Abstract
Increased intestinal permeability has been identified as one of the many pathophysiological factors associated with the development of irritable bowel syndrome (IBS), a common disorder of gut–brain interaction. The layer of epithelial cells that lines the intestine is permeable to a limited degree, and the amount of paracellular permeability is tightly controlled to enable the absorption of ions, nutrients, and water from the lumen. Increased intestinal permeability to macromolecules can be triggered by a variety of insults, including infections, toxins from food poisoning, or allergens, which in turn cause an inflammatory response and are associated with abdominal pain in patients with IBS. This review article discusses increased intestinal permeability in IBS, focusing on IBS with constipation (IBS-C) through the lens of a patient case with a reported prior diagnosis of “leaky gut syndrome” upon initial contact with a gastrointestinal specialist. We review advantages and disadvantages of several methods of measuring intestinal permeability in patients and discuss when measuring intestinal permeability is appropriate in the therapeutic journey of patients with IBS-C. Furthermore, we discuss a possible mechanism of restoring the intestinal barrier to its healthy state through altering intracellular pH by inhibiting sodium-hydrogen exchanger isoform 3 (NHE3). Tenapanor is a minimally absorbed, small-molecule inhibitor of NHE3 that has been approved by the US Food and Drug Administration for the treatment of IBS-C in adults. Preclinical studies showed that tenapanor may restore the intestinal barrier in IBS-C by affecting the conformation of tight junction proteins via NHE3 inhibition to block the paracellular transport of macromolecules from the intestinal lumen. Testing for increased permeability in patients with IBS-C who experience abdominal pain may help inform the choice of therapeutics and alter patients’ misconceptions about “leaky gut syndrome”.
Introduction
Irritable bowel syndrome (IBS) is a disorder of gut–brain interaction defined by recurrent abdominal pain at least once a week associated with two of the following: defecation, changes in stool frequency or changes in stool form, occurring for at least 6 months, according to Rome IV criteria.Citation1 The gut–brain interaction refers to the bidirectional communication between the central nervous system and the enteric nervous system that links the cognitive and emotional centers of the brain with peripheral intestinal functions.Citation2 Based on the Rome IV criteria, the global prevalence of IBS is an estimated 4%, with a higher prevalence in women and individuals younger than 65 years.Citation3 Although not life-threatening, the disease burden significantly negatively affects patients’ daily functioning, health-related quality of life (QoL), and health care resource utilization and costs.Citation4–6
There are four distinct subtypes of IBS based on the prominent stool consistency: IBS with predominant constipation (IBS-C), IBS with predominant diarrhea (IBS-D), IBS with mixed bowel habits, and IBS unclassified (ie, without a significant pattern of abnormal stool).Citation1 According to the Rome IV criteria, IBS-C and IBS-D account for 31.5% and 29.3% of patients with IBS, respectively.Citation7 Patients with IBS-C report greater symptom burden and impaired functioning than other subtypes.Citation8 Abdominal pain has been reported as the most troublesome abdominal symptom,Citation9 with lack of improvement in abdominal pain being reported as a common reason for treatment discontinuation by 29–49% of patients in the CONTOR study.Citation10 In this review, we discuss a patient case of IBS-C, review intestinal permeability (ie, how it is measured and how it may contribute to abdominal pain in patients with IBS-C) and discuss how restoring intestinal barrier function with tenapanor, a locally acting inhibitor of sodium-hydrogen exchanger isoform 3 (NHE3), may improve abdominal pain.
Patient Case
A 31-year-old male, referred to a gastroenterologist for a second opinion, reported an 18-month history of lower abdominal pain, bloating, and infrequent, hard to evacuate stools. Symptoms began after an apparent episode of food poisoning. While initially troubled with loose, watery, non-bloody bowel movements, for the past year the patient’s bowel movements were hard, difficult to evacuate, and only occurred every 2 to 3 days. His spouse had similar symptoms but recovered completely. Laboratory tests, including a complete blood count, metabolic profile, liver chemistries, thyroid tests, celiac serologies, and C-reactive protein were normal on two separate occasions. Because of persistent symptoms, his gastroenterologist performed a colonoscopy, which was normal. A lactulose breath test ruled out both small intestinal bacterial overgrowth and intestinal methanogen overgrowth. Over-the-counter products, including docusate sodium, bisacodyl, and polyethylene glycol, did not relieve constipation symptoms and some caused abdominal cramps. He recently saw a naturopath who told him that he had “leaky gut syndrome” and suggested a trial of several different dietary supplements. The patient has since requested another opinion regarding “leaky gut”.
