Abstract
Background:
This paper describes a Phase 1, single-center, randomized, open-label, two-period crossover study which compared the pharmacodynamic effects of single doses of dexlansoprazole modified-release 60 mg and esomeprazole 40 mg on 24-hour intragastric pH in healthy adult subjects.
Methods:
Forty-four subjects aged 20–54 years were randomized in a 1:1 ratio to two sequence groups defining the order in which they received dexlansoprazole and esomeprazole in periods 1 and 2. Primary pharmacodynamic end points over 24 hours postdose were percentage of time with intragastric pH > 4 and mean pH, and secondary pharmacodynamic end points were percentage of time intragastric pH > 4, and mean pH at 0–12 hours, and at >12–24 hours postdose. Each drug was given after an overnight fast and one hour before breakfast. Continuous pH recording began immediately before dosing through to 24 hours postdose.
Results:
At 0–24 hours postdose, the mean percentage of time with pH > 4 for dexlansoprazole and esomeprazole was 58% and 48%, respectively; the difference was statistically significant (P = 0.003). The average of mean pH values at 0–24 hours postdose for dexlansoprazole and esomeprazole were 4.3 and 3.7, respectively; the difference was statistically significant (P < 0.001). At >12–24 hours postdose, mean percentage of time with pH > 4 and average of mean pH were greater for dexlansoprazole (60% and 4.5, respectively) compared with esomeprazole (42% and 3.5, respectively); the difference was statistically significant (P < 0.001 for both intervals). At 0–12 hours postdose, the difference in dexlansoprazole and esomeprazole values for the pharmacodynamic end points was not statistically significant.
Conclusion:
For the entire 24-hour postdose period, predominantly resulting from the >12–24-hour postdose interval, the average intragastric pH following a single dose of dexlansoprazole 60 mg was higher compared with that observed following a single dose of esomeprazole 40 mg, and the difference was statistically significant.
Introduction
Dexlansoprazole and esomeprazole belong to the class of drugs known as proton pump inhibitors. Proton pump inhibitors inhibit the secretion of hydrogen ions in the stomach by inhibiting the (H+,K+)-ATPase enzyme (proton pump) at the secretory surface of the gastric parietal cell, resulting in potent inhibition of gastric acid secretion with prolonged elevation of intragastric pH. Both drugs are marketed in the United States for healing of and maintenance of healed erosive esophagitis and treatment of heartburn associated with symptomatic, nonerosive gastroesophageal reflux disease.
One of the limitations to the use of proton pump inhibitors on a once-daily basis has been incomplete acid suppression over the 24-hour postdose interval.Citation1 Dexlansoprazole modified-release is a formulation that uses an innovative dual delayed-release (DDR™) delivery system. DDR technology is designed to provide an initial drug release in the proximal small intestine followed by another drug release at more distal regions of the small intestine several hours later.Citation2 As a result, dexlansoprazole modified-release produces a plasma concentration-time profile with two distinct peaks, whereby the first peak occurs 1–2 hours after administration, followed by a second peak at 4–5 hours postdose.Citation2–Citation4 Esomeprazole is a delayed-release formulation with single-release characteristics that produces maximum plasma concentrations at approximately 1.6 hours postdose.Citation5 Dexlansoprazole modified-release may be taken without regard to meals.Citation4,Citation6 In comparison, esomeprazole is recommended to be taken at least one hour before a meal to achieve maximal efficacy.Citation5
The pharmacodynamic, pharmacokinetic, and safety profiles of various proton pump inhibitors following administration in humans have been extensively studied.Citation2,Citation3,Citation7–Citation11 However, this is the first clinical study reported in the literature as a head-to-head comparison of the pharmacodynamics of dexlansoprazole modified-release and esomeprazole after a single dose. Because the study population consisted of healthy adult subjects, no efficacy endpoints were evaluated in this study. Comparison of the single-dose pharmacodynamics of dexlansoprazole modified-release and esomeprazole in a well controlled crossover study adds to the knowledge base for proton pump inhibitors without having to compare across studies. It is known from the literature that these two proton pump inhibitors have pharmacokinetic differences. Esomeprazole exhibits a dose-dependent and time-dependent pharmacokinetic profile that results in an approximate 2.5-fold increase in bioavailability at steady state and increased pharmacodynamic effects after five days of once-daily dosing.Citation12 The pharmacokinetics of dexlansoprazole modified-release are time-independent and the pharmacokinetic and pharmacodynamic profiles after five days of once-daily dosing are similar (less than 10% difference) to those observed after a single dose.Citation2,Citation4
The objective of the current trial was to evaluate the pharmacodynamic effects of single doses of dexlansoprazole modified-release 60 mg and esomeprazole 40 mg on 24-hour intragastric pH in healthy subjects. The dosage strengths chosen for this study were the highest approved for healing of erosive esophagitis (60 mg for dexlansoprazole modified-release and 40 mg for esomeprazole).
