Abstract
Inflammatory bowel disease (IBD) is an important cause of morbidity and mortality for millions of patients worldwide. Current treatment options include corticosteroids, 5-aminosalicylates, immunosuppressants, and TNFα antagonists. However, these are frequently ineffective in achieving sustained response and remission over time. At present, gastroenterologists lack safe and effective treatments if patients fail anti-TNF therapy. Vedolizumab is a promising new agent for IBD patients refractory to anti-TNF therapy. Vedolizumab is an integrin antagonist which is thought to act by reducing inflammation by selectively inhibiting leukocyte migration in the gut. Emerging evidence from clinical trials suggests a potential role for vedolizumab in both ulcerative colitis (UC) and Crohn’s disease (CD), particularly in patients who have previously failed biological therapy. The safety profile of vedolizumab appears reasonable, possibly because it has a “gut-selective” mode of action, with no reported cases of progressive multifocal leukoencephalopathy, a condition which has been linked to another integrin antagonist, natalizumab. This review discusses the available evidence for integrin antagonists and their potential role in the management of IBD.
Introduction
Inflammatory bowel disease (IBD) affects approximately 3.6 million individuals in the US and in Europe.Citation1 Although the incidence and prevalence of IBD varies geographically, the overall incidence has been increasing worldwide.Citation1,Citation2 The cost burden can be substantial, as many patients are diagnosed at a relatively young age and management often requires life-long medical and surgical input.Citation3 Current treatment modalities for ulcerative colitis (UC) and Crohn’s disease (CD) include 5-aminosalicates, corticosteroids, immunosuppressants (including thiopurines, methotrexate, tacrolimus, cyclosporine, and anti-TNF therapies), and surgery.
Over the last decade, the use of anti-TNF-α medications, such as infliximab, adalimumab, certolizumab pegol, and golimumab, have been used in patients with moderate to severe CD,Citation4–Citation7 and in those with acute severe colitisCitation8–Citation11 who have failed to respond to corticosteroids. Although anti-TNFα treatments are thought to be effective, in real terms, 40% of UC patients and 20%–40% of CD patients will fail to respond to infliximab therapy.Citation12,Citation13
Adalimumab is indicated for the treatment of moderate-to-severe CD.Citation14 Adalimumab has a response rate of between 36%–41% in maintaining remission in CD patients at 56 weeks when given to those patients who have previously failed infliximab.Citation15 It also has a reported failure rate of 72% in maintaining remission in UC patients at 2 years.Citation16 Certolizumab pegol has been shown to maintain clinical remission at week 26 in 29% of patients with moderate-to-severe CD versus 18% of those treated with placebo.Citation7
Despite an initial response to anti-TNF therapy, there are a group of patients, ie, “secondary non-responders”, who will lose their ability to respond over time. It has been proposed that the development of endogenous antibodies to these drugs, accelerated drug clearance, and ongoing fibrosis or aberrant immune pathways are responsible for this effect.Citation17,Citation18
Despite their clear efficacy, it is thought that between 30%–40% of UC patients and 40% of CD patients who are treated with anti-TNF therapies will lose response with time, at a rate of around 10%–13% per year.Citation17,Citation19,Citation20 In addition, the efficacy of a second anti-TNF therapy is lower in those who have previously received an anti-TNF therapy, when compared to those who were previously anti-TNF naïve.Citation21 They can also be associated with significant safety issues, including infusion reactions, increased susceptibility to infection, and worsening of congestive cardiac failure.Citation22 Consequently, there is an urgent requirement for alternative treatments in patients who fail to respond to anti-TNF therapies.
A new class of therapy, the integrin inhibitors, is being developed and has shown promising results to date. This group of drugs is thought to target and disrupt the leukocyte adhesion and trafficking systems, thereby reducing inflammation.Citation23 Vedolizumab is a “gut selective” integrin antagonist and its potential role in IBD is discussed in this review.
Methodology
Search strategy
We conducted a search using PubMed (1947–present) and Medline (1946–present) using the following keywords: vedolizumab, [MLN002, MLN0002, integrin antagonist], AND UC [ulcerative colitis], CD [Crohn’s disease], IBD [inflammatory bowel disease]. Keywords were also exploded and selected from MeSH terms for PubMed. References from relevant articles were also searched manually.
Data analysis
The level and quality of evidence were determined by the study design, sample size, potential bias, statistical analysis, use of controls, and data collection strategy.
Standard protocol approvals, registrations, and patient consent
Published data were used for this systematic review; therefore, no ethical approval was sought.
