare cluster of glandular cells with high nuclei/cytoplasm ration, irregular nuclear contour, prominent nucleoli, hyperchromasia and overlapping. (E and F) EAC: The specimen consists of multiple clusters of glandular cells with high nuclei/cytoplasm ration, prominent nucleoli, irregular nuclear contour, hyperchromasia, mitosis (see arrowhead), overlapping and the single cells.
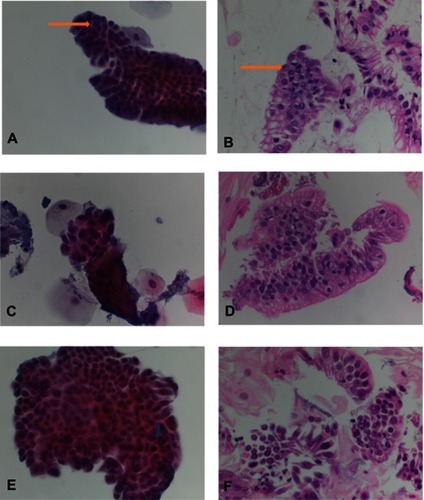
Suspicious for EAC (SFEAC) consisted of rare atypical cells with hyperchromasia, pleomorphism, prominent nucleoli, irregular nuclear contour and thickened membrane, overlapping, loss of polarity and rare mitosis ( and ). EAC had similar morphologic changes, but abundant cells or clusters ( and ). In addition, EAC consisted of frequent mitosis and focal necrosis (tumor diathesis). Some single, loose tumor cells were identified in EAC, but not in benign glands. One atypical squamous cell (ASC) and one squamous cell carcinoma (SCC) were diagnosed. The cytology showed high nuclear/cytoplasm ratio, hyperchromatin, pleomorphic. However, focal goblet cells were also identified in its cell block.
Estimation of glandular cell abundance
The number and percentage of glandular cell groups in cell blocks were counted manually (). Thirty cases (22%) had more than 30 glandular clusters. Almost half of 136 cases (50%) had between 4 and 29 groups of glandular cells. Fifty-one cases (37%) had ≤3 glandular groups in cell blocks: 34 of 51 cases were diagnosed as NCC; 17 had more glandular cells in cytology smear or positive MUC2 immunostain in cell block to make diagnosis in glandular categories. The percentages of glandular cells in cell blocks were also counted, and the range was from 0.1% to 80%. The average of glandular cells was 7.1%.
Table 3 Number of glandular cell (GC) clusters in cell block
Evaluation of MUC2 IHC on TMA
MUC2 was reported as highly specific markers for goblet cells metaplasia in distal esophagus and gastroesophageal junction (GEJ).Citation33 We further performed MUC2 IHC in multiple cases of IM, CM, SE, LGD, HGD, and EAC on TMA and evaluated its sensitivity and specificity for detection of IM and beyond in surgical specimens ( and ). IM cases were 100% positive for MUC2 IHC but only positive in 50.5% of the cases of EAC. We calculated sensitivity, specificity and accuracy in two ways. First, we set BE as positive and CM, SE and SCC as negative. The sensitivity and specificity and accuracy were 100%, 99.5% and 99.5%. Second, we set IM, LGD, HGD and EAC as positive and CM, SE and SCC as negative. The sensitivity and specificity and accuracy were 66.9%, 99.5% and 83.4% due to lower positive rate of EAC cases.
Figure 4 MUC2 immunohistochemical study (IHC) in esophageal tissue microarray (TMA). (A) Columnar cell metaplasia with negative MUC2 immunostain; (B) Barrett’s esophagus with positive MUC2 immunostain; (C) Low-grade dysplasia with positive MUC2 immunostain; (D) High-grade dysplasia with positive MUC2 immunostain; (E) Esophageal adenocarcinoma with negative MUC2 immunostain; (F) Esophageal adenocarcinoma with positive MUC2 immunostain.
