Abstract
During the last 5 decades, liver transplantation has witnessed rapid development in terms of both technical and pharmacologic advances. Since their discovery, calcineurin inhibitors (CNIs) have remained the standard of care for immunosuppression therapy in liver transplantation, improving both patient and graft survival. However, adverse events, particularly posttransplant nephrotoxicity, associated with long-term CNI use have necessitated the development of alternate treatment approaches. These include combination therapy with a CNI and the inosine monophosphate dehydrogenase inhibitor mycophenolic acid and use of mammalian target of rapamycin (mTOR) inhibitors. Everolimus, a 40-O-(2-hydroxyethyl) derivative of mTOR inhibitor sirolimus, has a distinct pharmacokinetic profile. Several studies have assessed the role of everolimus in liver transplant recipients in combination with CNI reduction or as a CNI withdrawal strategy. The efficacy of everolimus-based immunosuppressive therapy has been demonstrated in both de novo and maintenance liver transplant recipients. A pivotal study in 719 de novo liver transplant recipients formed the basis of the recent approval of everolimus in combination with steroids and reduced-dose tacrolimus in liver transplantation. In this study, everolimus introduced at 30 days posttransplantation in combination with reduced-dose tacrolimus (exposure reduced by 39%) showed comparable efficacy (composite efficacy failure rate of treated biopsy-proven acute rejection, graft loss, or death) and achieved superior renal function as early as month 1 and maintained it over 2 years versus standard exposure tacrolimus. This review provides an overview of the efficacy and safety of everolimus-based regimens in liver transplantation in the de novo and maintenance settings, as well as in special populations such as patients with hepatocellular carcinoma recurrence, hepatitis C virus-positive patients, and pediatric transplant recipients. We also provide an overview of ongoing studies and discuss potential expansion of the role for everolimus in these settings.
Introduction to liver transplantation
During the 5 decades since the first human liver transplant in 1963,Citation1 there have been a number of important technical and pharmacological advances in liver transplantation. Early results were poor, with a survival of only 13 months,Citation2,Citation3 but they started to improve after the introduction of more-effective immunosuppressive agents. Today, better recipient selection (based on Model for End-Stage Liver Disease scoreCitation4 and Milan criteria)Citation5,Citation6 and evolving surgical techniques and perioperative management have resulted in improved short-term outcomes. Surgical advances include reduced-sized liver grafts in 1981,Citation7 followed by split liver transplantation in 1988Citation8 and the use of living donors in the 1990s.Citation9,Citation10 Indications for liver transplantation have also changed over time. In addition to the common indications, including acute and chronic conditions such as chronic viral hepatitis C, hepatitis B, autoimmune conditions (primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis), and hepatic malignancies, patients with metabolic conditions such as nonalcoholic steatohepatitis are now more frequently being wait-listed for liver transplantation.Citation11
Immunosuppression after liver transplantation in the 1960s and 1970s mainly included use of chemical agents such as purine analog azathioprine and steroids. With this regimen, 1- and 5-year survival rates were 32.9% and 20.0%, respectively.Citation12 The introduction of cyclosporine in the early 1980s significantly improved both graft and patient survival,Citation12,Citation13 with a cyclosporine-based regimen achieving 1- and 5-year survival rates of 69.7% and 62.8%, respectively.Citation12
Despite the technological and pharmacological advances and improvement in short-term outcomes, management issues associated with surgery, immunosuppression, and recurrence of disease still remain a challenge in liver transplantation. Although the incidence of acute rejection has declined over the years as immunosuppressive regimens have developed, other complications associated with the surgical procedure or immunosuppression, such as hepatic artery thrombosis (HAT), biliary tract complications, and infections,Citation14,Citation15 are of concern. Potential problems related to chronic immunosuppression include systemic events (pulmonary, renal, or neurological) and malignancy.Citation14 In addition, recurrence of original diseases such as hepatitis C virus (HCV) infection, hepatocellular carcinoma (HCC), and primary sclerosing cholangitis represents a major clinical obstacle. Recurrent HCV infection after liver transplantation is universal and is associated with accelerated liver fibrogenesis, leading to allograft cirrhosis in at least 30% of patients within 5 years of transplantation.Citation16,Citation17 Posttransplant HCC recurrence has been reported in up to 20% of patients;Citation18 although lower incidence (<10%) has been reported among the patients within the Milan criteria.Citation19,Citation20 Recurrence of primary sclerosing cholangitis has been reported in 10%–38.7% of liver transplant recipients.Citation21
Role of immunosuppression in liver transplantation
At this time, the goal of immunosuppression in liver transplantation is to maintain graft function with a low rate of acute rejection while minimizing drug-related adverse effects. Under-immunosuppression can lead to an increased risk for graft rejection, whereas over-immunosuppression is associated with an increased risk for adverse events; notably, infections and malignancies. Thus, providing optimal immunosuppression is key. Calcineurin inhibitors (CNIs) represent the mainstay of immunosuppressive therapy in liver transplantation. With the introduction of CNI therapy, acute rejection rates have declined considerably. However, prolonged CNI exposure is associated with nephrotoxicity,Citation22,Citation23 neurotoxicity,Citation24–Citation26 increased risk for malignancies,Citation27–Citation29 metabolic complications,Citation30,Citation31 and hypertension.Citation32
CNI-induced nephrotoxicity is the leading cause of renal dysfunction after liver transplantationCitation22,Citation23,Citation33 and has been associated with significant morbidity and mortality.Citation22,Citation23 Incidence rates as high as 18.1% by 5 years posttransplant have been observed,Citation23 and in the Model for End-Stage Liver Disease era, the 5-year cumulative incidence of chronic renal failure has been reported to be 22%.Citation34 Such high rates of nephrotoxicity, along with steroid-induced growth retardation, can have a major effect, particularly in pediatric recipients,Citation35–Citation37 who have a longer exposure to immunosuppressive therapy. Indeed, renal dysfunction has been reported in as many as 32% of pediatric liver transplant recipients at an average follow-up of 7.6 years after transplantation.Citation38 In addition, CNI-induced neurological adverse effects have been reported in up to 40% of patients receiving cyclosporine.Citation25 Both cyclosporine and tacrolimus are associated with an increased risk for HCC recurrence in liver transplant recipients.Citation39,Citation40 Metabolic adverse events, such as hyperlipidemia and new-onset diabetes mellitus (NODM), are also common with CNIs,Citation30 although the incidence rates vary; for example, a significantly higher incidence of NODM has been reported with tacrolimus than with cyclosporine.Citation31
Delaying the introduction of CNIs or reducing CNI exposure are the strategies that have been explored to lower the adverse events associated with this class of drug. One approach in adult recipients has been to administer short-term induction therapy (polyclonal or monoclonal antibodies, or interleukin 2 receptor antibodies such as basiliximab) with delayed introduction of CNIs.Citation41–Citation44 Use of induction therapy is also known to reduce the rates of acute rejection in children receiving CNI-based immunosuppression.Citation45–Citation48