Integrin antagonists for treatment in IBD
Although the exact cause of IBD remains unknown, recent advances in understanding the pathogenesis of UC and CD have led to greater interest in biologic therapies. The most widely used and effective therapies for IBD are monoclonal antibodies targeted against the pro-inflammatory cytokine TNFα; however, a significant number of patients fail to respond or lose response over time to these therapies. This has led to research in understanding alternative pathways involved in the inflammation process so as to provide new and alternative targets for therapies.Citation23 The migration of leukocytes and other inflammatory cells into intestinal vasculature and disruption of the intestinal barrier function are important in the pathogenesis of IBD.Citation24 The high recruitment of T-cells to the intestinal mucosa and subsequent cytokine production has been shown to be key in the pathogenesis of IBD by affecting the endothelial barrier and inducing cell apoptosis in endothelial cells.Citation25 Integrin antagonists are antibody-mediated therapies which aim to block the interaction between leukocytes and endothelial cells, and as a result, disrupt trafficking of T-lymphocytes into the inflamed gut ().Citation26,Citation27
Figure 1 Blockade of α-integrins inhibits leukocyte migration into gut mucosa.
Abbreviation: ICAM-1, Intercellular Adhesion Molecule 1; MadCAM-1, mucosal addressin cell adhesion molecule; PSGL, P-selectin glycoprotein ligand; VCAM-1, vascular cell adhesion molecule 1.
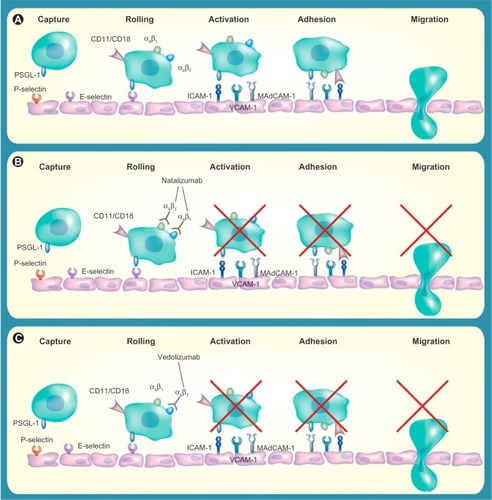
Natalizumab
The first of the integrin antagonists to emerge was natalizumab, a monoclonal antibody targeted against the adhesion molecule, alpha 4 integrin. Although first used in the treatment of multiple sclerosis, it was subsequently approved for use in CD in 2008.Citation28
The efficacy of natalizumab in moderate-to-severe CD was reported in the ENCORE trial where it achieved a clinical response rate of 48% at weeks 8 to 12 compared to 32% in the placebo group (P<0.001).Citation29 The ENACT-2 trial reported that patients who responded to initial treatment with natalizumab were more likely to maintain clinical response (61% versus [vs] 28%; P<0.001) and remission (44% vs 26%; P=0.003) with continued natalizumab treatment when compared with patients receiving placebo maintenance at week 36.Citation30
Natalizumab remains a second-line treatment of CD and its use is limited by an associated increased incidence of progressive multifocal leukoencephalopathy (PML), a central demyelinating illness caused by the opportunistic human polyoma John Cunningham (JC) virus.Citation31 Natalizumab targets the α4 monomer, thereby antagonizing both the α4β1 and α4β7 integrins. It is hypothesized that through inhibition of the α4β1 subunit and its interactions, there is reduced immune surveillance in the central nervous system, and consequently, an increased risk of PML.Citation32 The incidence rate has been estimated to be between 0.09 to 11 per 1,000 patients, with higher risk being associated with longer use of natalizumab, prior use of immunosuppressants, and evidence of JC virus infection.Citation33 Although natalizumab-associated PML has an improved survival compared with PML in other populations, the mortality rate is up to 29%, with many of the surviving patients suffering from a degree of disability.Citation34
Vedolizumab, by selectively targeting the α4β7 integrin heterodimer, is thought to be “super-selective” and may not cross the blood–brain barrier.Citation35 It appears to specifically inhibit lymphocyte migration within the gut.Citation36 Studies have reported that it does not affect the levels of T-cells in the cerebrospinal fluid of healthy volunteers after a single dose, and nor does it inhibit immune surveillance of the central nervous system in non-human primates.Citation34,Citation36,Citation37 To date, there have been no reported cases of PML in patients treated with vedolizumab for UC or CD.Citation38–Citation42 The precise targeting of vedolizumab to leukocyte trafficking systems within the gut may provide an improved risk–benefit profile.Citation36,Citation43
Vedolizumab