Materials and methods
Ethics
The institutional review board (IntegReview, Austin, TX) reviewed and approved the study protocol, protocol amendment 1, and the informed consent form prior to enrollment of subjects; all subjects were enrolled under amendment 1. This study was conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice, the ethical principles of the World Medical Association Declaration of Helsinki, and local regulations.
Study population
Eligible subjects were adult men or women, aged 18–55 years inclusive, and in good health. Women of childbearing potential were required to have negative urine pregnancy tests at screening and at day -1 of period 1. Sexually active women and men agreed to use acceptable contraception for a period of time starting from signing of the informed consent throughout the duration of the study and for 30 days following the last dose of study drug.
Women who were pregnant or lactating, and any subjects with an uncontrolled, clinically significant disorder or abnormality which may have impacted the ability of a subject to participate in the study or potentially confound the study results were excluded as per the study exclusion criteria. In addition, subjects were excluded if they had a hypersensitivity to any component of dexlansoprazole modified-release, esomeprazole, or related compounds, had any significant findings from physical examination or clinical laboratory test results, had positive test results on urine screens for alcohol and drugs of abuse, a positive serum caffeine screen, a positive breath test for Helicobacter pylori at screening, or consumed any medication or foods contraindicated by the protocol.
Medication or dietary products including grapefruit or Seville oranges, nicotine-containing products, prescription medication (except for hormonal contraceptives and hormone replacement therapy, if on a stable dose for at least 90 days prior to day 1 of period 1), hepatic or renal clearance altering agents, over the counter medications, vitamin supplements, and alcohol or caffeine-containing products were excluded during the screening and treatment periods. Occasional use of acetaminophen (≤2 g/day) was allowed, except on day 1 of each period.
Study design
This open-label, randomized, two-period crossover study was conducted at a single study site. During each period, subjects were confined to the study site from day -1 until all study procedures were completed on day 2. A washout interval of at least seven days separated doses of study drugs in periods 1 and 2. This washout interval was considered sufficient because the half-lives are 1–2 hoursCitation4 and approximately 1.6 hoursCitation5 for dexlansoprazole and esomeprazole, respectively, and it allowed intragastric pH to return to baseline levels between doses of study drugs.
On day 1 of each period, study drug was administered at approximately 8 am after an overnight fast of at least eight hours and followed by a 60-minute postdose fast. Breakfast, lunch, dinner, and an evening snack were served at hours 1, 4, 9, and 12 postdose, respectively. During confinement in period 2, subjects received meals identical to those received by the confined subjects in period 1. Each meal was standardized to contain approximately 25% fat. Blood samples were collected at scheduled time points up to 24 hours postdose to quantify dexlansoprazole and esomeprazole plasma concentrations. Intragastric pH recording was performed for 24 hours beginning immediately prior to study drug administration on day 1 of each period. Subjects fasted overnight for at least eight hours prior to collection of blood and urine samples for safety laboratory tests on day 2 of each period.