This is a familiar story for internists and gastroenterologists, as this case is representative of many patients with IBS. This syndrome commonly affects individuals less than 50 years old,Citation11 with more than 50% of patients with IBS exhibiting a change in predominant subtype over a 1-year period,Citation12–14 and the use of over-the-counter products typically fails to provide relief.Citation15 Infectious gastroenteritis is a known cause of IBS;Citation16 however, the exact mechanisms that led to this patient developing IBS-C, while his spouse recovered completely, remain unknown. Current evidence indicates that multifactorial pathophysiological disturbances may lead to the development of IBS-C, including motility disturbances, visceral hypersensitivity, an increase in intestinal permeability, and disruptions to the gastrointestinal (GI) microbiome.
What is Intestinal Permeability?
The GI tract serves as a functional barrier between the body and the surrounding environment that is essential for maintaining human health. Consisting of a complex system of physical and chemical layers (epithelial cells, immune cells, commensal microbiota, mucus, and digestive secretions), the intestinal tract protects the internal milieu from exposure to pathogens and toxins while facilitating the uptake of nutrients and water. Perhaps, the most important physical barrier in the intestine is the epithelium, which consists of a single layer of epithelial cells interconnected by tight junctions, adherens junctions, and desmosomes ().Citation17 Movement of solutes and fluid across the intestinal epithelium is regulated by tight junctions between epithelial cells as well as across the apical membrane of epithelial cells and is essential for normal health.Citation17
Figure 1 Schematic diagram of the intestinal epithelium with (A) normal permeability, and (B) increased permeability. (A) In normal intestinal epithelium, nutrients are absorbed from the lumen of the intestine into systemic circulation by passive, or paracellular, transport between the intercellular spaces. Tight junction proteins interconnecting the epithelial cells stop the transport of macromolecules from the lumen into systemic circulation. (B) In intestinal epithelium with increased permeability, the tight junction complex is disrupted and allow paracellular transport of macromolecules. They may activate mucosal mast cells to produce an inflammatory response, which can excite TRPV1-expressing sensory neurons, leading to increased visceral hypersensitivity and abdominal pain.
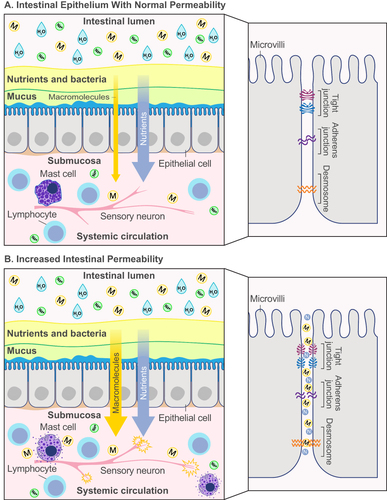
Infections in the GI tract can change intestinal permeability, both acutely and chronically, disrupting ionic gradients and osmotic pressure and allowing systemic infiltration of macromolecules (eg, toxins, bacteria, food allergens).Citation17,Citation18 A recent study showed that acute Clostridioides difficile infection in mice can cause significant intestinal epithelial damage and increase intestinal permeability through tight junction-dependent mechanisms.Citation19 Increased intestinal permeability can, in turn, activate epithelial immune cells, followed by cytokine release and inflammation, which can modify neural control of GI sensory-motor and secretory functions and increase visceral hypersensitivity ().Citation20,Citation21 Patients with IBS without an infectious etiology have also displayed aberrant long-term increases in intestinal permeability associated with alterations in tight junction proteins and adhesion molecules, similar to postinfection IBS (PI-IBS).Citation20 Factors positively associated with the development of IBS and increased intestinal permeability include psychological distress, depression, anxiety, and food intolerance ().Citation22,Citation23
Table 1 Factors Associated with Abnormal Intestinal Permeability
Table 2 Methods for Measuring Intestinal Permeability
Why is Intestinal Permeability Important, but Not “Leaky Gut”?