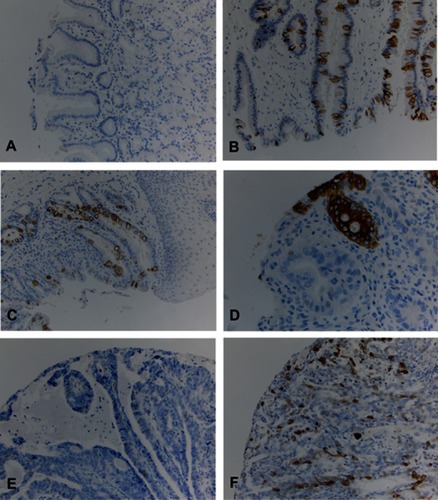
Table 4 MUC2 immunohistochemistry in esophageal tissue microarray
Combined EsophaCap cytology and MUC2 IHC for screening for BE and beyond
For this phase of analysis, we asked how accurate combined cytology and MUC2 IHC would be for screening IM and beyond, in which the goal is to detect IM and more advanced disease in a patient population. We defined a “negative” biopsy as patients with surgical pathology diagnoses of CM, SE, and a negative cytology diagnosis as NCC, CCNGC in combination with a negative MUC2 IHC result. We defined a “positive” biopsy as IM, LGD, HGD and EAC, and a positive cytology as IMNHGD, AGC, SFEAC, EAC or any positive MUC2 IHC result.
By comparing the biopsy result to the cytological/IHC diagnosis, we determined that the sensitivity, specificity and accuracy for detecting IM and beyond were 67.77% (95% CI: 58.67–75.98%), 90.91% (95% CI: 58.72–99.77%) and 69.70 (95% CI: 61.10–77.4%), respectively. The positive predictive value (PPV) and negative predictive value (NPV) were 98.80% (95% CI: 92.65–99.81%) and 20.41% (95% CI: 10.71–34.00%). MUC2 IHC alone had very high specificity to identify IM and beyond (100%), but reasonable sensitivity (79.27%; 95% CI: 68.89–87.43%) if we only calculate the cases with >4 groups of glandular cells in cell blocks. If we count all cases regardless of cellularity, the specificity to identify IM and beyond is 100%, but sensitivity is 54.17% since glandular cells may or may not present in cell blocks (sample error), combined with the observation that dysplasia and EAC are often negative for MUC2 IHC.
EsophaCap cytology for surveilling BE patients for HGD and EAC
In the second phase of analysis, we divided the diagnostic categories in a manner compatible with a hypothetical surveillance test designed to identify the presence of HGD or EAC in a population of patients with known IM. We defined a “negative” biopsy as one of CM, SE, IM and LGD, and a “positive” biopsy as HGD or EAC. We defined a negative cytology as one showing NCC, CCNGC and IMNHGD, and a positive cytology as AGC, SFEAC or EAC. The sensitivity, specificity and accuracy of the cytological diagnosis for distinguishing negative from positive biopsies were 40.43% (95% CI: 26.37–55.73%), 71.76% (95% CI: 60.96–81.00%) and 60.60% (95% CI: 51.70–69.00%), respectively. The PPV and NPV were 44.19% (95% CI: 32.77–56.25%) and 68.54% (95% CI: 62.43–74.06%).
Discussion
In the present study, we used paired biopsy and cytology specimens gathered from a cohort of 169 patients to investigate the potential utility of the EsophaCap swallowable encapsulated cytology sponge for IM screening, as well as for the surveillance of IM patients for progression to HGD and EAC. In the IM screening context, we found that with the combined cytology and MUC2 IHC, the sensitivity, specificity and accuracy for detecting IM and beyond were 67.77%, 90.91% and 69.70, respectively. In the IM progression surveillance context, we were able to identify HGD/EAC with a sensitivity of 40% and a specificity of 71%.
Cytological examination of esophageal cells has been used for screening and early detection of esophageal carcinoma in many countries including China,Citation19–Citation22 South Africa,Citation34 United States,Citation22–Citation24 Britain,Citation34 IranCitation35 and Switzerland.Citation28 Application of a sponge-on-a-string for cytology sample collection from esophagus was reported in several studies.Citation25,Citation26,Citation29,Citation34 The Cytosponge(™) has been heavily studied as a tool for non-endoscopic diagnosis of BE.Citation25–Citation27 Using TFF3 immunostain in cell blocks, the overall sensitivity of the test for BE was 79.9%, increasing to 87.2% for patients with ≥3 cm of circumferential BE.Citation25,Citation26 In our study, we used EsophaCap to collect esophageal samples. We focused on screening IM and beyond instead of IM only. The sensitivity is 68% relatively low for diagnosing IM and beyond, but the specificity is 91%, relatively high. A lower level of sensitivity is probably due to the scant cellularity from some EsophaCap samples. Fifty-one cases (37%) had ≤3 glandular groups in cell blocks. Further improvement of the EsophaCap sampling could increase the sensitivity of this test. The Pap smear as a cervical cancer screening test is one of the most successful cancer screening tests with pooled sensitivity and specificity as 43–84% and 88–95% and HPV test has 58–94% sensitivity and 88–90% specificity.Citation36–Citation38 Our result has similar range of the sensitivity and specificity to screen IM and beyond compared to Pap smear and HPV tests for cervical cancer,Citation37 which indicate that the EsophaCap combined with MUC2 IHC is a potentially good screening approach for IM and beyond.