Vedolizumab (also known as MLN0002, LDP02, and MLN02) is a highly selective monoclonal antibody targeting the α4β7 integrin molecule.Citation44 The α4β7 integrin is a cell surface glycoprotein variably expressed on lymphocytes and is thought to be partly responsible for T-cell homing into lymphoid tissues in the gastrointestinal tract through its binding to the mucosal addressin cell adhesion molecule (MAdCAM-1).Citation45 These bound lymphocytes then migrate from the endothelium of the intestinal vasculature into the lamina propria and tissues, propagating inflammation.Citation46 Higher levels of α4β7 integrin and MAdCAM-1 have been shown to be present in the colons of those with IBD than in patients with irritable bowel syndrome.Citation47 It is also thought that there are lower numbers of T-lymphocytes with the α4β7 integrin circulating in the peripheral blood in patients with colonic inflammation.Citation47 As these agents are considered “gut selective”, the α4β7 integrin molecules provide an opportunity to attenuate the pathological gut inflammation seen in patients with IBD.Citation48
Vedolizumab: pharmacokinetics and immunogenicity
Pharmacokinetics
During Phase II trials, it was reported that vedolizumab exhibited dose proportional pharmacokinetics, with maximally saturated α4β7 receptors on peripheral serum lymphocytes over a minimal test dose range of 2 mg/kg.Citation40 As the serum concentration of vedolizumab fell below the level of detection of the assay, α4β7 integrin-mediated trafficking was restored. The mean elimination half-life of vedolizumab is 15–22 days, with the levels of vedolizumab at one infusion every 8 weeks detectable at a steady level throughout the study, with nearly full inhibition of α4β7 receptors.Citation40
Immunogenicity
During early Phase II trials of MLN0002, up to 38% patients developed auto-antibodies.Citation39 This unexpectedly high rate of immune sensitization led to an improved formulation of α4β7 integrin inhibitor to be developed. The new preparation of vedolizumab was developed using the Chinese hamster ovary cell-based system instead of the mouse myeloma cell line that MLN 0002 had been derived from. This new formulation of vedolizumab has been associated with antibody formation in 3.7%–4.1% of patients.Citation49,Citation50 The presence of these antibodies is thought to be associated with reduced efficacy of the drug.Citation39,Citation46 A dose-related antibody response has also been reported, with a lower proportion of patients who received a higher dose of vedolizumab (2 mg/kg vs 0.5 mg/kg) developing antibodies.Citation41 The presence of auto-antibodies was also associated with reduced saturation of the α4β7 binding site.Citation14 In a recent Phase III trial, concomitant immunosuppressive therapy was shown to be associated with decreased immunogenicity, which may improve efficacy and increase response rates.Citation49
Vedolizumab and UC
UC is a chronic condition and can be associated with significant morbidity and disability.Citation51 UC is the most common type of IBD and its frequency in developed countries has been increasing since the mid-20th century.Citation52 Approximately 25% of people with UC will have one or more episodes of acute severe colitis in their lifetime. Although mortality rates have improved steadily over the past 30 years, acute severe colitis still has an average mortality rate of 2%, which can rise to 13% if an emergency colectomy is required.Citation53,Citation54
Vedolizumab in UC: early clinical trials
The inhibition of α4β7 integrin was first shown to be effective in inducing remission in colitis in a study on cotton-top tamarin monkeys in 1996.Citation55 The authors reported that there was reduced inflammatory activity and rapidly improved stool consistency when a monoclonal antibody against the α4β7 integrin was given to eight tamarin monkeys who were chronically affected with colitis. Furthermore, they found that antibody therapy reduced the mucosal density of α4β7 positive lymphocytes and α4β7 neutrophils and macrophages. These positive results allowed for vedolizumab to progress to Phase I clinical trials. provides a summary of results for vedolizumab.
Table 1 A summary of vedolizumab results in Phase I–III clinical trials to date
Vedolizumab in UC: Phase I trial
In 2000, a double-blind, placebo-controlled Phase I trial using a humanized α4β7 antibody was carried out in 29 patients with moderate-to-severe UC.Citation38,Citation46 The inclusion criteria were that patients had a modified Baron gradeCitation56 of 2 and a Mayo scoreCitation57 of 5 or more, endoscopic evidence of UC for at least 25 cm from anal verge and had at least three bowel movements a day. A single dose of the humanized antibody was given to participants in an ascending dose, 0.15 mg/kg subcutaneous injection (SC), 0.15 mg/kg intravenous injection (IV), 0.5 mg/kg IV, and 3 mg/kg IV or placebo. A dose of 0.5 mg/kg IV was found to be enough to give an endoscopic response at day 30, a two grade improvement in the modified Baron score, and to completely saturate the antibody receptors. Complete endoscopic and clinical remission was seen in 40% of those patients receiving 0.5 mg/kg IV. These promising results led to several Phase II trials being commissioned.