“Leaky gut syndrome” is the theory that toxins and bacteria freely permeate, or pass through, the intestinal epithelial barrier into the body, causing symptoms of chronic GI distress. While this theory makes a great visual to help explain increased intestinal permeability in plain language, it is not an accurate description of what truly happens. “Leaky gut” is not a definitive medical diagnosis according to most medical professionals, and despite lay claims to the contrary, tests of blood, stool, hair, and nail cannot accurately diagnose “leaky gut”. Importantly, a normal, healthy gut always “leaks” to some degree; epithelial cells contain transmembrane pores and transporters that regulate passive or active transport of molecules and ions, tight junctions open selectively to allow passage of nutrients, and temporary gaps regularly form when epithelial cells are renewed every 5 days in humans (ie, cell sheddingCitation17). Furthermore, small exogenous or endogenous agents (eg, antigens) can cause immune cells in the mucosa and lamina propria (the thin layer of connective tissue lining the intestines) to produce an inflammatory response, independent of macromolecule (eg, bacteria, toxins) permeation.Citation24
Intestinal permeability can be accurately measured, as discussed below, and changes in intestinal permeability are associated with a number of different disease states.Citation17 Intestinal permeability more accurately accounts for the dynamic function of the GI tract as a complex system of multiple defensive layers that are normally selectively permeable. A systematic review of IBS studies published through 2020 showed that 37–62% of patients with IBS-D and 16–50% of patients with PI-IBS had measurable increases in intestinal permeability versus healthy controls.Citation25 In IBS-C studies, increased intestinal permeability was found in 4–25% of patients.Citation25 Importantly, these data highlight that many, but not all, patients with IBS may have a pathologically “leaky gut” as the main pathophysiological feature.
How is Intestinal Permeability Measured?
Methods for measuring intestinal permeability have not evolved much in the past decade, and in vivo permeability assays such as the lactulose/mannitol test, measures of sucralose, sucrose, PEG4000/400, and 51Cr-EDTA are still the most widely used (). Other methodologies are available, such as ex vivo measurement using an Ussing chamber, which evaluates short-circuit current as a marker for the transportation of ions across the epithelium. However, this approach is somewhat limited due to the requirement for fresh intestinal biopsy tissue. The measurement of bacteria-related markers within blood, feces, or biopsy material is another alternative to in vivo permeability assays. While this approach holds promise for the future, it is currently not well established due to limited data, unclear/low assay specificity, and the need for some assays to be carried out within specialist laboratories ().
In vivo permeability assays typically employ noninvasive oral administration of large oligosaccharides (eg, lactulose) and small sugars (eg, mannitol).Citation17 While the small sugars can freely permeate a healthy intestinal epithelium, the large oligosaccharides can only traverse an intestinal barrier that is compromised. Thus, lactulose is not absorbed to any significant degree in a healthy individual, while mannitol is readily absorbed, which can then be measured after being filtered by the kidneys. The ratio of these molecules (lactulose:mannitol) is measured in the urine after a specified time and reflects the status of the GI epithelium. A diagnostic benefit of using lactulose and mannitol is that neither molecule is metabolized extensively, and both are excreted largely unchanged in the urine in proportion to the quantities absorbed into the circulation.Citation26 Another advantage of these molecules is that they are relatively inert, enabling widespread clinical use. Nonetheless, measures of intestinal permeability in vivo are relatively rare in clinical trials.