Other treatment strategies focus on optimizing immune modulation by combining immunosuppressants with different mechanisms of action to minimize the adverse events while maintaining immunosuppressive efficacy.Citation49 Maintenance immunosuppression in liver transplant recipients typically includes combinations of drugs that target complementary pathways, most frequently a CNI plus an inosine monophosphate dehydrogenase inhibitor, with or without steroids.Citation50
Unlike the wide array of immunosuppressants approved for use in kidney transplantation, until recently, only the CNIs cyclosporine and tacrolimus, as well as mycophenolate mofetil, were approved for liver transplantation; moreover, cyclosporine plus mycophenolate mofetil was the only combination regimen approved in this setting.Citation51–Citation53 Development of the mammalian target of rapamycin (mTOR) inhibitors everolimus and sirolimus generated considerable interest among transplant physicians, especially in view of their potential to reduce or eliminate CNIs. The mTOR inhibitors exert their immunosuppressive effect via a separate mechanism and exhibit a different pharmacological profile to CNIs and inosine monophosphate dehydrogenase inhibitors, providing a new option in the immunosuppressive armamentarium for transplant recipients. Of the two mTOR inhibitors, sirolimus was introduced first in the late 1990s for prophylaxis of rejection in solid organ transplantation. Everolimus is a 40-O-(2-hydroxyethyl) derivative,Citation54 which was developed to improve the pharmacokinetic profile of sirolimus. The hydroxyethyl group provides a pharmacokinetic advantage, conferring faster absorption and a shorter half-life than sirolimus.Citation55–Citation57 Unlike sirolimus, no loading dose is required for everolimus, and the twice-daily dosing schedule allows better dose adjustments.Citation57
Everolimus (in combination with cyclosporine and corticosteroids) was first approved in 2003 for the prophylaxis of organ rejection in kidney and heart transplant recipients in many European countries, followed by a US Food and Drug Administration (FDA) approval for kidney transplantation in 2010. Everolimus, in combination with reduced-dose tacrolimus and steroids, is the first approval by US FDA for an immunosuppressive agent in liver transplantation for more than a decade.Citation58 Of note, sirolimus is not approved for use in liver transplantation and carries a black box warning from the US FDA because of a high incidence of HAT, graft loss, and death.Citation59 The review focuses on the use of everolimus-based immunosuppression in liver transplantation.
Everolimus
Mechanism of action
At an intracellular level, everolimus binds to FKB12. The resulting complex blocks the activation of the TOR complex 1 complex, a cycle-specific kinase that activates p70 ribosomal S6 kinase (p70S6k).Citation60,Citation61 Inhibition of the mTOR pathway prevents progression of the cell cycle from G1 into the S phase, thus suppressing interleukin (IL)-driven T-cell differentiation. Inactivation of the p70S6k in lymphocytes results in selective inhibition of ribosomal protein synthesis, thereby deactivating the immune response.Citation60,Citation62 mTOR plays an important role in several physiological processes, such that inhibition by everolimus also leads to various downstream consequences via an effect on nonimmune cells such as vascular smooth muscle cells, tubular epithelial cells, and fibroblasts. Antiangiogenic activity of mTOR inhibitors has been associated with a decrease in vascular endothelial growth factor production.Citation63–Citation65 Anticancer effects are mediated via the phosphatidylinositol 3-kinase/AKT/mTOR pathway,Citation66 and antifibrotic activity has been linked to the mTOR/p70S6k/procollagen 1 pathway.Citation67
Everolimus is metabolized in the gut and liver by CYP3A4; therefore, concomitant administration of other CYP3A inhibitors such as verapamil, ketoconazole, and erythromycin may lead to drug–drug interactions.Citation68–Citation70 Routine therapeutic drug monitoring is recommended to guide dose adjustments when everolimus is coadministered with other CYP3A inhibitors.Citation71 Everolimus also has shown synergism with cyclosporine in terms of the immunosuppressive effect.Citation56,Citation72 Tacrolimus and cyclosporine differ significantly in their interaction when coadministered with everolimus. Lower doses of everolimus are needed when coadministered with cyclosporine (so that reducing the dose of cyclosporine may decrease everolimus concentrations), whereas tacrolimus has a minimal effect on everolimus blood levels.Citation73
Efficacy studies
The efficacy of everolimus has been demonstrated in de novo and maintenance liver transplant recipients.Citation74–Citation79 A summary of key randomized controlled trials of everolimus in liver transplant recipients is presented in .
Table 1 Summary of randomized controlled trials of everolimus in liver transplantation
Phase 1 and dose-finding studies of everolimus in combination with standard-dose cyclosporine in de novo liver transplant recipients revealed that everolimus at a dose of 2–4 mg/day was well-tolerated in this population.Citation80,Citation81
In maintenance liver transplant recipients, a feasibility study (N=40) showed that conversion from CNI to everolimus with or without antimetabolite therapy was feasible in 75% of patients. This study also found that the improvement in renal function after conversion to everoli-mus directly correlated with baseline creatinine clearance.Citation75 A multicenter, retrospective study of maintenance liver transplant patients conducted at nine French centers demonstrated that 12 months after conversion to everolimus, more than 60% of patients were CNI-free, and that conversion was associated with a low risk for acute rejection. In addition, a significant improvement in renal function was observed: mean estimated glomerular filtration rate (eGFR) increased from 64.2±30 mL/minute at day 0 to 68.4±32.5 mL/minute at month 12 (P=0.007).Citation82 In a randomized, open-label, multicenter study by De Simone et al, 145 maintenance liver transplant recipients (time since transplantation, >3 years) with CNI-related renal impairment were randomized either to start concentration-controlled everolimus with CNI reduction or discontinuation or to continue a standard-exposure CNI regimen.Citation74 Everolimus was compared with the standard-exposure CNI. Results showed that everolimus allows CNI withdrawal or reduction without compromising efficacy. CNI elimination was achieved in 80% and 85% of patients at 6 and 12 months, respectively, with a very low incidence of biopsy-proven acute rejection (BPAR). The incidence of BPAR was identical (1.4%) at month 6 in both treatment groups and was 4.2% and 1.3% at month 12 in the everolimus and standard-exposure CNI groups, respectively. Despite safe conversion to everolimus without compromising efficacy, no significant improvement in renal function was observed. Low CNI exposure at baseline in both everolimus and standard-exposure CNI groups, higher CNI dose reductions in the control group, and the extended time since transplantation (>3 years) were identified as the main contributing factors. The authors, therefore, hypothesized that earlier conversion to everolimus with subsequent CNI reduction may provide an increased benefit for renal function.Citation74
In de novo liver transplant recipients, Masetti et al described the use of everolimus in a single-center, randomized, phase 2 trial.Citation78 After treatment with cyclosporine for first 10 days, 78 liver transplant recipients were randomized either to receive everolimus plus cyclosporine for up to 30 days, followed by everolimus monotherapy, or to continue receiving cyclosporine with or without mycophenolate mofetil, depending on the presence of chronic kidney disease. Early withdrawal of cyclosporine with subsequent everolimus monotherapy was associated with a significant improvement in renal function (mean eGFR–Modification of Diet in Renal Disease [MDRD]Citation83 at month 12 was 87.7±26.1 mL/minute versus 59.9±12.6 mL/minute in the cyclosporine group; P<0.001). Furthermore, a higher proportion of patients had stage 3 or higher chronic kidney disease (eGFR <60 mL/minute) in the cyclosporine group versus in the everolimus group (52.2% versus 15.4%; P=0.005). Comparable rates of BPAR (5.7% versus 7.7%) were observed in both treatment groups ().Citation78
Figure 1 Incidence of biopsy-proven acute rejection at month 12 in comparative trials of everolimus.