Pharmacodynamic assessments
On day -1 of period 1, a single-channel antimony probe attached to a Digitrapper® data recorder (Sierra Scientific Instruments, Los Angeles, CA) was inserted intranasally into the stomach to a distance of approximately 10 cm past the lower esophageal sphincter. This procedure verified that the subject could tolerate probe insertion and obtained the length of the probe insertion that was used on day 1 of periods 1 and 2. The unit for measurement of intragastric pH was calibrated with standard buffers (pH approximately 1 and 7) before each use. On day 1 of periods 1 and 2, intragastric pH was recorded every four seconds over the 24-hour postdose interval; however, median intragastric pH values over 15-minute intervals were determined and used for the calculation of pharmacodynamic parameters.
The primary pharmacodynamic parameters calculated for each treatment regimen over 24 hours postdose were percentage of time with intragastric pH > 4 (ie, percentage of time that the medians over 15-minute intervals had pH values > 4) and mean intragastric pH (ie, the average of the medians over 15-minute intervals). Secondary pharmacodynamic parameters were percentage of time with pH > 4 and mean intragastric pH calculated for the time intervals 0–12 hours postdose and >12–24 hours postdose.
Pharmacokinetic assessments
Blood samples for the determination of dexlansoprazole and esomeprazole concentrations in plasma were collected into chilled Vacutainers® containing dipotassium ethylenediaminetetraacetic acid (K2EDTA) according to the following schedule: baseline (within 30 minutes prior to day 1 dosing) and at hours 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, 16, and 24 postdose. Plasma concentrations of dexlansoprazole and esomeprazole were determined by liquid chromatography and tandem mass spectrometry at PPD Development (Middleton, WI), with validated concentration ranges of 2.00–2000 ng/mL for dexlansoprazole and 1.00–1000 ng/mL for esomeprazole. Plasma concentrations below the lower limit of quantification were set to zero for calculation of mean plasma concentrations and derivation of individual subject pharmacokinetic parameters.
The following pharmacokinetic parameters were calculated for dexlansoprazole and esomeprazole plasma concentration data: area under the plasma concentration-time curve from time 0 to the time of the last quantifiable concentration (AUCt) and to infinity (AUC∞); maximum observed plasma concentration (Cmax); time to reach Cmax (Tmax); elimination half-life; oral clearance; and apparent volume of distribution. Pharmacokinetic parameters were derived using noncompartmental methods with WinNonlin® Enterprise, Version 5.2 (Pharsight Corporation, Mountain View, CA). Actual sample times, rather than scheduled sampling times, were used in all pharmacokinetic computations involving sampling times.
Assessment of CYP2C19 metabolizer status
The cytochrome P450 (CYP) 2C19 isozyme is a polymorphic enzyme that is involved in the metabolism of dexlansoprazoleCitation4 and esomeprazole.Citation5 One blood sample for DNA isolation was collected before dosing on day 1 of period 1 from each subject in the study into plastic K2EDTA spray-coated tubes and stored under frozen conditions. A portion of the DNA sample was analyzed for the presence of CYP2C19 allelic variants (Covance Central Laboratory, Indianapolis, IN). Genotyping and phenotyping analysis for CYP2C19 was performed for all subjects to determine CYP2C19 metabolizer status.
Statistical analysis
A sample size of 44 subjects, 22 subjects per sequence group, allowed for up to four dropouts (an approximate 9% dropout rate) and still provided at least 93% power to detect a 10% difference in the percentage of time with intragastric pH > 4 over 24 hours between the two treatment regimens. The sample size was calculated using 159.13 as the intrasubject variance for the percentage of time with intragastric pH > 4, which was estimated from a previous dexlansoprazole modified-release study.Citation6 The power for detecting a difference of 0.5 in mean 24-hour pH between the two treatment regimens was expected to be greater than 95%. Differences were deemed statistically significant if P was ≤0.05.