Zhou et alCitation27 used the lactulose/mannitol test with 24-hour urine collection to assess intestinal permeability changes after dietary glutamine supplementation for treatment of PI-IBS. This clinical trial not only indicated that increased intestinal permeability may be an underlying etiological factor for patients with PI-IBS, it also demonstrated the utility and safety of lactulose/mannitol testing in conjunction with other agents.Citation27 However, the lactulose/mannitol test has its limitations, one being that it can only assess the small intestine.Citation17 In fact, all currently available intestinal permeability tests are site-specific,Citation17 which is a possible confounding factor, among others, for studies attempting to discern the prevalence of increased intestinal permeability in vivo.
Can Increased Intestinal Permeability Lead to Pain in IBS?
The confluence of increased intestinal permeability, inflammation, and visceral pain in IBS has been extensively reviewed by Camilleri et al.Citation28 A study by Zhou et alCitation23 showed that patients with IBS-D and increased intestinal permeability had greater hypersensitivity to both somatic and visceral stimuli than patients with IBS-D with normal intestinal permeability and healthy controls.Citation29 Although similar studies have not been performed in patients with IBS-C, the mechanisms are likely similar. Increased intestinal permeability allows macromolecules to infiltrate the bloodstream and activate mast cells, initiating an inflammatory cascade;Citation17 the degree of intestinal permeability has been directly associated with the inflammatory response and IBS symptom severity.Citation22,Citation25,Citation30 Greater numbers of activated mast cells close to nerve endings correlate with the severity and frequency of abdominal pain in patients with IBS-C and IBS-D.Citation31–33 In addition to the intestinal upsurge in activated mast cells and other immune cells in the intestinal submucosa,Citation34,Citation35 a study reported that a three-fold increase in intestinal transient receptor potential vanilloid type-1 (TRPV1) immunoreactive sensory nerve fibers can be a predictor of pain in patients with IBS, regardless of subtype.Citation33 These observations highlight the relationship between increased intestinal permeability and the development and severity of abdominal pain in patients with IBS-C.
Why is It Important to Treat Increased Intestinal Permeability in IBS?
IBS is associated with considerable disease burden that markedly disrupts a patient’s daily life.Citation5,Citation6,Citation8,Citation25,Citation36 Chronic visceral pain of varied severity is a key disease characteristic that constitutes part of the ROME IV diagnostic criteria for IBS.Citation1 Patients with IBS experience abdominal pain at least once a week on average,Citation1,Citation37 and those with IBS-C more frequently experience diffuse abdominal pain of greater severity than those with other subtypes.Citation8 Patients with IBS report having worse health-related QoL than patients with diabetes or end-stage renal diseaseCitation36 and would give up an average of 15 years of life to be symptom-free.Citation5 Consequently, patients are willing to take considerable medication risks to alleviate their IBS symptoms, including an average 10% risk of sudden death, for a hypothetical cure.Citation38,Citation39 Considering the worldwide prevalence of IBS is estimated at between 4% (Rome IV criteria) and 10% (Rome III criteria),Citation3 a thorough investigation into the root causes of related abdominal pain and effective treatment methods is warranted.
Evidence indicates that modulating intestinal permeability may improve GI symptoms, especially abdominal pain, in some patients with IBS. Although no guidelines recommend testing for intestinal permeability in patients meeting criteria for IBS, it may be useful for gastroenterologists to consider intestinal permeability testing (eg, lactulose/mannitol) with patients with IBS-C upon diagnosis to determine whether increased intestinal permeability could be contributing to their symptoms (). Routine measuring of intestinal permeability both in clinical practice and for research studies may be helpful to guide patient therapy (discussed below). This positive diagnostic strategy may better inform health care practitioners on the optimal treatment option for each patient with IBS-C. The challenge is that diagnostic testing is not always readily available to the large number of patients with IBS who might benefit from this approach. Moreover, large studies would be needed to specify standards for a positive or negative test.
Figure 2 Clinical treatment decision guide for IBS-C.