Vedolizumab in UC: Phase II trials
A Phase II trial of α4β7 antibody (MLN002) was subsequently carried out in 2005 on patients with active UC, a multi-center, double-blind, placebo-controlled trial involving 181 patients.Citation39 Patients were randomized to receive either 0.5 mg/kg, 2 mg/kg of MLN002, or placebo intravenously on day 1 and day 29. The authors reported that vedolizumab was significantly more likely to induce clinical remission at 6 weeks compared with placebo. Clinical remission rates (defined as a UC clinical score of 0–1 and a modified Baron grade of 0–1 with no evidence of rectal bleeding) at week 6 were 33%, 32%, and 14% for the groups receiving 0.5 mg/kg, 2.0 mg/kg, and placebo, respectively (P=0.03). The corresponding proportion of patients who improved by at least three points on the UC clinical score was also significant between the MLN002 groups (66% in the 0.5 mg/kg, 53% in the 2 mg/kg) and the placebo group (33%, P=0.002). Furthermore, 28% of patients receiving 0.5 mg/kg and 12% of those receiving 2.0 mg/kg had endoscopically evident remission, as compared with 8% of those receiving placebo (P=0.007). Both groups had statistically significant results compared to placebo; however, it is unclear why the clinical and endoscopic remission and response rates were higher in the 0.5 mg/kg dose group than in the 2 mg/kg group. Although the authors have not provided a hypothesis for this, it is possible that the higher withdrawal rate in the 2 mg/kg group (8% vs 2%) could have influenced results as those who withdrew early were classified as not achieving remission. No important differences were observed among the three groups in the reasons for withdrawal.
In 2012, the results of a Phase II dose-ranging study were reported by Parikh et al using an improved formulation of vedolizumab.Citation40 The study recruited 46 adults with UC (defined as a partial Mayo score >1) randomized to receive vedolizumab (2, 6, or 10 mg/kg) or placebo on days 1, 15, 29, and 85, and were followed until day 253. This trial involved higher doses of vedolizumab and shorter frequency between treatment doses than in previous trials. They reported that the clinical response rate of the combined cohort who received vedolizumab was over 50% compared to between 22%–33% in those treated with placebo. Vedolizumab was also shown to reduce fecal calprotectin levels as compared to placebo.
Vedolizumab in UC: Phase III trials
In August 2013, the results of the GEMINI I trial were published. This randomized, double-blinded, Phase III trial studied the efficacy and safety of vedolizumab in patients with moderate-to-severe UC.Citation50,Citation58 Feagan et alCitation50 reported two integrated trials involving 895 patients, to assess the efficacy of vedolizumab for both induction and maintenance therapy in UC.
Patients who had previously failed first-line treatments, including anti-TNFα therapy, as well as those who were anti-TNFα naïve were eligible for this study. Other eligibility criteria included active UC, a Mayo score of ≥6 and an endoscopic subscore of ≥2 despite glucocorticoids, thiopurines, or anti-TNFα. The primary outcome for the induction phase of the trial was clinical response to vedolizumab at week 6. Patients were randomized to receive either vedolizumab 300 mg IV or placebo on days 1 and 15. The authors reported that a significantly greater proportion of patients who received vedolizumab achieved clinical response, remission, and mucosal healing at 6 weeks, compared with placebo. Clinical response was achieved in 47% of the vedolizumab group compared to 26% in the placebo group (P<0.0001). Clinical remission was seen in 17% of the vedolizumab group compared with 5% of those treated with placebo (P=0.0009). Mucosal healing rates were seen in 41% of those treated with vedolizumab compared with 25% of the placebo group (P=0.0012). Clinical response and remission rates were higher with vedolizumab treatment among both those with prior anti-TNF failure and those with no prior anti-TNF exposure.
GEMINI I also reported the efficacy and safety of maintenance therapy with vedolizumab in moderate-to-severe UC.Citation50,Citation59 Those patients achieving clinical response after induction therapy at 0 and 2 weeks, were randomized to receive vedolizumab 300 mg IV at 4-week intervals, vedolizumab 300 mg IV at 8-week intervals, or placebo for 46 weeks.
The results showed that vedolizumab was associated with significantly higher clinical remission rates than placebo at 52 weeks (42% in the vedolizumab 8-weekly group, 45% in vedolizumab 4-weekly group, and 16% in the placebo group; P<0.0001). Vedolizumab was also associated with higher mucosal healing rates (52% in the vedolizumab 8-weekly group, 56% in vedolizumab 4-weekly, and 20% placebo group; P<0.0001). The number of patients who were on concomitant glucocorticoids at 52 weeks was significantly lower in those treated with vedolizumab than in those who received placebo (31% of the vedolizumab 8-weekly group, 45% of vedolizumab 4-weekly group, and 14% placebo group; P=0.0120/P<0.0001, respectively). No clear differences in efficacy were observed between the two vedolizumab regimens. Clinical response and remission rates were greater with vedolizumab in both anti-TNF therapy naïve patients, as well as those with a history of exposure to anti-TNFα agents.