An analysis of variance model that included fixed effects of sequence, period, and regimen, as well as a random effect of subject nested within sequence was fitted to the pharmacodynamic parameters. Pairwise comparisons between treatment regimens were conducted. Intragastric pH values > 0 but ≤8 were included in the median calculations. Only subjects who had valid pharmacodynamic parameters estimated for both periods were included in the pharmacodynamic analyses for that parameter. The effect of CYP2C19 metabolizer status on the pharmacodynamics of dexlansoprazole and esomeprazole was assessed by performing an additional analysis of variance that excluded the subjects identified as CYP2C19 poor metabolizers.
Descriptive statistics were used to summarize the plasma concentrations of dexlansoprazole and esomeprazole and their single-dose pharmacokinetic parameters from time of dose (0 hour) to 24 hours postdose for all subjects who completed at least one treatment period.
All data analyses were performed using SAS® Version 9.1 (SAS Institute, Cary, NC). There was no imputation of incomplete or missing data.
Safety analysis
All subjects who received at least one dose of study drug were included in the safety analysis. All safety assessments, including adverse events, clinical laboratory evaluations, 12-lead electrocardiogram results, vital sign measurements, and physical examination findings were summarized by treatment regimen with descriptive statistics, where deemed appropriate. A treatment-emergent adverse event was defined as an adverse event or serious adverse event that started or worsened after receiving the first dose of study drug and within 30 days after the last dose of study drug. Adverse event verbatim reported terms were coded to system organ class and then to the first listed preferred term using the Medical Dictionary for Regulatory Activities, Version 13.1.
Results
Study population
This study was conducted between September 2010 and October 2010. Forty-four subjects, comprising 21 (47.7%) men and 23 (52.3%) women were enrolled, of whom 43 completed the study in accordance with the protocol and had complete pharmacodynamic and pharmacokinetic data for both treatment periods. Ten of the 44 subjects (22.7%) were black or African American, while the race of the remaining 34 subjects (77.3%) was Caucasian. The mean age was 36.7 (range 20–54) years, weight 73.1 kg, height 170.4 cm, and body mass index 25.1 kg/m2. One subject voluntarily withdrew consent for personal reasons after receiving esomeprazole in period 1, but prior to administration of dexlansoprazole modified-release in period 2. Pharmacokinetic and safety data for this subject from period 1 were included in the summaries. However, because the analysis of variance required pharmacodynamic data from both treatment regimens, this subject was not included in the pharmacodynamic analysis. Forty-two of 44 subjects (95.5%) were extensive CYP2C19 metabolizers and two subjects (4.5%) were poor CYP2C19 metabolizers. Data from all subjects, regardless of CYP2C19 metabolizer status, were included in the pharmacodynamic analyses and pharmacokinetic summary because this was a crossover study where subjects received both treatment regimens and the subjects acted as their own control.
Pharmacodynamics
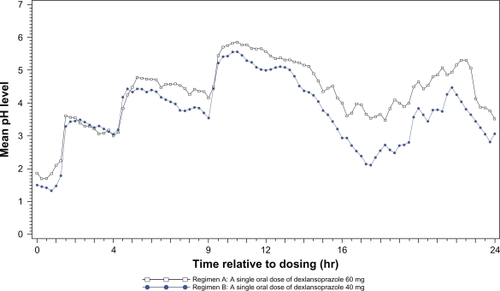
Mean intragastric pH over 24 hours after single doses of dexlansoprazole modified-release and esomeprazole are presented in . Period and sequence effects were not found to be statistically significant. The pharmacodynamic profiles at 0–24 hours after a single dose of dexlansoprazole modified-release or esomeprazole were generally similar to that reported in the literature.Citation2,Citation3,Citation9,Citation10,Citation12
Figure 1 Mean intragastric pH from 0 to 24 hours postdose after single oral doses of dexlansoprazole modified-release 60 mg (n = 43) and esomeprazole 40 mg (n = 44) delayed-release capsules.