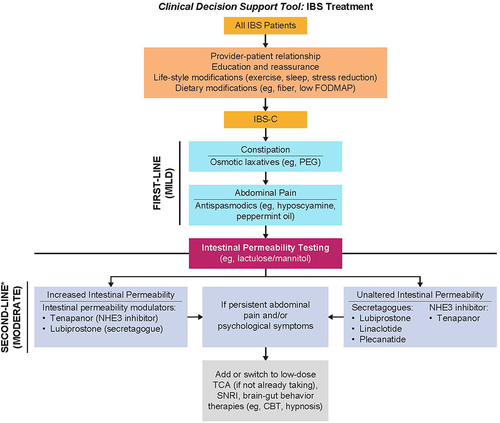
How Can Increased Intestinal Permeability in IBS-C Be Treated?
Current guidelines recommend the use of secretagogues (eg, lubiprostone, linaclotide, and plecanatide) and the NHE3 inhibitor, tenapanor, for patients with IBS-C.Citation37,Citation40 Of note, the 5-hydroxytryptamine receptor 4 agonist tegaserod is US Food and Drug Administration (FDA)-approved and recommended for women younger than age 65 years with one or fewer cardiovascular risk factors who have not adequately responded to secretagogues,Citation37,Citation40 although this agent has been removed from the US market for commercial reasons and will not be discussed further in this review.Citation41 While all of the recommended drugs have evidence to support their use, only lubiprostone and tenapanor are reported to modulate intestinal permeability.Citation43,Citation44 In the absence of head-to-head studies and with the lack of treatment options for patients who are refractory to secretagogues, this review will focus on how tenapanor could improve abdominal pain in patients with IBS-C by aiding in the restoration of normal intestinal permeability.
Tenapanor is a first-in-class, minimally absorbed, small-molecule inhibitor of NHE3 approved by the FDA for the treatment of IBS-C in adults.Citation45 It is also FDA approved as an add-on therapy for hyperphosphatemia in adults with chronic kidney disease on dialysis who have an inadequate response to phosphate binders or who are intolerant of phosphate binders.Citation44,Citation46 NHE3 is located on the apical surface of epithelial cells in the small intestine and proximal colon and is primarily responsible for absorbing dietary sodium and water and regulating acid-base homeostasis via proton exchange ().Citation47 Inhibition of NHE3 reduces dietary sodium absorption, which in turn retains luminal water, resulting in softer stools and accelerated intestinal transit.Citation44,Citation48,Citation49
Figure 3 Mechanism of action of tenapanor. Tenapanor inhibits NHE3 located on the apical surface of the intestinal epithelial cells. NHE3 inhibition reduces sodium absorption, leading to water retention in the lumen that in turn softens stools and accelerates intestinal transit. NHE3 inhibition also causes retention of intracellular protons in the epithelial cell, which decreases the intracellular pH and increases TEER. This increase is thought to result from a conformational change in the tight junction proteins that decreases intestinal permeability to macromolecules. Tenapanor also normalizes sensory neuronal excitability and TRPV1 currents and reduces visceral hypersensitivity. This figure is published under Creative Commons license CC-BY: King AJ et al. Am J Physiol Gastrointest Liver Physiol. 2024. doi: 10.1152/ajpgi.00233.2023. Copyright © 2024, American Journal of Physiology-Gastrointestinal and Liver Physiology.Citation50
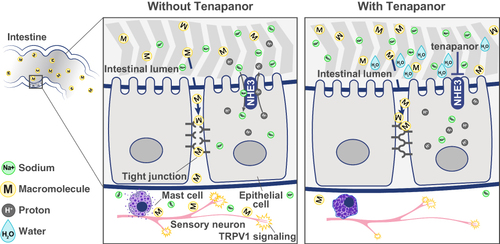
In vivo studies in rodents and translational experiments with human small intestinal stem cell-derived enteroid monolayers showed that blocking NHE3 with tenapanor reduces sodium absorption and increases sodium excretion in the stool.Citation44 The reduction in sodium absorption led to intracellular proton retention,Citation44 which decreased the intracellular pH and increased transepithelial resistance (TEER).Citation44 This increase was thought to result from a conformational change in tight junction proteins that reduces intestinal permeability ().Citation44 Supporting this proposed mechanism of action, a study using human colonic epithelial monolayers demonstrated that inflammatory cytokines reduced TEER and increased macromolecule permeability, and this increase could be attenuated by tenapanor.Citation50 Interestingly, in a rat model of IBS-like colonic hypersensitivity, tenapanor also normalized colonic sensory neuronal excitability and TRPV1 currents and reduced visceral hypersensitivity.Citation50