Taken together, these results demonstrate good response and remission rates, mucosal healing, low rate of immunogenicity, and accepted tolerability and safety for vedolizumab in UC in patients who were naïve and in those who had failed anti-TNF therapy.
Vedolizumab and CD
CD is a condition that is characterized by trans-mural inflammation, which can affect any area of the gastrointestinal tract, along with many systemic manifestations. It has a relapse rate of 67% at 5 years, and 10% of patients will have chronically active disease.Citation60 Although the rate of surgical intervention is falling with the increased use of biologics, the lifetime risk of surgery in CD patients remains high, with up to 60%–70% of patients requiring surgery, dependent on disease severity and location.Citation61 Biologics, such as vedolizumab, may assist to improve response, and remission rates may reduce morbidity, mortality, disability, and the cost burden of CD.
Vedolizumab in CD: Phase II trials
In 2008, Feagan et al reported the results of a randomized, double-blinded, placebo-controlled Phase II trial, which was conducted to assess the efficacy and safety of vedolizumab (then named MLN0002), in patients with active CD.Citation42 Adult patients with CD of the ileum and/or colon, naïve to biologic therapy, and with a CD Activity Index (CDAI) score of 220–400 at screening, were eligible to participate in this study.Citation62 One hundred and eighty-three patients were randomized to receive MLN0002 2.0 mg/kg, MLN0002 0.5 mg/kg, or placebo by IV infusion on days 1 and 29. The randomization was stratified to include concomitant mesalazine use. The primary outcome measure was clinical response at day 57, defined as an equal or greater than 70-point decrement in the CDAI score. The authors reported that there was no significant difference in clinical response rates between patients who received vedolizumab and those who received placebo. Clinical response rates at day 57 were 53%, 49%, and 41% in the MLN0002 2.0 mg/kg, MLN0002 0.5 mg/kg, and placebo groups, respectively. The proportion of patients achieving a more stringently defined enhanced clinical response (≥100 point decrement in CDAI from baseline) at day 57 were 47% and 31%, in the MLN0002 2.0 mg/kg and placebo groups respectively (P=0.05). Although there was failure to achieve the primary outcome, the results suggested a possible dose-dependent effect of vedolizumab in CD.
Vedolizumab in CD: Phase III trials
More recently, the results of the Gemini II were published. Sandborn et al reported a randomized, double-blind, placebo-controlled Phase III trial, which assessed the efficacy and safety of vedolizumab as induction and maintenance therapy in moderate-to-severe CD.Citation49 Patients were randomized to receive either vedolizumab 300 mg IV or placebo on days 1 and 15. As with GEMINI I, the randomization was stratified to take into account past or concomitant use of glucocorticoids, immunosuppressants, and anti-TNFα agents. The primary outcome was considered to be clinical remission (CDAI score = 150 points) and enhanced clinical response (100 point decrease in CDAI from baseline) at 6 weeks. A secondary outcome was mean change in serum CRP at 6 weeks in patients with elevated C-reactive protein (CRP) at baseline.Citation63
The authors reported that a significantly greater proportion of patients receiving vedolizumab achieved clinical remission at week 6 compared to those receiving placebo (15% in the vedolizumab group compared to 7% of placebo group, P=0.0206). However, vedolizumab was not associated with a significant difference in clinical response rates compared with placebo at 6 weeks. In those patients who had elevated baseline CRP levels, there was no significant difference in the mean change of CRP level between the groups. Of the intention-to-treat (ITT) population, 48% had prior anti-TNFα failure; of these, 55% were primary failures. In addition, 27% of the ITT population had failed at least two anti-TNF preparations. Positive trends were observed for clinical remission and enhanced clinical response rates in vedolizumab versus placebo patients, irrespective of prior anti-TNF treatment status.
For the maintenance phase of the trial, patients who had a clinical response with vedolizumab at week 6 were randomly assigned to receive 8-weekly vedolizumab, 4-weekly vedolizumab, or placebo regime for up to 52 weeks. The patients that did not have a clinical response to vedolizumab induction therapy received 4-weekly vedolizumab and were followed up to 52 weeks. The primary endpoint was clinical remission at week 52. At the end of week 52, 39% of patients who received 8-weekly vedolizumab and 36% of patients receiving 4-weekly vedolizumab were in clinical remission, as compares to 22% of those who received placebo (P<0.001 and P=0.004 when comparing each group with placebo, respectively). The proportion of patients who had a 100-point reduction in CDAI score and glucocorticoid-free remission were significantly greater in both vedolizumab groups; however, rates of durable clinical remission showed no significant differences.
Why are there differences in the efficacy of vedolizumab between UC and CD?