Abbreviations: EVR, everolimus; CNI, calcineurin inhibitor; BPAR, biopsy-proven acute rejections; NR, not reported; NS, not significant.
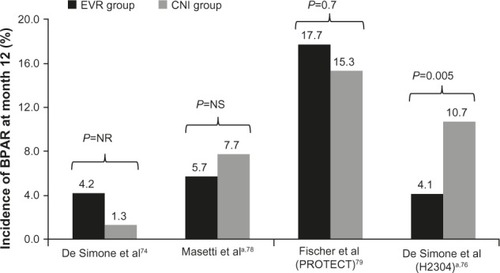
PROTECT, another study employing early conversion to everolimus, was a multicenter, open-label, randomized controlled trial in which 203 de novo liver transplant recipients were randomized at 30–60 days posttransplant to either continue a CNI-based regimen or start everolimus with CNI discontinuation.Citation79 All patients received basiliximab induction, with or without maintenance corticosteroids. Results showed that conversion from a CNI-based to an everolimus-based regimen can be achieved safely, with beneficial effects on renal function. The incidences of a composite efficacy failure endpoint (BPAR, graft loss, death, or loss to follow-up; everolimus, 20.8%, versus CNI, 20.4%) and BPAR (everolimus, 17.7%, versus CNI, 15.3%) were similar between the two treatment groups. At month 11, GFR estimated using the Modification of Diet in Renal Disease formula (considered the appropriate formula for GFR calculation in liver transplant recipients)Citation84 showed a significant difference between the everolimus and CNI groups (least square [LS] mean difference of 7.8 mL/minute in favor of everolimus; P=0.021). However, using the Cockroft-Gault formula, the mean difference in GFR between the everolimus and CNI groups (2.9 mL/minute; P=0.46) did not reach the protocol-defined superiority criterion (a between-group difference in GFR of 8 mL/minute).Citation79
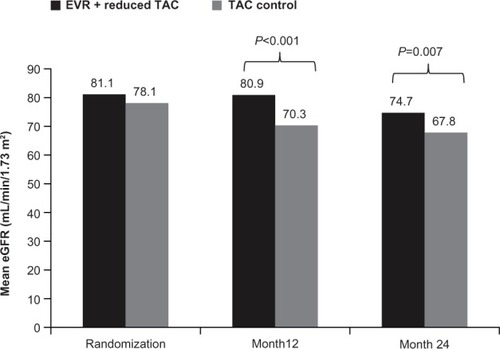
In the pivotal registration study (H2304) that led to everolimus approval in liver transplantation, 719 de novo liver transplant recipients were randomized after a 30±5-day run-in period to everolimus plus reduced tacrolimus, standard- exposure tacrolimus, or everolimus with tacrolimus elimination. Tacrolimus exposure was reduced by 39% in the everolimus plus reduced tacrolimus group compared with in the standard-exposure tacrolimus group.Citation77 Everolimus with reduced tacrolimus proved to be noninferior to standard-exposure tacrolimus, showing a numerically lower incidence rate of the primary endpoint of efficacy failure, defined as treated BPAR, graft loss, or death at month 12.Citation76 The study also showed that immunosuppression with everolimus plus reduced tacrolimus was associated with significantly fewer BPAR episodes than standard-exposure tacrolimus (4.1% versus 10.7%; P=0.005 at month 12 and 6.1% versus 13.3%; P=0.010 at month 24), and that BPAR was less severe.Citation76,Citation77 A high incidence of acute rejections led to premature termination of enrollment to the tacrolimus elimination group, in which tacrolimus was completely withdrawn by the end of month 4 after transplantation. Most of these acute rejections occurred around the time of tacrolimus elimination. Abrupt cessation of tacrolimus might have contributed to the high rate of acute rejections. The authors speculated that an mTOR inhibitor regimen without induction therapy and with no additional immunosuppressive comedication might not be a viable option until 90 days after liver transplantation. In terms of renal function, everolimus plus reduced tacrolimus was associated with superior renal function (eGFR [MDRD-4]) at 12 and 24 months compared with standard tacrolimus (). The improvement in eGFR from randomization to month 24 was significantly higher in the everolimus plus reduced tacrolimus group versus in the standard tacrolimus group (mean difference, 6.7 mL/minute/1.73 m2; 97.5% confidence interval, 1.9–11.4 mL/minute/1.73 m2; P=0.002). For patients who continued their randomized treatment, the mean difference in eGFR was 11.5 mL/minute/1.73 m2 in favor of everolimus plus reduced tacrolimus. Furthermore, the benefit in eGFR was also evident using different formulas (Chronic Kidney Disease Epidemiology Collaboration, Nankivell, and Cockcroft-Gault). For the tacrolimus elimination group, the mean difference in GFR at month 24 versus that in the standard tacrolimus group was 10.4 mL/minute/1.73 m2; 97.5% confidence interval, 5.6–15.3 mL/minute/1.73 m2; P=0.002, intent-to-treat population).Citation77
Figure 2 Mean estimated glomerular filtration rate with everolimus + reduced tacrolimus versus tacrolimus control in the H2304 study.
Abbreviations: EVR, everolimus; TAC, tacrolimus; eGFR, estimated glomerular filtration rate.