Clinical trial data in patients with IBS-C demonstrated that oral administration of tenapanor 50 mg twice a day (BID) versus placebo significantly improved key IBS-C symptoms, including increased complete spontaneous bowel movements and decreased abdominal pain.Citation51,Citation52 In fact, tenapanor was associated with improvement in abdominal pain and other abdominal symptoms, independent of changes in bowel movements in a subset of patients.Citation53 Tenapanor 50 mg BID had an acceptable safety profileCitation51,Citation52,Citation54 and was generally well tolerated when administered for up to 55 consecutive weeks.Citation54 The most common treatment-emergent adverse event (TEAE) in all tenapanor trials was mild to moderate transient diarrhea (11%), with no notable increase or change in TEAEs with long-term use.Citation54 The low incidence of serious TEAEs (1%)Citation51,Citation52,Citation54 and tenapanor-related TEAEs (diarrhea) leading to tenapanor discontinuation (3.5%)Citation54 further supports its long-term safety and tolerability in patients with IBS-C. Current American Gastroenterological Association guidelines recommend tenapanor for adult patients with IBS-C.Citation40
Conclusions
There is growing evidence that suggests that increased intestinal permeability and associated inflammatory processes contribute to abdominal pain in patients with IBS-C. Tenapanor, a minimally absorbed NHE3 inhibitor, is an FDA-approved treatment for adults with IBS-C that effectively relieves constipation and abdominal pain. The beneficial clinical effect of tenapanor on abdominal pain may be mediated partly by reducing intestinal permeability, related inflammation, and visceral hypersensitivity. Based on results from non-clinical studies, following inhibition of NHE3, the intracellular proton retention decreased the intracellular pH and increased TEER, which reduced intestinal permeability to macromolecules. To guide treatment selection, it may be useful for gastroenterologists to consider intestinal permeability testing (eg, lactulose/mannitol) with patients with IBS-C upon diagnosis to determine whether increased intestinal permeability could be contributing to the severity of their symptoms (). Treatment targeting intestinal permeability could bring significant relief to patients with IBS-C.
Abbreviations
BID, twice a day; IBS, irritable bowel syndrome; IBS-C, IBS with constipation; IBS-D, IBS with predominant diarrhea; FDA, US Food and Drug Administration; GI, gastrointestinal; NHE3, sodium-hydrogen exchanger isoform 3; PI-IBS, postinfectious IBS; QoL, quality of life; TRPV1, transient receptor potential vanilloid type-1; TEAE, treatment-emergent adverse event; TEER, transepithelial resistance.
Author Contributions
All authors made a significant contribution to the work reported; participated in the conception, study design, execution, and acquisition of data, analysis, and interpretation; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work. All authors read and approved the final version of the manuscript.
Disclosure
David C. Kunkel has received support from Evoke Pharma, Pfizer, ReStalsis, Phathom Pharmaceuticals, Ardelyx, Mahana Therapeutics, Takeda, GI Supply and Laborie, Regeneron, and Vanda Pharmaceuticals. Brian E. Lacy is a consultant for Allakos, Allergan, Ironwood, Salix, and Viver; received grants from Bausch for a gastroparesis research study; and participates in a scientific advisory board yearly meeting for Sanofi, Takeda and Ardelyx. Susan Edelstein, David Rosenbaum, and Laura Williams are employees of Ardelyx, Inc. Kenji Kozuka is an employee of Ardelyx and owns stock. The authors report no other conflicts of interest in this work.
Acknowledgments
Medical writing and editorial support for the development of this manuscript, under the direction of the authors, were provided by Ashfield MedComms (US), an Inizio company, and funded by Ardelyx, Inc.
Additional information
Funding
References
- Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterology. 2016;150(6):1393–1407.e1395.
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–209.
- Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160(1):99–114 e113.
- Nellesen D, Yee K, Chawla A, Lewis BE, Carson RT. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm. 2013;19(9):755–764.