A central aim of the current study was to establish morphological criteria for evaluating cytology obtained using the EsophaCap (™) swallowable encapsulated sponge. A challenge in interpreting the cytology is that the sponge almost certainly samples some gastric epithelium from the GE junction before its passage through the esophagus. However, neither cytology nor histology can differentiate columnar cells originating in the stomach from columnar cell metaplasia in the esophagus. Therefore, we require the presence of goblet cells to diagnose IM and prefer to use terms such as columnar cells with no goblet cell metaplasia (CCNGC) to describe MUC2 negative glandular cells. Another challenge is the differentiation of inflammation-related reactive atypia in columnar cell metaplasia and IM from HGD and EAC. We created a category of “atypical glandular cells” (AGC) to fit these cases (). However, 11 of 36 cytological cases called AGC had corresponding biopsies diagnosed as HGD or EAC. Therefore, we included AGC among the classes of “positives”.
The diagnosis of LGD in biopsy tissues has high inter-observer variability,Citation38,Citation39 and the diagnosis of LGD in esophageal cytology from brushing and ballooning is even more of a challenge.Citation31,Citation32,Citation40 Three studies have reported that the sensitivities for detection of LGD in esophageal cytology range from 20% to 31%.Citation31,Citation32,Citation40 In our study, we confirmed that it was difficult to identify LGD in Thin Pap smear and cell blocks. Therefore, we created the cytological category “intestinal metaplasia with no high-grade dysplasia” (IMNHGD) which includes specimens suspicious for low-grade dysplasia as well as IM. Cytology diagnosed as IMNHGD were concordant with a histological diagnosis of IM and LGD in 27(69.23%) of 39 cases; nevertheless the matched biopsy of these cases showed HGD in 6/39 (15.38%) cases and EAC in 6/39 (15.38%) of cases. The morphology criteria for IMNHGD may need to be made more stringent in order to further reduce miscalling of HGD and EAC.
The cytological criteria for “EAC” are abundant obvious malignant cells with focal necrosis (tumor diathesis) and atypical single cells. The criteria of “suspicious for adenocarcinoma” (SEAC) is similar to EAC, but with scant cellularity and without necrosis. The sensitivity for the cytological diagnosis of HGD/EAC in esophageal brushing/ballooning in most studies ranges from 82% to 100%.Citation31,Citation32,Citation40 In contrast, our observed sensitivity of 40% is much lower compared to most studies. The major problem is the lower cellularity in our study. As high as 37% (18/49) of HGD and EAC cases showed only 0–3 glandular groups in cell blocks, which presented difficulties in making definite cytological diagnosis. As we mentioned before, the improvement of sample collection is our major challenge for increasing the sensitivity of current tests. The cytological studies reporting greatest sensitivity uniformly used visually guided endoscopic brushings to collect the cytological samples. However, the need for endoscopy increases the cost and decreases the convenience of the test compared to the swallowable encapsulated sponge.
The patients in our study were from gastroenterological clinics, which is not representative of population scenario. The analysis of sensitivity and specificity also is limited since our patients are at high risk for BE or dysplasia. In future, we will screen the people in our population with several risk factors such as over 50-year-old, obese, GERD, smoking or alcoholic. Then, we can decide the sensitivity and specificity of our new method to screen the population.
Conclusion
Combining EsophaCap (™) cytology with MUC2 immunohistochemistry has reasonable sensitivity and specificity for screening IM and beyond. However, the EsophaCap has low to moderate accuracy for detection of HGD and EAC, and would be unsuitable for use in a surveillance setting. In the future, combining cytology, MUC2 IHC and molecular (eg, genetic) tests may improve the accuracy for surveilling high-risk patients for HGD and EAC. In addition, improving EsophaCap sampling would also improve sensitivity and specificity.
