Abstract
Extraskeletal Ewing’s sarcoma (E-EWS)/peripheral primitive neuroectodermal tumor (pPNET) is a rare soft tissue tumor that arises from a multipotent progenitor cell and is considered to be of neuroectodermal origin. Although soft tissue E-EWS/pPNETs are common, they are exceedingly rare in the small bowel. Only 30 cases of E-EWS/pPNET of the small bowel have been reported. However, only one case of gastrointestinal perforation has been reported till today. Here, we report the second case of E- EWS/pPNET with gastrointestinal perforation.
Introduction
Ewing sarcoma (EWS) is a highly malignant bone tumor, mostly arising from long bones. The American pathologist James Ewing first described it in 1921. It is most commonly seen in children and young adults between 10 and 30 years of age. The tumor has been found in most organs including the liver, pancreas, lung, penis, prostate, vagina, esophagus, etc. However, Extraskeletal Ewing’s sarcoma/peripheral primitive neuroectodermal tumor (E-EWS/pPNET) of the small bowel is exceedingly rare with only 31 cases reported in the literature including this case. Only one case has been reported till today with gastrointestinal perforationCitation1 Here, we report a second case of primary E-EWS/pPNET of the small bowel presenting with gastrointestinal perforation with a brief review of the literature.
Case history
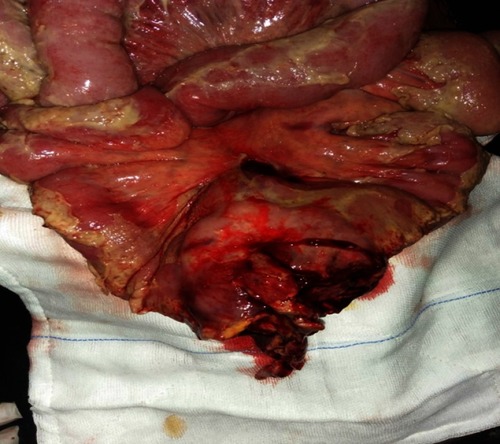
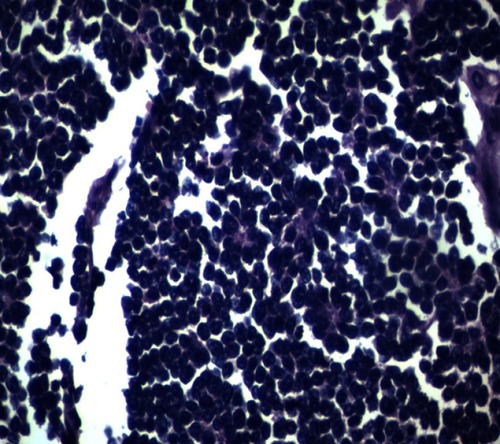
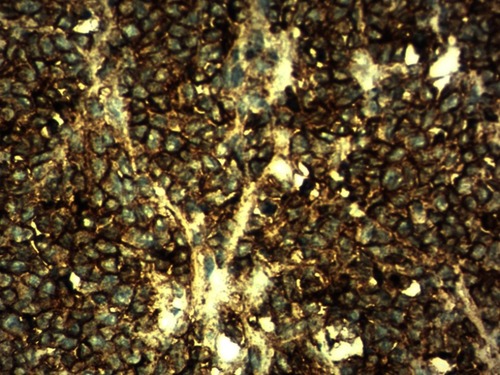
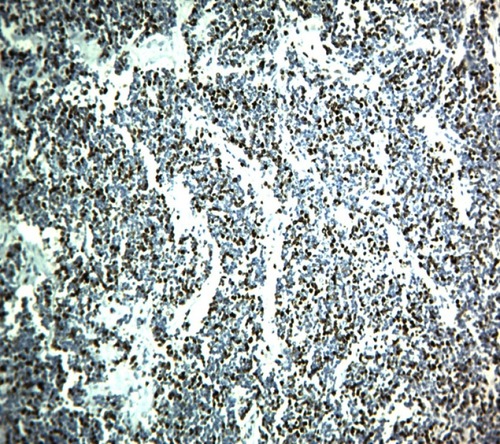
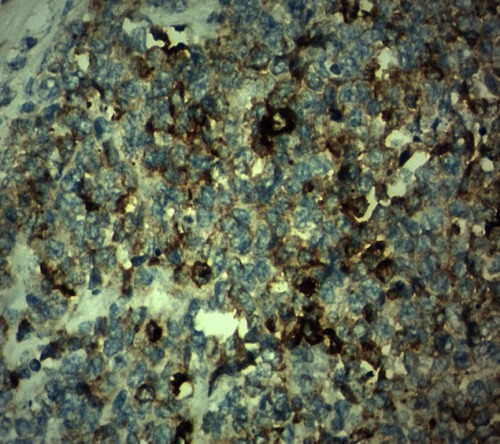
A 42-year-old male patient presented to the emergency room with chief complaints of severe abdominal pain, vomiting and not passing stool for 3 to 4 days. Physical examination revealed a pulse rate of 120/min and blood pressure of 90/60 mmHg. Abdominal examination revealed distension, tenderness, with guarding and rigidity suggesting peritonitis. Laboratory investigations revealed hemoglobin 5.2 g/dl, leukocytosis with left shift (20,000/cu mm with 90% polymorphs), alanine transaminase (ALT)90IU/L, aspartate aminotransferase (AST)20IU/L, an average random blood glucose (100 mg/dl), elevated serum creatinine (2.84 mg/dl), serum amylase (40 U/L), serum lipase (45 U/L), serum bilirubin 2.5 mg/dl, and increased C-reactive protein levels (140 mg/L). X-ray of the abdomen in erect position revealed pneumoperitoneum. Abdominal ultrasonography revealed a large amount of free fluid with internal echoes suggestive of gross peritonitis. As the patient presented in sepsis with pneumoperitoneum and severe anemia, we decided against performing computed tomography (CT) abdomen as it would not change our decision for exploratory laparotomy and only add to the delay. Initial resuscitation with packed cells (2 