Safety and tolerability
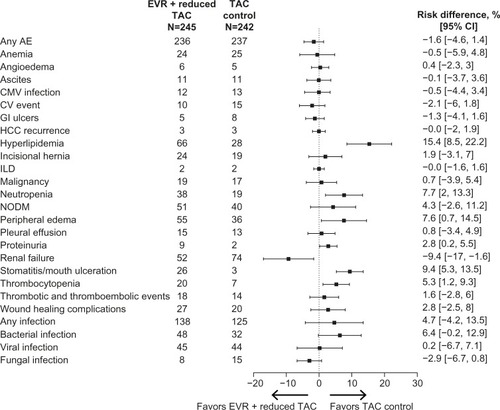
Overall, everolimus showed a comparable safety profile to standard-of-care treatment in the largest clinical trial comparing everolimus plus reduced tacrolimus with standard-exposure tacrolimus in de novo liver transplant recipients ().Citation77 In clinical trials, specific events such as infections, hyperlipidemia, neutropenia, peripheral edema, stomatitis/mouth ulceration, arterial hypertension, anemia, leukopenia, thrombocytopenia, dry skin, eczema, and rash were more frequent in patients receiving an everolimus-based regimen. More patients randomized to everolimus discontinued study drug than in the control groups, mainly because of adverse events.Citation74,Citation77–Citation79 The following sections summarize the key safety events reported from comparative trials of everolimus in liver transplant recipients.
Figure 3 Key adverse events with everolimus + reduced tacrolimus versus tacrolimus control in the H2304 study.
Abbreviations: AE, adverse event; CI, confidence interval; CMV, cytomegalovirus; CV, cardiovascular; EVR, everolimus; GI, gastrointestinal; HCC, hepatocellular carcinoma; ILD, interstitial lung disease; NODM, new-onset diabetes mellitus; TAC, tacrolimus.
Urinary protein excretion
mTOR inhibitor treatment in general is associated with a net increase in urinary protein excretion.Citation85 In the H2304 study, in de novo liver transplant recipients, the incidence of proteinuria reported as an adverse event was low overall but was higher in the everolimus plus reduced tacrolimus group than in the standard tacrolimus group (3.7% versus 0.8%, respectively; P=0.063), and proteinuria was the leading cause of study drug discontinuation (eight versus one patient). However, the mean urine protein-to-creatinine ratio remained below the 500 mg/day threshold. Of note, renal failure excluding proteinuria was more frequent in the standard tacrolimus group (30.6% versus 21.2% in the everolimus group; P=0.023).Citation77 After CNI withdrawal in the PROTECT study, proteinuria was reported in 9.9% of everolimus-treated patients versus 2.0% of the patients in the CNI group (P≤0.05). None of the cases of proteinuria were considered severe or serious.Citation79 In the study of maintenance patients by De Simone et al, two patients discontinued everolimus because of proteinuria, which in both cases was suspected to be related to study medication.Citation74
Incisional hernia and wound healing complications
A recent systematic review of randomized controlled trials in solid organ transplant recipients reported increased risk for poor wound healing and lymphoceles with immediate use of mTOR inhibitors posttransplant.Citation86 Willems et al showed that loss of wound strength can be prevented by delaying postoperative administration of everolimus.Citation87 In the study by Masetti et al, in which everolimus was initiated 10 days after transplantation, incisional hernias were reported more frequently in the everolimus group than with cyclosporine (46.1% versus. 26.9%), although the difference was not statistically significant (P=0.16).Citation78 Comparable rates of wound healing complications (11.0% versus 8.3%; P=0.36) and incisional hernias (9.8% versus 7.9%; P=0.52) with everolimus plus reduced tacrolimus versus standard tacrolimus in the H2304 study suggest that a delay in everolimus introduction can reduce the risk of wound healing complications.Citation77 Similar results were seen in the PROTECT study, in which rates of incisional hernias (11.9% versus 9.8%) and wound healing complications (2.0% versus 3.9%) were comparable in the everolimus and the CNI groups.Citation79
HAT
Early HAT is a well-known complication after liver transplantation. In the H2304 study, 14 cases of HAT were reported during the prerandomization run-in phase. After randomization, one case of HAT was reported in the everolimus plus reduced tacrolimus group, after introduction of everolimus at 30 days posttransplant. This patient had previously experienced an episode of HAT during the run-in period, requiring re-anastomosis of the hepatic artery and stent placement.Citation76 In the PROTECT study, and in the study by Masetti et al, no cases of HAT were reported in patients converted from a CNI-based to everolimus-based immunosuppression.Citation78,Citation79
Lipid disorders and new-onset diabetes mellitus
Dyslipidemia has been reported in 50%–60% of patients at 3–4 years after liver transplantation.Citation32,Citation88 In the H2304 study, hyperlipidemia as an adverse event was reported in a significantly higher proportion of patients in the everolimus group compared with in the tacrolimus control group (26.9% versus 11.6%; P<0.001).Citation77 Similarly, in the PROTECT study, both hyperlipidemia and hypercholesterolemia were reported more frequently in the everolimus-treated patients than in CNI-treated patients (11.9% versus 2.0% and 22.8% versus 10.8%, respectively; both P≤0.05).Citation79 The relationship between increased dyslipidemia during mTOR inhibitor administration and cardiovascular outcomes has not been systematically evaluated, and thus the clinical effect of these adverse events is not fully understood. However, the proportion of patients receiving lipid- lowering treatment was similar with everolimus plus reduced tacrolimus or the standard-of-care tacrolimus treatment group (23.3% versus 17.8%; P=0.944) at 1 year posttransplant in the H2304 study.Citation76 Furthermore, the incidence of cardiovascular events at month 24 did not differ between the two treatment groups (4.1% versus 6.2%; P=0.31).Citation77
Among maintenance patients, hypercholesterolemia and hyperlipidemia were reported more frequently in the everolimus group compared with the CNI group (13.9% versus 2.7% and 9.7% versus 2.7%, respectively), and the difference was significant for hypercholesterolemia (P=0.017).Citation74
NODM has been reported in 26% of liver transplant recipients, and the type of immunosuppressive therapy is one of the predictive factors for NODM after liver transplantation.Citation89 NODM was reported in 20.8% of patients receiving everolimus plus reduced tacrolimus versus 16.5% of patients receiving standard-exposure tacrolimus at month 24 in the H2304 study (P=0.25).Citation77 Similarly, a comparable proportion of patients reported diabetes mellitus with everolimus and CNI (4.0% versus 7.8%) in the PROTECT study.Citation79
Infections