Abbreviation list
AGC, atypical glandular cells; CCNGC, columnar cell with no goblet cell; CM, columnar metaplasia; EAC, esophageal adenocarcinoma; HGD, high-grade dysplasia; ID, indefinite dysplasia; IHC, immunohistochemistry; IM, intestinal metaplasia; IMNHGD, intestinal metaplasia with no high-grade dysplasia; LGD, low-grade dysplasia; NCC, no or rare columnar cell; ND, non-diagnostic; SFEAC, suspicious for esophageal adenocarcinoma.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We want to thank all support from cytology technician, immunohistochemistry (Qi Yang and Loralee McMahon) and histology staffs at University of Rochester.
Disclosure
The authors report no conflicts of interest in this work.
References
- Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19(6):1468–1470. doi:10.1158/1055-9965.EPI-10-001220501776
- Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol. 2016;31(6):1141–1146. doi:10.1111/jgh.1328926749521
- Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–146. doi:10.1093/jnci/dji02415657344
- Reynolds JV, Donohoe CL, McGillycuddy E, et al. Evolving progress in oncologic and operative outcomes for esophageal and junctional cancer: lessons from the experience of a high-volume center. J Thorac Cardiovasc Surg. 2012;143(5):1130–1137 e1131. doi:10.1016/j.jtcvs.2011.12.00322244551
- Modiano N, Gerson LB. Barrett‘s esophagus: incidence, etiology, pathophysiology, prevention and treatment. Ther Clin Risk Manag. 2007;3(6):1035–1145.18516262
- Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett‘s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125(6):1670–1677.14724819
- Jankowski J, Bennett C, Jankowski JA. Management of Barrett esophagus: a practical guide for clinicians based on the BADCAT and BoB CAT recommendations. Pol Arch Med Wewn. 2015;125(10):765–770.26397112
- Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett‘s esophagus. N Engl J Med. 2011;365(15):1375–1383. doi:10.1056/NEJMoa110304221995385
- Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett‘s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103(13):1049–1057. doi:10.1093/jnci/djr20321680910
- Pohl H, Pech O, Arash H, et al. Length of Barrett‘s oesophagus and cancer risk: implications from a large sample of patients with early oesophageal adenocarcinoma. Gut. 2016;65(2):196–201. doi:10.1136/gutjnl-2015-30922026113177
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American college of G. ACG clinical guideline: diagnosis and management of Barrett‘s Esophagus. Am J Gastroenterol. 2016;111(1):30–50;quiz 51. doi:10.1038/ajg.2015.322
- Iwakiri K, Kinoshita Y, Habu Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol. 2016;51(8):751–767. doi:10.1007/s00535-016-1227-827325300
- Inadomi JM. Surveillance in Barrett‘s esophagus: a failed premise. Keio J Med. 2009;58(1):12–18.19398879
- Gerson LB. Cost-analyses studies in Barrett‘s Esophagus: what is their utility? Gastroenterol Clin North Am. 2015;44(2):425–438. doi:10.1016/j.gtc.2015.02.01126021203
- Yu D, Hopman WM, Paterson WG. Wait time for endoscopic evaluation at a Canadian tertiary care centre: comparison with Canadian association of gastroenterology targets. Can J Gastroenterol. 2008;22(7):621–626.18629391
- Sharma P, Katzka DA, Gupta N, et al. Quality indicators for the management of Barrett‘s esophagus, dysplasia, and esophageal adenocarcinoma: international consensus recommendations from the American gastroenterological association symposium. Gastroenterology. 2015;149(6):1599–1606. doi:10.1053/j.gastro.2015.08.00726296479
- Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological A. American Gastroenterological association technical review on the management of Barrett‘s esophagus. Gastroenterology. 2011;140(3):e18–52;quiz e13. doi:10.1053/j.gastro.2011.01.031
- Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett‘s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122(1):26–33.11781277
- Shen O, Liu SF, Dawsey SM, et al. Cytologic screening for esophageal cancer: results from 12,877 subjects from a high-risk population in China. Int J Cancer. 1993;54(2):185–188.8486421