units), intravenous fluid and vasopressor agents was performed. Exploratory laparotomy revealed gross contamination of the peritoneal cavity with frank pus. After peritoneal lavage with warm saline, we noticed a large 8×8 cm perforated tumor in the distal jejunum (). En bloc resection of the tumor with segmental jejunal resection was performed. The proximal end was brought out as a stoma; the distal end was closed with a feeding tube placed for nutritional purposes as a feeding jejunostomy. The specimen was sent for histopathological evaluation. Histopathological examination was suggestive of malignant round cell tumor (), and Immunohistochemistry (IHC) was advised. Tumor cells were positive for CD99(diffuse strong) (), synaptophysin (focal) (), and Ki 67(80%) () and negative for CD3,CD20,CD5,CD10,TdT (terminal deoxynucleotidyl transferase), panCK (pancytokeratin), chromogranin and CD56. Overall the IHC profile suggested the diagnosis of pPNET. The postoperative course was unremarkable and the patient was discharged on the ninth postoperative day. The patient was referred to the oncologist for chemotherapy. A CT scan of the abdomen 2 months after surgery revealed intraperitoneal metastases without liver metastasis. The patient had under gone six cycles of chemotherapy with cyclophosphamide, vincristine and doxorubicin alternating every 3 weeks. He is at present asymptomatic and doing well on 9-month follow-up.
The patient provided written informed consent for publication of his case details and images.
Discussion
The Ewing sarcoma family of tumors, a subgroup of small-round-cell tumors characterized by specific recurrent translocations (usually EWSR1/FLI1) include Ewing Sarcoma and Primitive neuroectodermal tumors.Citation2 James Ewing was the first to describe EWS in 1921. PNET is an uncommon aggressive malignancy of the bone occurring mainly in childhood and adolescence.Citation3 It is difficult for pathologists to differentiate between EWS and PNET due to overlapping of the genetic abnormality. Therefore, they no longer categorize EWS and PNET as different tumors.Citation3 The distinction lies merely in their degree of differentiation; therefore the 2013 update to the World Health Organization pathology classification system has removed all distinction between PNET and ES.Citation4 The terms are used interchangeably as they represent opposite ends of a spectrum (with PNET being more primitive, despite the name) and because of their common biology, they are treated in common protocols. Batsakis et al divided PNET family of tumors into three groups based on the tissue of origin:Citation5 CNS primitive neuroectodermal tumors (PNETs), neuroblastoma and peripheral primitive neuroectodermal tumors (pPNETs). We shall focus on pPNET in this review.