Response and remission rates for vedolizumab vary between UC and CD. Clinical response rates in UC were reported between 47%–50%, with durable clinical remission rates between 41%–45% and mucosal healing rates of between 28%–56%.Citation50,Citation39 In CD, the results were less encouraging, with a response rate of 31%–53% and durable clinical remission rates of between 36%–39%.Citation49,Citation42 It has been suggested that the difference arises due to the “super-selective” nature of vedolizumab. CD is a systemic disorder, characterized by transmural inflammation which can affect any part of the gastrointestinal tract from mouth to anus, whereas UC is considered a more localized disease associated with sub-mucosal inflammation, more limited to the large bowel. Vedolizumab, due to its specificity for the α4β7 integrin heterodimer, is thought to exert a localized mode of action. This is in contrast to natalizumab, which has been shown to have good efficacy in CD and inhibits both the α4β7 and α4β1, integrins, possibly resulting in a greater systemic blockade on leukocyte trafficking.Citation24 Another hypothesis is that vedolizumab may require a longer time to produce a clinical response by inhibiting leukocyte migration in CD than in UC. This was reported in the GEMINI II trial results when vedolizumab failed to achieve its primary endpoint of clinical response at week 6 but showed efficacy at maintaining remission at week 52.Citation49 Overall, the evidence of vedolizumab in CD is promising, yet more data is required to fully characterize which subset of CD patients would derive most benefit.
Safety and tolerability of vedolizumab
The overall safety profile of vedolizumab throughout the clinical trials conducted has been positive and consistent with the concept of selective immunosuppression.Citation49,Citation64 Clinically important infusion reactions were rare.Citation40,Citation49,Citation50 Doses of 10 mg/kg of vedolizumab were well tolerated.Citation40 As stated before, no cases of PML have yet been reported despite more than 3,000 patients having been exposed to vedolizumab for periods of up to 6 years.Citation37,Citation39,Citation40,Citation64 In the large GEMINI I study, no significant difference was observed among the study groups in the most commonly reported adverse incidents.Citation50 The most common adverse advents reported were exacerbation of IBD, headache, and nasopharyngitis.Citation49,Citation50 The GEMINI II study, however, found that the incidence of infections and serious infections was higher in those who received vedolizumab than with placebo. They also found that the incidence of any serious event was higher among those who received vedolizumab than among those who received placebo (25% vs 15%). Long term observational data combined with data from Phase IV trials where large numbers of patients are exposed are needed to fully characterize the safety profile of vedolizumab, including the risk, if any, of developing PML. Vedolizumab is currently being assessed in a Phase III trial to determine its long-term safety (GEMINI LTS) and is due to be completed in March 2016.Citation65
The future role of vedolizumab in IBD
Vedolizumab appears to be an important, much needed new therapy for patients with IBD. Standard therapies are suboptimal, with many patients not responding, relapsing, or experiencing common side effects. In UC, vedolizumab has shown efficacy in inducing and maintaining remission in patients who have failed first-line treatment, as well as those who have failed anti-TNFα therapy. Therefore, one important role of vedolizumab may be in refractory UC prior to surgical intervention. Vedolizumab has also been shown to be effective in CD, although more research is required to identify a subset of patients who will achieve the most benefit from its initiation.
In the future, vedolizumab and other disease-modifying drugs may have a place in first-line therapy in those patients with IBD. Current medical management of IBD is based on a step-wise system with treatments being used sequentially and treatment escalated as patients fail to respond to each step of treatment. However, there is mounting evidence that focusing treatment on mucosal healing, rather than clinical symptoms, may result in a reduction in the need for surgery and reduced hospitalization rates for both UC and CD.Citation66 Similar to the treatment strategies currently used in rheumatoid arthritis, the elimination of disability and reduction of structural bowel damage has emerged as a new therapeutic goal.Citation13 The concept of identifying patients who have a high risk of morbidity early in their disease and introducing disease modifying anti-IBD drugs, including vedolizumab and other small molecule inhibitors is growing in favor with gastroenterologists.Citation13
To date, vedolizumab has shown a good level of patient acceptability and a reasonable safety profile; however, prospective studies are required to ascertain its long-term benefits and side-effect profile in the treatment of IBD. It seems probable that vedolizumab will become available as a second-line treatment for those patients who fail anti-TNF therapy, especially in UC. However, as more data becomes available, vedolizumab may become the first-line or adjunctive therapy in certain patients with moderate-to-severe IBD.
Disclosure
The authors report no conflicts of interest in this work.