- Liu SF, Shen Q, Dawsey SM, et al. Esophageal balloon cytology and subsequent risk of esophageal and gastric-cardia cancer in a high-risk Chinese population. Int J Cancer. 1994;57(6):775–780.8206671
- Adams L, Roth MJ, Abnet CC, et al. Promoter methylation in cytology specimens as an early detection marker for esophageal squamous dysplasia and early esophageal squamous cell carcinoma. Cancer Prev Res (Phila). 2008;1(5):357–361. doi:10.1158/1940-6207.CAPR-08-006119137073
- Korsten MA, Worner TM, Feinman L, Shaw S, Federman Q. Balloon cytology in screening of asymptomatic alcoholics for esophageal cancer, Part I. Dig Dis Sci. 1985;30(9):845–851.3896702
- Tsang TK, Hidvegi D, Horth K, Ostrow JD. Reliability of balloon-mesh cytology in detecting esophageal carcinoma in a population of US veterans. Cancer. 1987;59(3):556–559.3791164
- Falk GW, Chittajallu R, Goldblum JR, et al. Surveillance of patients with Barrett‘s esophagus for dysplasia and cancer with balloon cytology. Gastroenterology. 1997;112(6):1787–1797.9178668
- Kadri SR, Lao-Sirieix P, O‘Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett‘s oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. doi:10.1136/bmj.c437220833740
- Kadri S, Lao-Sirieix P, Fitzgerald RC. Developing a nonendoscopic screening test for Barrett‘s esophagus. Biomark Med. 2011;5(3):397–404. doi:10.2217/bmm.11.4021657849
- Paterson AL, Lao-Sirieix P, O‘Donovan M, et al. Range of pathologies diagnosed using a minimally invasive capsule sponge to evaluate patients with reflux symptoms. Histopathology. 2017;70(2):203–210. doi:10.1111/his.13039.
- Leoni-Parvex S, Mihaescu A, Pellanda A, Monnier P, Bosman FT. Esophageal cytology in the follow-up of patients with treated upper aerodigestive tract malignancies. Cancer. 2000;90(1):10–16.10692211
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13(1):77–83 e72. doi:10.1016/j.cgh.2014.06.02624997328
- Benaglia T, Sharples LD, Fitzgerald RC, Lyratzopoulos G. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett‘s esophagus. Gastroenterology. 2013;144(1):62–73 e66. doi:10.1053/j.gastro.2012.09.06023041329
- Kumaravel A, Lopez R, Brainard J, Falk GW. Brush cytology vs. endoscopic biopsy for the surveillance of Barrett‘s esophagus. Endoscopy. 2010;42(10):800–805. doi:10.1055/s-0030-125571020821361
- Geisinger KR, Teot LA, Richter JE. A comparative cytopathologic and histologic study of atypia, dysplasia, and adenocarcinoma in Barrett‘s esophagus. Cancer. 1992;69(1):8–16.1727677
- McIntire MG, Soucy G, Vaughan TL, Shahsafaei A, Odze RD. MUC2 is a highly specific marker of goblet cell metaplasia in the distal esophagus and gastroesophageal junction. Am J Surg Pathol. 2011;35(7):1007–1013. doi:10.1097/PAS.0b013e318218940d21602660
- Jaskiewicz K, Venter FS, Marasas WF. Cytopathology of the esophagus in Transkei. J Natl Cancer Inst. 1987;79(5):961–967.3479644
- Roshandel G, Merat S, Sotoudeh M, et al. Pilot study of cytological testing for oesophageal squamous cell dysplasia in a high-risk area in Northern Iran. Br J Cancer. 2014;111(12):2235–2241. doi:10.1038/bjc.2014.50625247319
- Wright TC Jr., Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46 e41–46 e11. doi:10.1016/j.ajog.2011.07.02421944226
- Mustafa RA, Santesso N, Khatib R, et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int J Gynaecol Obstet. 2016;132(3):259–265. doi:10.1016/j.ijgo.2015.07.02426851054
- Montgomery E, Goldblum JR, Greenson JK, et al. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol. 2001;32(4):379–388. doi:10.1053/hupa.2001.2351111331954
- Kerkhof M, van Dekken H, Steyerberg EW, et al. Grading of dysplasia in Barrett‘s oesophagus: substantial interobserver variation between general and gastrointestinal pathologists. Histopathology. 2007;50(7):920–927. doi:10.1111/j.1365-2559.2007.02706.x17543082
- Saad RS, Mahood LK, Clary KM, Liu Y, Silverman JF, Raab SS. Role of cytology in the diagnosis of Barrett‘s esophagus and associated neoplasia. Diagn Cytopathol. 2003;29(3):130–135. doi:10.1002/dc.1033412951679