Most pPNETs occur between the first and third decades of life with a peak incidence in the second decade. Our patient was, unusually, 42 years of age. It is traditionally believed that the outcome is worse in older patients based on stratification in younger cohorts. However, recent studies suggest that age may not be a negative determinant of survival prognosis.Citation6,Citation7 This may be due to intensified chemotherapy and wider use of surgery, but the question is still open.Citation8
The most common locations for pPNET are the chest wall, extremities, and retroperitoneal region. However, a tumor originating from the small intestine is rare.
We performed an extensive literature search using the terms:“E-EWS”, “PNET”, “small bowel”, “gastrointestinal perforation”, on various search engines including PubMed, Embase,GoogleScholar and Semantic Scholar. Since the first report in 2,000 by Horie et alCitation1 a total of 30 cases have been described worldwide. summarizes previously reported small bowel E-EWS/pPNET cases, including our case. We analyzed the cases reported till date using descriptive statistics. Among the reported cases, including our case, there were 16 males and 15 females, with an average age at presentation of 36.7 years (range: 9–63 years); 2 (6.45%) cases were aged <12years (child), while 8(25.80%) cases were in the age group of 12–18 years (adolescent).Eight patients(25.80%) were over 40 years. Thirteen patients (41.93%) were between 20 and 40 years of age. Its incidence (41.93%) is highest in the 20–40 years’ age-group and it is quite rare (6.45%) in children less than 12 years. The peak incidence of EWS is between 10 and 20 years of age. Our analysis does not match this traditional belief. We found that 67.74% cases were over 20 years of age. The location of the E-EWS/PNET within the small bowel in descending order was: ileum 19cases (61.29%), jejunum 7cases (22.58%) and duodenum 2 cases (6.45%); in 3 (9.67%) cases the exact location within the small bowel was not specified. The present example describes an unusual presentation, namely the perforation. The ileum is most commonly involved, and jejunal involvement is rare. The most common presentation in this analysis was abdominal lump/mass in 7 cases (22.58%) followed by iron deficiency anemia in 5(16.12%), intestinal obstruction in 4(12.90%), gastrointestinal perforation including our case 2 (6.45%), and liver metastases in 2(6.45%) cases.The presentation was not documented in 5 cases at the first visit. Therefore, the most common presentation in the review was abdominal lump/mass and the least common was gastrointestinal perforation. Perforation was considered to be caused by tumor necrosis or local ischemic changes. Our case is the second case of gastrointestinal perforation in small intestine E-EWS/pPNET. We also reviewed the management in such cases and found that 27 patients underwent surgical resection, two patients were treated from a core biopsy, while for one patient information was not available.
Table 1 Cases of E-EWS/pPNET of the small bowel, reported in the English literature to date
Histologically, Homer-Wright or Flexner-Wintersteiner rosettes and perivascular pseudorosettes may form from undifferentiated small round cells which constitute pPNETCitation27 The panel suggested by Mhawech-Fauceglia et alCitation30 for the diagnosis of EWS/PNET is a combination of CD99 and FLI1. A few other studies also establish the utility of CD99 for the determination of EWS/PNET.Citation31–Citation33 However, it has also been found in synovial sarcoma, rhabdomyosarcoma and desmoplastic small round cell tumor.Citation34,Citation35 EWS shows diffuse membranous positivity for CD99 whereas synovial sarcoma shows strong cytoplasmic positivity.Citation35 In the present case, we found a diffuse membranous positivity for CD99. In this review, CD99 was positive in 27 out of 29 cases, and in 2 cases CD 99 was negative while in two examples it was not documented. We found 93.10% positivity for CD 99 for the small bowel pPNET. FLI1 is a specific marker for ES. However, it is variably positive in lymphoblastic lymphomaCitation36 In the above analysis FLI1 was not done in 18 cases (58.06%). Kim et alCitation28 also observed that FLI1 was not done in 56% cases. Vimentin is also a useful marker and usually positive while S100, chromogranin A, synaptophysin, and neuron-specific enolase show variable sensitivity.Citation31 In small bowel PNET, Vimentin was documented in only 5 cases (16.1%). We did a panel of negative IHC markers like CD3, CD20, CD5, CD10,TdT to exclude lymphoma, panCK to rule our epithelial cell tumor and desmoplastic small round cell tumor, chromogranin and CD56 to rule out the neuroendocrine tumor and Desmin to rule out rhabdomyosarcoma. Recently, Yoshida et alCitation37 reported that the NKX2.2 gene is a valuable marker for PNET, with a sensitivity of 93% and a specificity of 89%. EWS/FLI1 fusion is sometimes advised to confirm the diagnosis particularly in older patients and/or at unusual location,although sometimes these tests may be negative;Citation38, Identification of the different gene fusion may add prognostic value to existing fluorescence in situ hybridization assays,Citation39 Recently, twenty-five cases of intestinal PNET were reviewed by Liao et alCitation29 they found that RT-PCR EWS-FLI1 was documented only in 16 patients (64%) and FISH break apart EWSR1 was documented in 14 cases (56%). Both of these were not documented in 24% cases.