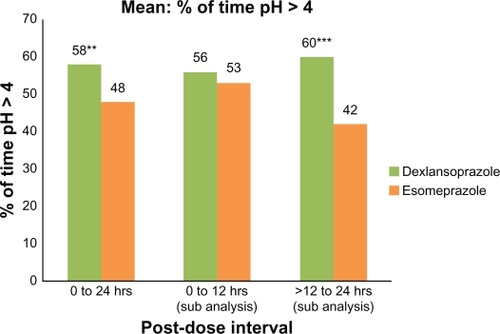
Over the 24-hour postdose period, the mean percentage of time with intragastric pH > 4 was 58% for dexlansoprazole compared with 48% for esomeprazole; the difference was statistically significant (P = 0.003, ). Similarly, >12–24 hours postdose, the mean percentage of time with intragastric pH > 4 was 60% for dexlansoprazole relative to 42% with esomeprazole, a difference that was statistically significant (P < 0.001). At 0–12 hours postdose, the mean percentage of time with pH > 4 for dexlansoprazole and esomeprazole was 56% and 53%, respectively, and the difference was not statistically significant.
Figure 2 Mean percentage of time with intragastric pH > 4.0 at 0–24 hours, 0–12 hours, and >12–24 hours after single oral doses of dexlansoprazole modified-release 60 mg and esomeprazole 40 mg delayed-release capsules (n = 43). Only subjects who had valid pharmacodynamic parameters estimated for both periods were included in the pharmacodynamic analyses for that parameter.
Notes: *P ≤ 0.05; **P < 0.01; ***P < 0.001.
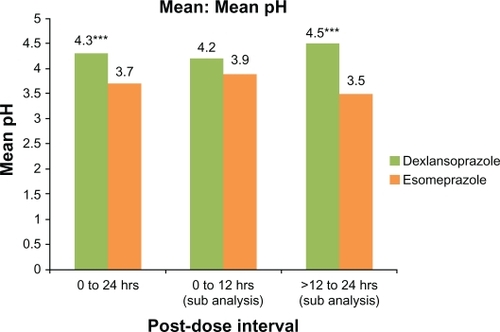
Over the 24-hour postdose period, the average of mean intragastric pH for dexlansoprazole was 4.3 compared with 3.7 for esomeprazole and the difference was statistically significant (P < 0.001, ). Likewise, >12–24 hours postdose, the average of mean intragastric pH was 4.5 for dexlansoprazole compared with 3.5 for esomeprazole and the difference was statistically significant (P < 0.001). At 0–12 hours postdose, the average of mean intragastric pH for dexlansoprazole was 4.2 compared with 3.9 for esomeprazole, and the difference was not statistically significant.
Figure 3 Mean intragastric pH at 0–24 hours, 0–12 hours, and >12–24 hours after single oral doses of dexlansoprazole modified-release 60 mg and esomeprazole 40 mg delayed-release capsules (n = 43). Only subjects who had valid pharmacodynamic parameters estimated for both periods were included in the pharmacodynamic analyses for that parameter.
Notes: *P ≤ 0.05; **P < 0.01; ***P < 0.001.
Pharmacokinetics
The mean plasma concentration-time curves for dexlansoprazole and esomeprazole in our study were generally similar to that reported in the literature.Citation2,Citation13–Citation15 Following administration of a single dose of dexlansoprazole modified-release 60 mg, the mean plasma concentration-time curve displayed two peaks at approximately 2 and 5 hours postdose, which are representative of the dual delayed-release characteristics of the dexlansoprazole modified-release capsule; in addition, plasma concentrations were generally detectable throughout the 24-hour postdose interval. For esomeprazole, the median Tmax occurred at approximately two hours after dosing, and plasma concentrations rapidly decreased thereafter (). Concentrations of dexlansoprazole were detectable in the plasma of all subjects (100%) at 12 hours postdose, in 38 of 43 subjects (88%) at 16 hours postdose, and 27 of 43 subjects (63%) at 24 hours postdose. In contrast, esomeprazole was detected in the plasma of 35 of 44 subjects (80%) at 12 hours postdose, 17 of 44 subjects (39%) at 16 hours postdose, and four of 44 subjects (9%) at 24 hours postdose. Plasma pharmacokinetic parameters for dexlansoprazole and esomeprazole are shown in .