References
- LoftusEVJrClinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influencesGastroenterology20041261504151715168363
- MolodeckyNASoonISRabiDMIncreasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic reviewGastroenterology20111424654 e4222001864
- ParkKTBassDInflammatory bowel disease-attributable costs and cost-effective strategies in the United States: a reviewInflamm Bowel Dis2011171603160921053357
- SandsBEAndersonFHBernsteinCNInfliximab maintenance therapy for fistulizing Crohn’s diseaseNew Eng J Med2004350987688514985485
- HanauerSBFeaganBGLichtensteinGRACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trialLancet200235993171541154912047962
- SandbornWJHanauerSBRutgeertsPAdalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trialGut20075691232123917299059
- SandbornWJFeaganBGStoinovSPRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn’s diseaseNew Eng J Med2007357322823817634458
- DignassALindsayJOSturmASecond European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current managementJ Crohns Colitis2012610991103023040451
- RutgeertsPSandbornWJFeaganBGInfliximab for induction and maintenance therapy for ulcerative colitisNew Eng J Med2005353232462247616339095
- ReinischWSandbornWJHommesDWAdalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trialGut201160678078721209123
- SandbornWJFeaganBGMaranoCPURSUIT-Maintenance Study Group. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitisGastroenterology2014146196109 e123770005
- FordACSandbornWJKhanKJHanauerSBTalleyNJMoayyediPEfficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysisAm J Gastroenterol20111064644659 quiz 66021407183
- AllenPBPeyrin-BirouletLMoving towards disease modification in inflammatory bowel disease therapyCurr Opin Gastroenterol201329439740423695427
- McLeanLPShea-DonohueTCrossRKVedolizumab for the treatment of ulcerative colitis and Crohn’s diseaseImmunotherapy20124988389823046232
- LichtensteinGRPanaccioneRMallarkeyGEfficacy and safety of adalimumab in Crohn’s diseaseTherap Adv Gastroenterol2008114350
- McDermottEMurphySKeeganDO’DonoghueDMulcahyHDohertyGEfficacy of Adalimumab as a long term maintenance therapy in ulcerative colitisJ Crohns Colitis20137215015322520592
- Ben-HorinSChowersYReview article: loss of response to anti-TNF treatments in Crohn’s diseaseAliment Pharmacol Ther201133998799521366636
- AfifWLoftusEVFaubionWAClinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel diseaseAm J Gastroenterol201010551133113920145610
- LeungYPanaccioneRAnti-adhesion molecule strategies for Crohn diseaseBio Drugs2008224259264
- GisbertJPPanésJLoss of response and requirement of infliximab dose intensification in Crohn’s disease: a reviewAm J Gastroenterol2009104376076719174781
- SandbornWJRutgeertsPEnnsRAdalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trialAnn Intern Med20071461282983817470824
- RutgeertsPvan AsscheGVermeireSOptimizing anti-TNF treatment in inflammatory bowel diseaseGastroenterology200412661593161015168370
- GhoshSPanaccioneRAnti-adhesion molecule therapy for inflammatory bowel diseaseTherap Adv Gastroenterol201034239258
- CominelliFInhibition of leukocyte trafficking in inflammatory bowel diseaseNew Eng J Med2013369877577623964940
- FussIJHellerFBoirivantMNonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitisJ Clin Invest20041131490149715146247
- BevilacquaMPEndothelial-leukocyte adhesion moleculesAnnu Rev Immunol1993117678048476577
- Van AsscheGRutgeertsPPhysiological basis for novel drug therapies used to treat the inflammatory bowel diseases. I. Immunology and therapeutic potential of antiadhesion molecule therapy in inflammatory bowel diseaseAm J Physiol Gastrointest Liver Physiol20052882G169G17415647604
- HolmesDIntegrin inhibitors go with the gutNat Rev Drug Discov201312641141223722331
- TarganSRFeaganBGFedorakRNInternational Efficacy of Natalizumab in Crohn’s Disease Response and Remission (ENCORE) Trial Group. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE TrialGastroenterology200713251672168317484865
- SandbornWJThe future of inflammatory bowel disease therapy: where do we go from here? Dig Dis201230Suppl 314014423295705
- DaneseSNew therapies for inflammatory bowel disease: from the bench to the bedsideGut201261691893222115827
- FDA Drug Safety CommunicationNew risk factor for Progressive Multifocal Leukoencephalopathy (PML) associated with Tysabri (natalizumab) [webpage on the Internet]Center for Drug Evaluation and Research2012 [cited January 20, 2012] Available from http://www.fda.gov/Drugs/DrugSafety/ucm288186.htmAccessed October 30, 2013
- BloomgrenGRichmanSHotermansCRisk of natalizumab-associated progressive multifocal leukoencephalopathyNew Eng J Med2012366201870188022591293
- VermerschPKapposLGoldRClinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathyNeurology201176201697170421576685