Infection is a well-recognized, frequent complication associated with immunosuppressive therapy. The incidence of infections varies between studies of everolimus in liver transplantation. In the H2304 study, the overall incidence was 56.3% in the everolimus group versus 51.7% in the tacrolimus control group, with no significant difference between groups. There was a trend toward more bacterial infections in the everolimus group (19.6% versus 13.2% with standard tacrolimus; P=0.067). Cytomegalovirus infection as an adverse event was reported in 4.9% and 5.4% of patients in the everolimus and tacrolimus treatment groups, respectively (P=0.84).Citation77 In the study by Masetti et al, the incidence of major episodes of infections was identical in the everolimus and cyclosporine groups (46.1%), but fungal infections were significantly more frequent with cyclosporine than everolimus (five cases versus one case; P=0.011).Citation78 Infections and infestations were more frequent under everolimus therapy than CNI therapy in the PROTECT study (73.3% versus 59.8%). Cytomegalovirus infection was reported in 7.9% of patients in the everolimus group compared with 10.8% patients in the CNI group.Citation79 In maintenance patients, De Simone et al reported infections in 31.9% of patients in the everolimus group versus 21.9% of patients in the CNI group.Citation74
Peripheral edema
Edema is a class effect observed with mTOR inhibitors. In the de novo setting (H2304 study), peripheral edema was reported more frequently in the everolimus plus reduced tacrolimus group versus the tacrolimus control group (22.4% versus 14.9%; P=0.036).Citation77 Masetti et al reported inferior limb edema in five patients in the everolimus group versus none in the cyclosporine group (P=0.16).Citation78 In maintenance patients as well, higher incidence of peripheral edema was reported in everolimus-treated patients compared with in CNI-treated patients (5.6% versus 1.4%), although the difference was not significant.Citation74
Interstitial lung disease
Interstitial lung disease is a rare but potentially fatal event associated with mTOR inhibitors as a class. In the H2304 study of de novo liver transplant recipients, interstitial lung disease was reported in two patients (0.8%) in both the everolimus plus reduced tacrolimus group and the standard tacrolimus group during the 24-month study period.Citation77 In the CNI withdrawal setting (PROTECT study), one case of interstitial lung disease was reported in the everolimus group.Citation79 In maintenance patients, one case of interstitial lung disease suspected to be related to study drug was reported in the everolimus group.Citation74
Stomatitis/mouth ulceration
Stomatitis/mouth ulceration is one of the common adverse events that has been reported with mTOR inhibitor therapy. In de novo setting (H2304 study), the incidence rate of stomatitis was 10.6% in patients receiving everolimus compared with 1.2% in patients receiving standard tacrolimus (P<0.001).Citation77 Masetti et al reported aphthous-type mouth ulceration in two everolimus-treated patients, which resolved with dose reduction.Citation78 In the maintenance setting, De Simone et al reported mouth ulceration in 26.4% of patients who were converted from a CNI-based regimen to everolimus with CNI reduction or discontinuation compared with none of the patients in the CNI continuation group (P<0.010).Citation74
Special liver transplant populations
HCV cirrhosis
There is a lack of robust evidence regarding the ideal immunosuppressive regimen for HCV-positive liver transplant recipients. However, although data are limited, everolimus may offer a benefit for posttransplant HCV-related fibrosis progression. In a subgroup of HCV-positive transplant recipients in the H2304 study, fibrosis progression (defined as 1 or more stage on the Ishak/Knodell score)Citation90 occurred in fewer everolimus-treated patients compared with patients receiving standard tacrolimus (14/29 [48.3%] versus 22/35 [62.9%]; P=0.087).Citation91 In an another study conducted in 43 recurrent HCV-positive patients, Villamil et al reported fibrosis progression (1 or more Ishak/Knodell stage) in fewer patients in the everolimus group compared with in the CNI group (1/14 [7.1%] versus 5/18 [27.8%]; P=0.060).Citation92 Larger prospective studies in HCV-positive liver transplant recipients are needed to confirm these preliminary findings.
Pediatric liver transplantation
Although there are few reports specifically relating to everolimus in pediatric liver transplant recipients, several studies in renal transplant recipients have shown it can be used safely in children.Citation93–Citation98 Nielsen et al reported a single-center experience in which everolimus was given as rescue therapy in 18 pediatric liver transplant recipients, with a median follow-up of 13 months. The indications for use of everolimus were chronic graft dysfunction (N=12), CNI toxicity (N=3), hepatoblastoma (N=2), and recurrence of primary sclerosing cholangitis (N=1). Of the 12 patients treated with everolimus for chronic graft dysfunction, by the end of follow-up, four patients had normal liver function tests and six showed a partial improvement. An increase in GFR was noted in one of the three patients with suspected CNI nephrotoxicity, and patients with hepatoblastoma did not develop any metastasis. No new safety signals were observed.Citation99 The ongoing H2305 study (NCT01598987) is the first prospective trial of everolimus (with reduced-exposure cyclosporine or tacrolimus) in pediatric liver transplant recipients. In addition to efficacy and general safety assessments, linear growth, sexual maturation, and hormonal gonadal axis are being closely monitored in this study to assess the potential effect of mTOR inhibition on development in children.
Hepatocellular carcinoma
Everolimus also acts as an antineoplastic agent, and therefore it may exert a beneficial effect in liver transplant recipients with HCC. In a retrospective analysis of 57 patients converted to an everolimus-based regimen mainly because of HCC recurrence (53%) or development of de novo tumors (33%), everolimus was well tolerated in patients with recurrent or de novo malignancies and provided a significant improvement in renal function along with a low rate of acute rejection.Citation100 Prospective randomized studies are needed to further evaluate the role of everolimus immunosuppression in HCC.
Living donor liver transplantation
Transplantation of a partial liver from a healthy donor to a patient with end-stage liver disease can help address the ongoing problem of donor organ shortage. Studies reporting everolimus use in living-donor liver transplant recipients are scarce. Majeed et al used everolimus as a part of immunosuppressive protocol in patients with HCC undergoing living-donor liver transplantation. However, the focus of the study was to assess predictors of posttransplant survival.Citation101 In living-donor liver transplant recipients, the efficacy and safety of everolimus plus reduced tacrolimus versus standard-exposure tacrolimus is being evaluated in a 24-month, phase 3, randomized, multicenter study (H2307; NCT01888432).
Quality of life
A systematic review showed that quality of life improves posttransplantation; however, compared with the general population, it still remains impaired.Citation102 Adverse events associated with long-term exposure to immunosuppressive agents can contribute to this effect. Studies focusing on immunosuppression with everolimus in relation to quality of life and patient satisfaction/acceptability are limited. It will be interesting for future research to focus on the effect of immunosuppressive regimens on health-related quality of life in transplant recipients.