Figure 4 Mean plasma concentration-time curves of dexlansoprazole and esomeprazole after single oral doses of dexlansoprazole modified-release 60 mg (n = 43) and esomeprazole 40 mg (n = 44) delayed-release capsules in healthy subjects, linear scale.
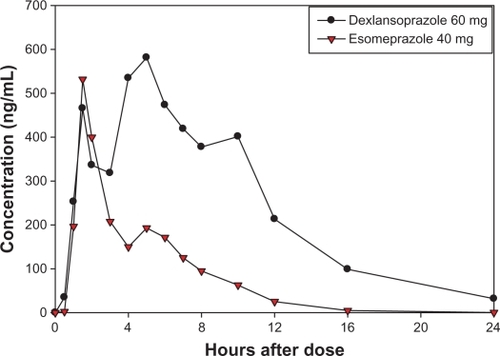
Table 1 Summary of plasma pharmacokinetic parameters for dexlansoprazole and esomeprazole after single oral doses of dexlansoprazole MR 60 mg and esomeprazole 40 mg
Effect of CYP2C19 status on pharmacodynamics and pharmacokinetics
Administration of a proton pump inhibitor with CYP2C19-dependent metabolism may result in higher plasma concentrations in subjects who are CYP2C19 poor metabolizers. Two of the 44 enrolled subjects were determined to be CYP2C19 poor metabolizers. Both subjects had AUC values that were substantially higher than the overall means for dexlansoprazole and esomeprazole. Data for these subjects were included in the pharmacodynamic analyses and pharmacokinetic summary because this was a crossover study where subjects received both treatment regimens, and subject metabolizer status was not expected to affect the overall conclusions of the study. To confirm this assumption, an analysis of variance model that was similar to the analysis for the complete data was fitted to the pharmacodynamic data, excluding the data from the two poor metabolizer subjects. Exclusion of the pharmacodynamic data from the poor metabolizers did not alter the statistical results obtained from the complete data set. Additional descriptive statistics were calculated for the pharmacodynamic and pharmacokinetic parameter data, excluding the data from the poor metabolizers, and indicated that inclusion of data from the poor metabolizers did not affect the overall pharmacodynamic or pharmacokinetic conclusions of the study (data on file at Takeda).
Safety
The incidence of treatment-emergent adverse events was 21% (nine of 43 subjects) and 14% (six of 44 subjects) after single-dose administration of dexlansoprazole modified-release and esomeprazole, respectively. All of the adverse events were rated mild or moderate in severity and only one adverse event (nausea) was considered related to the study drug (dexlansoprazole modified-release) by the investigator. The most common adverse events reported by at least two subjects with dexlansoprazole modified-release were headache (four of 43 subjects, 9%), flatulence (two of 43 subjects, 5%), and joint injury (two of 43 subjects, 5%). No adverse event was reported by two or more subjects with esomeprazole. There were no serious adverse events, deaths, or premature study discontinuations as a result of an adverse event. No clinically meaningful changes in clinical laboratory values, physical examination findings, vital signs, or 12-lead electrocardiograms were reported over the course of study.