- Drossman DA, Morris CB, Schneck S, et al. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol. 2009;43(6):541–550.
- Ballou S, McMahon C, Lee HN, et al. Effects of Irritable Bowel Syndrome on Daily Activities Vary Among Subtypes Based on Results From the IBS in America Survey. Clin Gastroenterol Hepatol. 2019;17(12):2471–2478 e2473.
- Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(10):908–917.
- Shah ED, Almario CV, Spiegel BM, Chey WD. Presentation and Characteristics of Abdominal Pain Vary by Irritable Bowel Syndrome Subtype: results of a Nationwide Population-Based Study. Am J Gastroenterol. 2020;115(2):294–301.
- Lacy BE, Parikh M, Taylor DCA. Prevalence and impact of abdominal symptoms in patients with IBS-C. Am J Gastroenterol. 2021;116:S229–S230.
- Taylor DCA, Abel JL, Martin C, et al. Comprehensive assessment of patients with irritable bowel syndrome with constipation and chronic idiopathic constipation using deterministically linked administrative claims and patient-reported data: the Chronic Constipation and IBS-C Treatment and Outcomes Real-World Research Platform (CONTOR). J Med Econ. 2020;23(10):1072–1083.
- Palsson OS, Whitehead W, Tornblom H, Sperber AD, Simren M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158(5):1262–1273 e1263.
- Drossman DA, Morris CB, Hu Y, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005;128(3):580–589.
- Garrigues V, Mearin F, Badia X, et al. Change over time of bowel habit in irritable bowel syndrome: a prospective, observational, 1-year follow-up study (RITMO study). Aliment Pharmacol Ther. 2007;25(3):323–332.
- Palsson OS, Baggish JS, Turner MJ, Whitehead WE. IBS patients show frequent fluctuations between loose/watery and hard/lumpy stools: implications for treatment. Am J Gastroenterol. 2012;107(2):286–295.
- Rangan V, Ballou S, Shin A, Camilleri M, Lembo A. Use of Treatments for Irritable Bowel Syndrome and Patient Satisfaction Based on the IBS in America Survey. Gastroenterology. 2020;158(3):786–788 e781.
- Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: a Systematic Review and Meta-analysis. Gastroenterology. 2017;152(5):1042–1054 e1041.
- Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189.
- Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20(11–12):1317–1322.
- Peritore-Galve FC, Kaji I, Smith A, et al. Increased intestinal permeability and downregulation of absorptive ion transporters Nhe3, Dra, and Sglt1 contribute to diarrhea during Clostridioides difficile infection. Gut Microbes. 2023;15(1):2225841.
- Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1(2):133–146.
- Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58(2):196–201.
- Shulman RJ, Jarrett ME, Cain KC, Broussard EK, Heitkemper MM. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J Gastroenterol. 2014;49(11):1467–1476.
- Vazquez-Roque MI, Camilleri M, Smyrk T, et al. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am J Physiol Gastrointest Liver Physiol. 2012;303(11):G1262–G1269.
- Krystel-Whittemore M, Dileepan KN, Wood JG. Mast Cell: a Multi-Functional Master Cell. Front Immunol. 2015;6:620.
- Hanning N, Edwinson AL, Ceuleers H, et al. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol. 2021;14:1756284821993586.
- Sequeira IR, Lentle RG, Kruger MC, Hurst RD. Standardising the lactulose mannitol test of gut permeability to minimise error and promote comparability. PLoS One. 2014;9(6):e99256.
- Zhou Q, Verne ML, Fields JZ, et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut. 2019;68(6):996–1002.
- Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303(7):G775–G785.
- Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146(1–2):41–46.
- Vivinus-Nebot M, Dainese R, Anty R, et al. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am J Gastroenterol. 2012;107(1):75–81.
- Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702.
- Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37.
- Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57(7):923–929.
- Salzmann JL, Peltier-Koch F, Bloch F, Petite JP, Camilleri JP. Morphometric study of colonic biopsies: a new method of estimating inflammatory diseases. Lab Invest. 1989;60(6):847–851.