For E- EWS/pPNET developing in the small bowel, there are no standard protocols. Surgery alone is associated with poor outcome. Therefore, enbloc resection with systemic adjuvant chemotherapy is primarily chosen for local and systemic control as for other ES/PNET.Citation25 The longest reported survival was 204 monthsCitation23 and the shortest was 3 months.Citation26 The commonest cause of death was metastatic disease.
Conclusion
E-EWS/pPNET of the small bowel is exceedingly rare. Gastrointestinal perforation has been reported in only one case till today and this is the second case. Contrary to traditional belief,we observed that E-EWS/pPNET of the small bowel is common in patients over 20 years of age (67.74%). We found abdominal lump to be the most common presentation, and perforation the least common.CD99 was positive in 93.10% and FLI1 was not done in majority of cases (58.06%). Although, CD99 was positive in other round cell tumors, but diffuse membranous positivity along with negative lymphoma, epithelial cell, neuroendocrine, and rhabdomyosarcoma markers suggest E-EWS/pPNET.EWS-FLI1 gene fusion has additional prognostic value. En bloc resection with systemic adjuvant chemotherapy is primarily chosen for local and systemic control as for other E-EWS/pPNET.
Institutional approval
Institutional approval is neither applicable nor required for publication of this manuscript.
Acknowledgments
We would like to acknowledge the help provided by Dr Bhavna Mehta, consultant histopathologist, Supratech Micropath laboratory, Ahmedabad (Gujarat, India) for providing wonderful images of histopathology and IHC.
Disclosure
The authors report no conflicts of interest in this work.
References
- Horie Y, Kato M. Peripheral primitive neuroectodermal tumor of the small bowel mesentery: a case showing perforation at onset. Pathol Int. 2000;50:398–403. doi:10.1046/j.1440-1827.2000.01045.x10849329
- Delattre O, Zucman J, Melot T, et al. The Ewing family of tumors—a subgroup of small-round-cell tumors defined by specific chimeric transcripts. New Engl J Med. 1994;331(5):294–299. doi:10.1056/NEJM1994070733101038022439
- Dehner LP. Primitive neuroectodermal tumor and Ewing’s sarcoma. Am J Surg Pathol. 1993;17:1–13. doi:10.1097/00000478-199301000-000018383465
- WHO. International Classification of Disease for Oncology (ICD-O). 3rd ed. Geneva, Switzerland: World Health Organization; 2013.