Perspectives
Before the approval of everolimus for use in liver transplantation, perceptions of mTOR inhibitors in this population were predominantly based on experience with sirolimus. Early studies with sirolimus (administered within 48 hours posttransplantation) reported high incidence of serious adverse events including HAT and wound healing complications, which may have been related to the use of loading doses and high maintenance doses (eg, a loading dose of 15 mg followed by 5 mg/day).Citation59,Citation103,Citation104 Importantly, most of the HAT events in sirolimus-treated patients were reported within the first 30 days posttransplantation. As a consequence, sirolimus received a black box warning for use in liver transplantation.Citation59 Although sirolimus is not approved in liver transplantation, recent studies using lower doses have shown an improved safety profile.Citation105,Citation106 Today’s target ranges for everolimus (trough level [C0] 3–8 ng/mL in combination with reduced tacrolimus)Citation58 and the delay in administration of 30 days posttransplantation have reduced the risk for complications reported in early sirolimus trials. However, in almost all studies, the rate of study drug discontinuations was higher with everolimus than with CNI. This can be partly explained by the nature of open-label studies testing a new drug against an established drug. The opportunity to achieve CNI reduction with a related improvement in renal function with everolimus-based immunosuppression shown in the large randomized pivotal trialCitation76–Citation77 suggests a potential advantage for mTOR inhibitor immunosuppression when introduced at 30 days after liver transplantation.
In adult liver transplant recipients, everolimus has proven to be safe and effective over the course of 2 years’ follow-up.Citation77,Citation79 Longer-term outcomes are yet to be investigated in prospectively conducted long-term follow-up studies. The extension phase of H2304 study (H2304E1; NCT01150097) will provide the longer-term renal function and safety outcomes in everolimus-treated patients. Tacrolimus elimination remains an attractive concept, but at present, a benefit has not been proven unequivocally, based on the difference between findings from the H2304 and PROTECT trials. It remains to be confirmed whether everolimus can help to address other unmet needs such as attenuating the progression of graft fibrosis and inhibiting HCC recurrence. Robust safety data will also be needed before everolimus can be used in pediatric patients and living donor transplant recipients. A summary of ongoing trials of everolimus in liver transplantation that will potentially address these unanswered questions is presented in .
Table 2 Summary of key ongoing trials of everolimus in liver transplantation
Because everolimus has been shown to provide immunosuppressive efficacy with preservation of renal function preservation in both kidney and liver transplant recipients, its use in combined kidney and liver transplantation may be beneficial considering that a significant proportion of such patients develops renal dysfunction.Citation107
Conclusion, place in therapy
Everolimus provides a new therapeutic option for liver transplant recipients, particularly with respect to posttransplant nephrotoxicity and other adverse events associated with long-term administration of CNIs. Clinical trials have shown that everolimus provides improved protection against renal dysfunction while maintaining immunosuppressive efficacy, particularly when introduced early after liver transplantation. Compared with standard-exposure tacrolimus, everolimus in combination with reduced-dose tacrolimus provides a significant improvement in renal function, sustained over 2 years. The available evidence supports the potentially beneficial effects of everolimus in HCC recurrence and HCV-positive and pediatric liver transplant recipients, although further data from prospective trials are awaited.
Acknowledgments
The authors thank Seema Dimri, Novartis Healthcare Pvt Ltd, India, for providing medical writing/editorial support. The first draft was prepared based on direction from the authors. All authors provided significant input and substantially contributed to the scientific contents. All authors approved the final draft submitted.
Disclosure
Rainer Ganschow and Jörg-Matthias Pollok have received travel grants from Novartis. Guido Junge is an employee of Novartis Pharma AG, Basel, Switzerland. Martin Jankofsky has nothing to disclose.
References
- StarzlTEMarchioroTLVonkaullaKNHermannGBrittainRSWaddellWRHomotransplantation of the Liver in HumansSurg Gynecol Obstet196311765967614100514
- StarzlTEGrothCGBrettschneiderLOrthotopic homotransplantation of the human liverAnn Surg196816833924154877589
- ZarrinparABusuttilRWLiver transplantation: past, present and futureNat Rev Gastroenterol Hepatol201310743444023752825
- WiesnerREdwardsEFreemanRModel for end-stage liver disease (MELD) and allocation of donor liversGastroenterology20031241919612512033
- MazzaferroVRegaliaEDociRLiver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosisN Engl J Med1996334116936998594428
- ShettyKTimminsKBrensingerCLiver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcomeLiver Transpl200410791191815237377
- BismuthHHoussinDReduced-sized orthotopic liver graft in hepatic transplantation in childrenSurgery19849533673706367125
- PichlmayrRRingeBGubernatisGHaussJBunzendahlHTransplantation einer Spenderleber auf zwei Empfänger (Splitting-Transplantation) - eine neue Methode in der Weiterentwicklung der LebersegmenttransplantationLangenbecks Arch Chir1983732127130 German
- RaiaSNeryJRMiesSLiver transplantation from live donorsLancet1989286614972570198
- StrongRWLynchSVOngTHMatsunamiHKoidoYBaldersonGASuccessful liver transplantation from a living donor to her sonN Engl J Med199032221150515072336076
- CharltonMRBurnsJMPedersenRAWattKDHeimbachJKDierkhisingRAFrequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United StatesGastroenterology201114141249125321726509
- GordonRDShawBWJrIwatsukiSEsquivelCOStarzlTEIndications for liver transplantation in the cyclosporine eraSurg Clin North Am19866635415563520895
- StarzlTEDemetrisAJVan ThielDLiver transplantation (2)N Engl J Med198932116109210992677722
- MorenoRBerenguerMPost-liver transplantation medical complicationsAnn Hepatol200652778516807513
- RomeroFARazonableRRInfections in liver transplant recipientsWorld J Hepatol201134839221603030
- BerenguerMFerrellLWatsonJHCV-related fibrosis progression following liver transplantation: increase in recent yearsJ Hepatol200032467368410782918
- CrespoGMarinoZNon-invasive diagnosis of liver fibrosis in the transplant settingDigestive Liver Dis Suppl2011512325