- MilchCWyantTXuJVedolizumab, a monoclonal antibody to the gut homing α4β7 integrin, does not affect cerebrospinal fluid T-lymphocyte immunophenotypeJ Neuroimmunol20132641–212312624067534
- AllenPBAnti-adhesion molecules: is gut specificity the key for a good safety profile? Curr Drug Deliv2012933333722762276
- FedykERWyantTYangL-LExclusive antagonism of the α(4) β(7) integrin by vedolizumab confirms the gut-selectivity of this pathway in primatesInflamm Bowel Dis2012182107211922419649
- FeaganBMacdonaldJGreenbergGAn ascending dose of a humanized alpha 4 beta 7 antibody in ulcerative colitis (UC)Gastroenterology20001184A874.21
- FeaganBGGreenbergGRWildGTreatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrinNew Eng J Med2005352242499250715958805
- ParikhALeachTWyantTVedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging studyInflammat Bowel Dis20121814701479
- ParikhALeachTXuJFeaganBLong-term clinical experience with vedolizumab (VDZ) in patients with mild to moderate ulcerative colitis (UC)J Crohns Colitis20126S103
- FeaganBGGreenbergGRWildGTreatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrinClin Gastroenterol Hepatol20086121370137718829392
- SolerDChapmanTYangL-LWyantTEganRFedykERThe binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseasesJ Pharmacol Exp Ther200933086487519509315
- MarshallJKLDP-02 (Millenium)Curr Opin Investig Drugs20012502504
- PetrovicAAlpdoganOWillisLMLPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host diseaseBlood20041031542154714563643
- GledhillTBodgerKNew and emerging treatments for ulcerative colitis: a focus on vedolizumabBiologics2013712313023723689
- SouzaHSEliaCCSpencerJMacDonaldTTExpression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel diseaseGut19994585686310562584
- MeenanJSpaansJGroolTAAltered expression of alpha 4 beta 7, a gut homing integrin, by circulating and mucosal T cells in colonic mucosal inflammationGut1997402412469071939
- SandbornWJFeaganBGRutgeertsPGEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s diseaseNew Eng J Med2013369871172123964933
- FeaganBGRutgeertsPSandsBEGEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitisNew Eng J Med2013369869971023964932
- AllenPBKammMAPeyrin-BirouletLDevelopment and validation of a patient-reported disability measurement tool for patients with inflammatory bowel diseaseAliment Pharmacol Therap201337443844423278192
- DaneseSFiocchiCUlcerative colitisNew Eng J Med2011365181713172522047562
- NICEManagement of ulcerative colitisNICE Guidelines62013 Available from http://publications.nice.org.uk/ulcerative-colitis-cg166Accessed September 14, 2013
- BittonABuieDEnnsRCanadian Association of Gastroenterology Severe Ulcerative Colitis Consensus Group. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statementsAm J Gastroenterol20121072179194 author reply 19522108451
- HesterbergPEWinsor-HinesDBriskinMJRapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7Gastroenterology19961115137313808898653
- BaronJHConnellAMLennard-JonesJEVariation between observers in describing mucosal appearances in proctocolitisBMJ196415375899214075156
- SchroederKWTremaineWJIlstrupDMCoated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized studyNew Eng J Med198731726162516293317057
- FeaganBGRutgeertsPJSandsBE943b Induction therapy for ulcerative colitis: results of GEMINI I, a randomized, placebo-controlled, double-blind, multicenter phase 3 trialGastroenterology20121425S160S161
- RutgeertsPJVedolizumab maintenance therapy for ulcerative colitis (uc): results of GEMINI I, a randomized, placebo-controlled, double-blind, multicenter phase 3 trialGut201261supp 3A65
- LapidusABernellOHellersGLöfbergRClinical course of colorectal Crohn’s disease: a 35-year follow-up study of 507 patientsGastroenterology19981146115111609609751
- MowatCColeAWindsorAIBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adultsGut201160557160721464096
- BaumgartDCVeto on vedolizumab (MLN0002) for Crohn’s diseaseInflammat Bowel Dis2010163537538
- SandbornWSandsBEColombelJFVedolizumab induction therapy for Crohns Disease: results of GEMINI II, a randomized, placebo controlled, double-blind, multi-center phase 3 trial. P395Annual Scientific Meeting Abstracts2012Las Vegas, NVAmerican College of Gastroenterology
- MosliMHFeaganBGVedolizumab for Crohn’s diseaseExpert Opin Biol Ther20131345546323394379
- Millennium Pharmaceuticals IncAn open-label study of vedolizumab (MLN0002) in patients with ulcerative colitis and Crohn’s disease Available from: http://clinicaltrials.gov/show/NCT00790933. NLM identifier: NCT00790933Accessed October 15, 2013
- Peyrin-BirouletLFerranteMMagroFScientific Committee of the European Crohn’s and Colitis Organization. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel diseaseJ Crohns Colitis20115547748321939925
- FiorinoGCorrealeCFriesWRepiciAMalesciADaneseSLeukocyte traffic control: a novel therapeutic strategy for inflammatory bowel diseaseExpert Rev Clin Immunol20106456757220594130