- Batsakis JG, Mackay B, el-Naggar AK. Ewing‘s sarcoma and peripheral primitive neuroectodermal tumor: an interim report. Ann Otol Rhinol Laryngol. 1996;105(10):838–843. doi:10.1177/0003489496105010148865780
- Valdes M, Nicholas G, Verma S, Asmis T. Systemic Therapy outcomes in adult patients with ewing sarcoma family of tumors. Case Rep Oncol. 2017;10(2):462–472. doi:10.1159/00047580628626407
- Casey DL, Meyers PA, Alektiar KM, et al. Ewing sarcoma in adults treated with modern radiotherapy techniques. Radiother Oncol. 2014;113:248–253. doi:10.1016/j.radonc.2014.11.02325613397
- Ahmed SK, Robinson SI, Okuno SH, Rose PS, Laack NNI. Adult Ewing sarcoma: survival and local control outcomes in 102 patients with localized disease. Sarcoma. 2013;1–7. Article ID 681425. doi:10.1155/2013/681425
- Graham DK, Stork LC, Wei Q, et al. Molecular genetic analysis of a small bowel primitive neuroectodermal tumor. Pediatr Dev Pathol. 2002;5:86. doi:10.1007/s10024-001-0192-111815873
- Sarangarajan R, Hill DA, Humphrey PA, Hitchcock MG, Dehner LP, Pfeifer JD. Primitive neuroectodermal tumors of the biliary and gastrointestinal tracts: clinicopathologic and molecular diagnostic study of two cases. Pediatr Dev Pathol. 2001;4(2):185–191. doi:10.1007/s10024001014111178636
- Shek TW, Chan GC, Khong PL, Chung LP, Cheung AN. Ewing sarcoma of the small intestine. J Pediatr Hematol Oncol. 2001;23(8):530–532. doi:10.1097/00043426-200111000-0001311878783
- Adair A, Harris SA, Coppen MJ, Hurley PR. Extraskeletal Ewings sarcoma of the small bowel: case report and literature review. J R Coll Surg Edinb. 2001;46:372–374.11768578
- Balasubramanian B, Dinakarababu E, Molyneux AJ. Primary primitive neuroectodermal tumour of the small bowel mesentery: case report. Eur J Surg Oncol. 2002;28(2):197–198. doi:10.1053/ejso.2001.115511884059
- Kie J-H, Lee M-K, Kim C-J, Lee K, Kwon K-W, Yang W-I. Primary Ewing’s sarcoma of the duodenum: a case report. Internat J Surgical Pathol. 2003;11(4):331–337. doi:10.1177/106689690301100416
- Boehm R, Till H, Landes J, Schmid I, Joppich I. Ileoileal intussusception caused by a Ewing sarcoma tumour. an unusual case report. Eur J Pediatr Surg. 2003;13:272–275.13680499
- Bala M, Maly A, Remo N, et al. Peripheral primitive neuroectodermal tumor of bowel mesentery in adults. Isr Med Assoc J. 2006;8:515–516.16889176
- Kim DW, Chang HJ, Jeong JY, et al. Ewing’s sarcoma/primitive neuroectodermal tumor (ES/PNET) of the small bowel: a rare cause of intestinal obstruction. Int J Colorectal Dis. 2007;22(9):1137–1138. doi:10.1007/s00384-006-0142-516683104
- Sethi B, Smith GT. Primary primitive neuroectodermal tumour arising in the small bowel. Histopathology. 2007;50(5):665–666. doi:10.1111/his.2007.50.issue-517394505
- Rodarte-Shade M, Palomo-Hoil R, Vazquez J, et al. Primitive neuroectodermal tumor (PNET) of the small bowel in a young adult with lower gastrointestinal bleeding. J Gastrointest Cancer. 2012;43(Suppl 1):S243–5. doi:10.1007/s12029-012-9409-y22760712
- Vignali M, Zacche MM, Messori P, Natale A, Busacca M. Ewing’s sarcoma of the small intestine misdiagnosed as a voluminous pedunculated uterine leiomyoma. Eur J Obstet Gynecol Reprod Biol. 2012;162(2):234–235. doi:10.1016/j.ejogrb.2012.02.00922410473
- Prasertvit S, Stoikes N. A rare case of Ewing’s sarcoma of the small intestine. Am Surg. 2013;79:E78–E79.23336641
- Kim JM, Chu YC, Choi CH, et al. Peripheral primitive neuroectodermal tumor with osseous component of the small bowel mesentery: a case study. Korean J Pathol. 2013;47:77–81. doi:10.4132/KoreanJPathol.2013.47.1.7723482293
- Milione M, Gasparini P, Sozzi G, et al. Ewing sarcoma of the small bowel: a study of seven cases, including one with the uncommonly reported EWSR1-FEV translocation. Histopathology. 2014;64(7):1014–1026. doi:10.1111/his.1235024898918
- Padma M, Lakshmi RR, Kumar CK. Extraskeletal Ewing’s sarcoma of the small bowel. Int J Med Sci Clin Invent. 2015;2:645–647.