- WelkerMWBechsteinWOZeuzemSTrojanJRecurrent hepatocellular carcinoma after liver transplantation – an emerging clinical challengeTranspl Int201326210911822994652
- FelgaGEvangelistaASSalvalaggioPRHepatocellular carcinoma recurrence among liver transplant recipients within the Milan criteriaTransplant Proc20124482459246123026620
- Pérez-SaboridoBde los GalanesSJMenéu-DíazJCTumor recurrence after liver transplantation for hepatocellular carcinoma: recurrence pathway and prognostic factorsTransplant Proc20073972304230717889172
- El-MasryMPuigCASaabSRecurrence of non-viral liver disease after orthotopic liver transplantationLiver Int201131329130221281429
- GonwaTAMaiMLMeltonLBEnd-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatmentTransplantation200172121934193911773892
- OjoAOHeldPJPortFKChronic renal failure after transplantation of a nonrenal organN Engl J Med20033491093194012954741
- HaubenMCyclosporine neurotoxicityPharmacotherapy19961645765838840363
- GijtenbeekJMvan den BentMJVechtCJCyclosporine neurotoxicity: a reviewJ Neurol1999246533934610399863
- BechsteinWONeurotoxicity of calcineurin inhibitors: impact and clinical managementTranspl Int200135313326
- GubaMGraebCJauchKWGeisslerEKPro- and anti-cancer effects of immunosuppressive agents used in organ transplantationTransplantation200477121777178215223891
- TjonASSint NicolaasJKwekkeboomJIncreased incidence of early de novo cancer in liver graft recipients treated with cyclosporine: an association with C2 monitoring and recipient ageLiver Transpl201016783784620583092
- WimmerCDAngeleMKSchwarzBImpact of cyclosporine versus tacrolimus on the incidence of de novo malignancy following liver transplantation: a single center experience with 609 patientsTranspl Int20132610999100623952102
- SubramanianSTrenceDLImmunosuppressive agents: effects on glucose and lipid metabolismEndocrinol Metab Clin North Am200736489190517983927
- VincentiFFrimanSScheuermannEDIRECT (Diabetes Incidence after Renal Transplantation: Neoral C Monitoring Versus Tacrolimus) InvestigatorsResults of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimusAm J Transplant2007761506151417359512
- BianchiGMarchesiniGMarzocchiRPinnaADZoliMMetabolic syndrome in liver transplantation: relation to etiology and immunosuppressionLiver Transpl200814111648165418975273
- PhamPTPhamPCWilkinsonAHManagement of renal dysfunction in the liver transplant recipientCurr Opin Organ Transplant200914323123919395967
- SharmaPWelchKEikstadtRMarreroJAFontanaRJLokASRenal outcomes after liver transplantation in the model for end-stage liver disease eraLiver Transpl20091591142114819718633
- CampbellKMBucuvalasJCRenal function in the long term after pediatric liver transplantation: is there a need for protocol kidney biopsies?Curr Opin Organ Transplant201015560861320733490
- VinerRMFortonJTColeTJClarkIHNoble-JamiesonGBarnesNDGrowth of long-term survivors of liver transplantationArch Dis Child199980323524010325703
- BartoshSMThomasSESuttonMMBradyLMWhitingtonPFLinear growth after pediatric liver transplantationJ Pediatr1999135562463110547252
- CampbellKMYazigiNRyckmanFCHigh prevalence of renal dysfunction in long-term survivors after pediatric liver transplantationJ Pediatr2006148447548016647407
- VivarelliMCucchettiALa BarbaGLiver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrenceAnn Surg2008248585786218948815
- VivarelliMCucchettiAPiscagliaFAnalysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppressionLiver Transpl200511549750315838913
- SolimanTHetzHBurghuberCShort-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantationLiver Transpl20071371039104417600336
- LiuCLFanSTLoCMInterleukin-2 receptor antibody (basiliximab) for immunosuppressive induction therapy after liver transplantation: a protocol with early elimination of steroids and reduction of tacrolimus dosageLiver Transpl200410672873315162466
- RamirezCBDoriaCdi FrancescoFIariaMKangYMarinoIRAnti-IL2 induction in liver transplantation with 93% rejection-free patient and graft survival at 18 monthsJ Surg Res2007138219820417292404
- TurnerAPKnechtleSJInduction immunosuppression in liver transplantation: a reviewTranspl Int201326767368323651083
- GrasJMGerkensSBeguinCSteroid-free, tacrolimus-basiliximab immunosuppression in pediatric liver transplantation: clinical and pharmacoeconomic study in 50 childrenLiver Transpl200814446947718383091
- SpadaMPetzWBertaniARandomized trial of basiliximab induction versus steroid therapy in pediatric liver allograft recipients under tacrolimus immunosuppressionAm J Transplant2006681913192116771811
- GanschowRGrabhornESchulzAVon HugoARogiersXBurdelskiMLong-term results of basiliximab induction immunosuppression in pediatric liver transplant recipientsPediatr Transplant20059674174516269045
- GanschowRBroeringDCStuerenburgIRogiersXHellwegeHHBurdelskiMFirst experience with basiliximab in pediatric liver graft recipientsPediatr Transplant20015535335811560755
- WiesnerRHFungJJPresent state of immunosuppressive therapy in liver transplant recipientsLiver Transpl201117Suppl 3S1S921850697
- KimWRSmithJMSkeansMAOPTN/SRTR 2012 Annual Data Report: liverAm J Transplant201414Suppl 1699624373168
- Neoral® (cyclosporine) [package insert]East Hanover, NJNovartis Pharmaceuticals Corporation2013
- Prograf® (tacrolimus) [package insert]Northbrook, ILAstellas Pharma US Inc2013
- CellCept® (mycophenolate) [package insert]South San Francisco, CAGenentech USA Inc2013
- SedraniRCottensSKallenJSchulerWChemical modification of rapamycin: the discovery of SDZ RADTransplant Proc1998305219221949723437
- SchulerWSedraniRCottensSSDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivoTransplantation199764136429233698
- KirchnerGIMeier-WiedenbachIMannsMPClinical pharmacokinetics of everolimusClin Pharmacokinet2004432839514748618
- SalvadoriMBertoniELong-term outcome of everolimus treatment in transplant patientsTranspl Res Risk Manag201137790
- Zortress® (everolimus) [package insert]East Hanover, NJNovartis Pharmaceuticals Corporation2013
- Rapamune® (sirolimus) [package insert]New York, NYPfizer, Inc2011
- HalloranPFImmunosuppressive drugs for kidney transplantationN Engl J Med2004351262715272915616206
- WullschlegerSLoewithRHallMNTOR signaling in growth and metabolismCell2006124347148416469695
- NashanBReview of the proliferation inhibitor everolimusExpert Opin Investig Drugs2002111218451857
- GubaMvon BreitenbuchPSteinbauerMRapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factorNat Med20028212813511821896
- ContrerasAGDormondOEdelbauerMmTOR-understanding the clinical effectsTransplant Proc200840Suppl 10S9S1219100913
- CruzadoJMNonimmunosuppressive effects of mammalian target of rapamycin inhibitorsTransplant Rev (Orlando)2008221738118631860
- FasoloASessaCTargeting mTOR pathways in human malignanciesCurr Pharm Des201218192766277722475451