Discussion
Proton pump inhibitors are the drugs of choice for the healing of erosive esophagitis, maintenance of healed erosive esophagitis, and sustained resolution of symptomatic, nonerosive reflux disease. Limitations to the use of proton pump inhibitors on a once-daily basis have been a delayed onset of action, incomplete acid suppression over the 24-hour postdose interval, and the need for ingestion before a meal to achieve maximal efficacy.Citation1,Citation3 To date, attempts to overcome these issues have included the development of isomeric proton pump inhibitors with stereoselective metabolism (ie, dexlansoprazole [the R-enantiomer of lansoprazole] and esomeprazole [the S-enantiomer of omeprazole]) and alterations in drug delivery to prolong the inhibition of gastric acid secretion.Citation3 The dexlansoprazole DDR technology is designed to provide an initial drug release in the proximal small intestine followed by another drug release at more distal regions of the small intestine several hours later. As a result, dexlansoprazole modified-release produces a dual-peaked pharmacokinetic profile that prolongs the plasma concentration-time profile of dexlansoprazole.Citation2 Unlike esomeprazole, which should be taken one hour prior to a meal,Citation5 dexlansoprazole modified-release can be taken without regard to food.Citation4 The aim of this study was to evaluate the pharmacodynamic effects of single doses of dexlansoprazole modified-release 60 mg and esomeprazole 40 mg on 24-hour intragastric pH in healthy subjects. The dosage strengths chosen for this study were the highest approved for healing of erosive esophagitis (60 mg for dexlansoprazole modified-release and 40 mg for esomeprazole).
The measurement of intragastric pH is a well accepted method for the assessment of the pharmacodynamic effects of a proton pump inhibitor.Citation16–Citation18 The present study is the first head-to-head comparison of the pharmacodynamic effects for a 24-hour period following single doses of dexlansoprazole modified-release and esomeprazole in healthy subjects, utilizing doses that are recommended for healing of erosive esophagitis.
The results of this study indicate that the DDR formulation technology of dexlansoprazole modified-release leads to an extended duration of gastric acid control on day 1 compared with the delayed-release formulation of esomeprazole. After a morning dose of each study drug, the extended duration in pharmacodynamic activity after dexlansoprazole modified-release was demonstrated 0–24 hours postdose, mainly due to the significant differences observed during the >12–24 hours postdose interval relative to esomeprazole. The two proton pump inhibitors had comparable pharmacodynamic activity 0–12 hours postdose.
Overall, the pharmacodynamic profiles in our study were similar to those observed in other published reports.Citation2,Citation9 The primary pharmacodynamic parameter, mean percentage of time with intragastric pH > 4 from time 0–24 hours postdose, was 58% for dexlansoprazole versus 48% for esomeprazole (). The literature reported that percentage of time with intragastric pH > 4 from time 0–24 hours after the same doses were approximately 60% for dexlansoprazole modified-releaseCitation2 and 54% and 52% for esomeprazole.Citation10,Citation12
A strength of this study is its randomized, crossover design, with each subject acting as his/her own control. A limitation is the single-dose design, preventing extrapolation of the results to a multiple-dose regimen. The study population of healthy volunteers does not allow for assessment of clinical efficacy, and no clinical significance is intended or implied. Nevertheless, the comparison of two proton pump inhibitor enantiomers with different formulation characteristics is of pharmacological interest. Although there are extensive data available detailing the multiple-dose pharmacodynamics and pharmacokinetics of proton pump inhibitors, this is the first clinical study reported in the literature to provide a comparison of the pharmacodynamics of dexlansoprazole modified-release and esomeprazole after a single dose in a well controlled crossover study.
In conclusion, a single dose of dexlansoprazole modified-release 60 mg provided statistically significantly greater pH control for the entire 24-hour postdose interval when compared with a single dose of esomeprazole 40 mg. This observed difference was mainly due to the statistically significant greater pH control from dexlansoprazole modified-release over the >12–24-hour postdose interval as compared with esomeprazole. The two proton pump inhibitors had comparable pharmacodynamic activity at 0–12 hours postdose.
Acknowledgements
The authors wish to acknowledge the contribution of the Jasper Clinic Inc.
Disclosure
MK, CE, and SN are employees of Takeda Global Research & Development Center Inc, Deerfield, IL. Writing assistance was funded by Takeda Pharmaceuticals North America Inc. This study was sponsored by Takeda Global Research and Development Center Inc.