- Peng L, Yang L, Wu N, Wu BO. Primary primitive neuroectodermal tumor arising in the mesentery and ileocecum: a report of three cases and review of the literature. Exp Ther Med. 2015;9:1299–1303. doi:10.3892/etm.2015.224225780425
- Liu Z, Xu YH, Ge CL, Long J, Du RX, Guo KJ. Huge peripheral primitive neuroectodermal tumor of the small bowel mesentery at nonage: a case report and review of the literature. World J Clin Cases. 2016;4(9):306–309. doi:10.12998/wjcc.v4.i9.30627672649
- Li T, Zhang F, Cao Y, et al. Primary Ewing’s sarcoma/primitive neuroectodermal tumor of the ileum: case report of a 16-year-old Chinese female and literature review. Diagn Pathol. 2017;12:37. doi:10.1186/s13000-017-0626-328472972
- Kim YS, Moon HM, Lee KS, et al. Pediatric Ewing’s sarcoma/primitive neuroectodermal tumor (ES/PNET) developed in the small intestine: a case report. Clin Pediatr Hematol Oncol. 2017;24:162–168. doi:10.15264/cpho.2017.24.2.162
- Liao YS. Chiang IH and Gao HW. A mesenteric primary peripheral Ewing’s sarcoma/primitive neuroectodermal tumor with molecular cytogenetic analysis: report of a rare case and review of literature. Indian J Pathol Microbiol. 2018;61:248–251. doi:10.4103/IJPM.IJPM_546_1729676369
- Mhawech-Fauceglia P, Herrmann F, Penetrante R, et al. Diagnostic utility of FLI-1 monoclonal antibody and dual-colour, break-apart probe fluorescence in situ (FISH) analysis in Ewing’s sarcoma/primitive neuroectodermal tumour (EWS/PNET).A comparative study with CD99 and FLI-1 polyclonal antibodies. Histopathology. 2006;49:569–575. doi:10.1111/j.1365-2559.2006.02535.x17163841
- Kempson RL, Fletcher CDM, Evans HL, et al. Tumors of the Soft Tissues. 3rd ed. Washington, DC: The Armed Forces Institute of Pathology; 2001:444–452.
- Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. MIC2 is a specific marker for Ewing’s sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing’s sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991;67:1886–1893.1848471
- Ramani P, Rampling D, Link M. Immunocytochemical study of 12E7 in small round-cell tumours of childhood: an assessment of its sensitivity and specificity. Histopathology. 1993;23:557–561. doi:10.1111/his.1993.23.issue-68314240
- Kumar R, Gautam U, Srinivasan R, et al.Primary Ewing’s sarcoma/Primitive neuroectodermal tumor of the kidney: report of a case diagnosed by fine needle aspiration cytology and confirmed by immunohistochemistry and RT-PCR along with review of literature. Diagn Cytopathol. 2012;(Suppl 2):E156–E161. doi:10.1002/dc.2171721548122
- Sanati S, Lu DW, Schmidt E, Perry A, Dehner LP, Pfeifer JD. Cytologic diagnosis of Ewing sarcoma/peripheral neuroectodermal tumor with paired prospective molecular genetic analysis. Cancer. 2007;111:192–199. doi:10.1002/cncr.2269217487851
- Lin O, Filippa DA, Teruya-Feldstein J. Immunohistochemical evaluation of [6] FLI-1 in acute lymphoblastic lymphoma (ALL): a potential diagnostic pitfall. Appl Immunohistochem Mol Morphol. 2009;17:409–412. doi:10.1097/PAI.0b013e3181972b6d19349856
- Yoshida A, Sekine S, Tsuta K, et al. NKX2.2 is a useful immunohistochemical marker for Ewing sarcoma. Am J Surg Pathol. 2012;36:993–999. doi:10.1097/PAS.0b013e31824ee43c22446943
- Lewis TB, Coffin CM, Bernard PS. Differentiating Ewing's sarcoma from other round blue cell tumors using a RT-PCR translocation panel on formalin-fixed paraffin-embedded tissues. Mod Pathol. 2007;20:397–40417334332
- Tanas MR, Rubin BP, Tubbs RR, Billings SD, Downs-Kelly E, Goldblum JR. Utilization of fluorescence in situ hybridization in the diagnosis of 230 mesenchymal neoplasms: an institutional experience. Arch Pathol Lab Med. 2010;134:1797–1803.21128778