- KawaharaTAsthanaSKnetemanNMm-TOR inhibitors: what role in liver transplantation?J Hepatol20115561441145121781947
- KovarikJMBeyerDBizotMNJiangQShenoudaMSchmouderRLEffect of multiple-dose erythromycin on everolimus pharmacokineticsEur J Clin Pharmacol2005611353815785960
- KovarikJMBeyerDBizotMNJiangQAllisonMJSchmouderRLPharmacokinetic interaction between verapamil and everolimus in healthy subjectsBr J Clin Pharmacol200560443443716187976
- KovarikJMBeyerDBizotMNJiangQShenoudaMSchmouderRLBlood concentrations of everolimus are markedly increased by ketoconazoleJ Clin Pharmacol200545551451815831774
- KovarikJMBeyerDSchmouderRLEverolimus drug interactions: application of a classification system for clinical decision makingBiopharm Drug Dispos200627942142616955532
- SchuurmanHJCottensSFuchsSSDZ RAD, a new rapamycin derivative: synergism with cyclosporineTransplantation199764132359233697
- KovarikJMCurtisJJHricikDEPescovitzMDScantleburyVVasquezADifferential pharmacokinetic interaction of tacrolimus and cyclosporine on everolimusTransplant Proc200638103456345817175302
- De SimonePMetselaarHJFischerLConversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: a prospective, randomized, multicenter trialLiver Transpl200915101262126919790150
- De SimonePCarraiPPrecisiAConversion to everolimus monotherapy in maintenance liver transplantation: feasibility, safety, and impact on renal functionTranspl Int200922327928619054383
- De SimonePNevensFDe CarlisLH2304 Study GroupEverolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trialAm J Transplant201212113008302022882750
- SalibaFDe SimonePNevensFH2304 Study GroupRenal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter studyAm J Transplant20131371734174523714399
- MasettiMMontaltiRRompianesiGEarly withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal functionAm J Transplant201010102252226220486905
- FischerLKlempnauerJBeckebaumSA randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation – PROTECTAm J Transplant20121271855186522494671
- LevyGSchmidliHPunchJSafety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12- and 36-month resultsLiver Transpl200612111640164816598777
- LevyGAGrantDParadisKCampestriniJSmithTKovarikJMPharmacokinetics and tolerability of 40-0-[2-hydroxyethyl] rapamycin in de novo liver transplant recipientsTransplantation200171116016311211186
- SalibaFDharancySLorhoRConversion to everolimus in maintenance liver transplant patients: a multicenter, retrospective analysisLiver Transpl201117890591321384525
- LeveyASBoschJPLewisJBA more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study GroupAnn Intern Med1999130646147010075613
- GonwaTAJenningsLMaiMLEstimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equationsLiver Transpl200410230130914762871
- LetavernierELegendreCmToR inhibitors-induced proteinuria: mechanisms, significance, and managementTransplant Rev (Orlando)200822212513018631865
- PengelLHLiuLQMorrisPJDo wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? A systematic review of randomized controlled trialsTranspl Int201124121216123021955006
- WillemsMCHendriksTde ManBMLommeRMvan der VlietJAEverolimus-induced loss of wound strength can be prevented by a short postoperative delay in its administrationWound Repair Regen201119668068622092838
- NealDATomBDLuanJIs there disparity between risk and incidence of cardiovascular disease after liver transplant?Transplantation2004771939914724441
- KuoHTSampaioMSYeXReddyPMartinPBunnapradistSRisk factors for new-onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing databaseTransplantation20108991134114020386364
- IshakKBaptistaABianchiLHistological grading and staging of chronic hepatitisJ Hepatol19952266966997560864
- SalibaFBrownRSMetselaarHJEverolimus based immunosuppression in hepatitis C virus positive de novo liver transplant recipients: 24-month results from a randomized controlled trial [abstract]Liver Transpl201319Suppl 1S100
- VillamilFGGadanoACZingaleFFibrosis progression in maintenance liver transplant patients with hepatitis C recurrence: a randomised study of everolimus vs calcineurin inhibitorsLiver Int Epub20131215
- GanschowRPapeLSturmEGrowing experience with mTOR inhibitors in pediatric solid organ transplantationPediatr Transplant201317769470624004351
- Van Damme-LombaertsRWebbNAHoyerPFEverolimus Study GroupSingle-dose pharmacokinetics and tolerability of everolimus in stable pediatric renal transplant patientsPediatr Transplant20026214715212000472
- HoyerPFEttengerRKovarikJMEverolimus Pediatric Study GroupEverolimus in pediatric de nova renal transplant patientsTransplantation200375122082208512829916
- KovarikJMNoeABerthierSClinical development of an everolimus pediatric formulation: relative bioavailability, food effect, and steady-state pharmacokineticsJ Clin Pharmacol200343214114712616666
- PapeLLehnerFBlumeCAhlenstielTPediatric kidney transplantation followed by de novo therapy with everolimus, low-dose cyclosporine A, and steroid elimination: 3-year dataTransplantation201192665866221804444
- PapeLAhlenstielTEhrichJHOffnerGReversal of loss of glomerular filtration rate in children with transplant nephropathy after switch to everolimus and low-dose cyclosporine APediatr Transplant200711329129517430485
- NielsenDBriem-RichterASornsakrinMFischerLNashanBGanschowRThe use of everolimus in pediatric liver transplant recipients: first experience in a single centerPediatr Transplant201115551051421696525
- AlegreCJiménezCManriqueAEverolimus monotherapy or combined therapy in liver transplantation: indications and resultsTransplant Proc20134551971197423769086
- MajeedTAWaiCTRajekarHExperience of the transplant team is an important factor for posttransplant survival in patients with hepatocellular carcinoma undergoing living-donor liver transplantationTransplant Proc20084082507250918929781
- TomeSWellsJTSaidALuceyMRQuality of life after liver transplantation. A systematic reviewJ Hepatol200848456757718279999
- WiesnerRKlintmalmGMcDiarmidSRapamune Liver Transplant Study GroupSirolimus immunotherapy results in reduced roles of acute rejection in de novo orthotopic liver transplant recipientsAm J Transpl20022464
- AsraniSKWiesnerRHTrotterJFDe novo sirolimus and reduced-dose tacrolimus versus standard-dose tacrolimus after liver transplantation: the 2000–2003 phase II prospective randomized trialAm J Transplant201414235636624456026
- ChinnakotlaSDavisGLVasaniSImpact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantationLiver Transpl200915121834184219938137
- McKennaGJTrotterJFKlintmalmELimiting hepatitis C virus progression in liver transplant recipients using sirolimus-based immunosuppressionAm J Transplant201111112379238721967703
- ChavaSPSinghBStangouASimultaneous combined liver and kidney transplantation: a single center experienceClin Transplant2010243E62E6820618811