References
- DeVaultKRTalleyNJInsights into the future of gastric acid suppressionNat Rev Gastroenterol Hepatol2009652453219713987
- VakilyMZhangWWuJAtkinsonSNMulfordDPharmacokinetics and pharmacodynamics of a known active PPI with a novel dual delayed release technology, dexlansoprazole MR: A combined analysis of randomized controlled clinical trialsCurr Med Res Opin20092562763819232037
- MetzDCVakilyMDixitTMulfordDReview article: Dual delayed release formulation of dexlansoprazole MR, a novel approach to overcome the limitations of conventional single release proton pump inhibitor therapyAliment Pharmacol Ther20092992893719298580
- Dexilant® (dexlansoprazole) delayed release capsules [package insert]Deerfield, ILTakeda Pharmaceuticals America Inc2011
- Nexium® (esomeprazole magnesium) delayed-release capsules [package insert]Wilmington, DEAstraZeneca Pharmaceuticals LP2011
- LeeRDVakilyMMulfordDWuJAtkinsonSNClinical trial: The effect and timing of food on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR, a novel dual delayed release formulation of a proton pump inhibitor – evidence for dosing flexibilityAliment Pharmacol Ther20092982483319243357
- MinerPJrKatzPOChenYSostekMGastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: A five-way crossover studyAm J Gastroenterol2003982616262014687806
- MinerPBJrTutuianRCastellDOLiuSSostekMBIntragastric acidity after switching from 5-day treatment with intravenous pantoprazole 40 mg/d to 5-day treatment with oral esomeprazole 40 mg/d or pantoprazole 40 mg/d: An open-label crossover study in healthy adult volunteersClin Ther20062872573316861094
- WarringtonSBaisleyKDunnKBoyceMMorocuttiAEffects of single doses of rabeprazole 20 mg and esomeprazole 40 mg on 24-h intragastric pH in healthy subjectsEur J Clin Pharmacol20066268569116850327
- MorelliGChenHRossiterGRegeBLuYAn open-label, parallel, multiple-dose study comparing the pharmacokinetics and gastric acid suppression of rabeprazole extended-release with esomeprazole 40 mg and rabeprazole delayed-release 20 mg in healthy volunteersAliment Pharmacol Ther20113384585421272047
- ThoringMHedenströmHErikssonLSRapid effect of lansoprazole on intragastric pH: A crossover comparison with omeprazoleScand J Gastroenterol19993434134510365892
- HunfeldNGTouwDJMathotRAA comparison of the acid-inhibitory effects of esomeprazole and pantoprazole in relation to pharmacokinetics and CYP2C19 polymorphismAliment Pharmacol Ther20103115015919785625
- BladhNBlychertEJohanssonKA new esomeprazole packet (sachet) formulation for suspension: In vitro characteristics and comparative pharmacokinetics versus intact capsules/tablets in healthy volunteersClin Ther20072964064917617287
- JunghardOHassan-AlinMHasselgrenGThe effect of area under the plasma concentration vs time curve on the maximum plasma concentration of esomeprazole on intragastric pHEur J Clin Pharmacol20025845345812389067
- Hassan-AlinMAnderssonTBredbergERöhssKPharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjectsEur J Clin Pharmacol20005666567011214773
- BellNJVBurgetDHowdenCWWilkinsonJHuntRHAppropriate acid suppression for the management of gastro-oesophageal reflux diseaseDigestion199251Suppl 159671397746
- ArmstrongDReview article: Gastric pH – the most relevant predictor of benefit in reflux disease?Aliment Pharmacol Ther200420Suppl 5192615456460
- KatzPOGinsbergGGHoylePESostekMBMonyakJTSilbergDGRelationship between intragastric acid control and healing status in the treatment of moderate to severe erosive oesophagitisAliment Pharmacol Ther20072561762817305763