- Yoshimoto T, Oshima T, Huang X, Tomita T, Fukui H, Miwa H. Microinflammation in the intestinal mucosa and symptoms of irritable bowel syndrome. J Gastroenterol. 2022;57(2):62–69.
- El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16(6):1171–1185.
- Lacy BE, Pimentel M, Brenner DM, et al. ACG Clinical Guideline: management of Irritable Bowel Syndrome. Am J Gastroenterol. 2021;116(1):17–44.
- Shah SL, Janisch NH, Crowell M, Lacy BE. Patients With Irritable Bowel Syndrome Are Willing to Take Substantial Medication Risks for Symptom Relief. Clin Gastroenterol Hepatol. 2021;19(1):80–86.
- Lacy BE, Everhart KK, Weiser KT, et al. IBS patients’ willingness to take risks with medications. Am J Gastroenterol. 2012;107(6):804–809.
- Chang L, Sultan S, Lembo A, Verne GN, Smalley W, Heidelbaugh JJ. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology. 2022;163(1):118–136.
- ZELNORM® (tegaserod) Notice of Withdrawal from Market (2022) [press release]. Available from: https://www.myzelnorm.com/assets/pdfs/Press%20Release%20on%20Notice%20of%20Withdrawal.pdf. Accessed November, 2023.
- AGA Institute. Clinical Decision Support Tool: IBS Treatment. Gastroenterology. 2022;163(1):152.
- Cuppoletti J, Blikslager AT, Chakrabarti J, Nighot PK, Malinowska DH. Contrasting effects of linaclotide and lubiprostone on restitution of epithelial cell barrier properties and cellular homeostasis after exposure to cell stressors. BMC Pharmacol. 2012;12:3.
- King AJ, Siegel M, He Y, et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med. 2018;10(456):eaam6474.
- Ardelyx, Inc. IBSRELA® (tenapanor). Ardelyx, Inc; 2022.
- Ardelyx, Inc. XPHOZAH® (tenapanor). Ardelyx, Inc; 2023.
- Xue J, Thomas L, Tahmasbi M, et al. An inducible intestinal epithelial cell-specific NHE3 knockout mouse model mimicking congenital sodium diarrhea. Clin Sci (Lond). 2020;134(8):941–953.
- Johansson S, Rosenbaum DP, Knutsson M, Leonsson-Zachrisson M. A Phase 1 study of the safety, tolerability, pharmacodynamics, and pharmacokinetics of tenapanor in healthy Japanese volunteers. Clin Exp Nephrol. 2017;21(3):407–416.
- Rosenbaum DP, Yan A, Jacobs JW. Pharmacodynamics, Safety, and Tolerability of the NHE3 Inhibitor Tenapanor: two Trials in Healthy Volunteers. Clin Drug Investig. 2018;38(4):341–351.
- King AJ, Chang L, Li Q, et al. NHE3 inhibitor tenapanor maintains intestinal barrier function, decreases visceral hypersensitivity, and attenuates TRPV1 signaling in colonic sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2024;326(5):G543–G554.
- Chey WD, Lembo AJ, Rosenbaum DP. Efficacy of Tenapanor in Treating Patients With Irritable Bowel Syndrome With Constipation: a 12-Week, Placebo-Controlled Phase 3 Trial (T3MPO-1). Am J Gastroenterol. 2020;115(2):281–293.
- Chey WD, Lembo AJ, Yang Y, Rosenbaum DP. Efficacy of Tenapanor in Treating Patients With Irritable Bowel Syndrome With Constipation: a 26-Week, Placebo-Controlled Phase 3 Trial (T3MPO-2). Am J Gastroenterol. 2021;116(6):1294–1303.
- Lembo A, Brenner DM, Lacy BE, Zhao S, Rosenbaum DP. Analysis of patient-reported treatment satisfaction and abdominal score in patients with irritable bowel syndrome with constipation (IBS-C) treated with tenapanor. Gastroenterology. 2023;164(6):S1059.
- Lembo AJ, Friedenberg KA, Fogel RP, et al. Long-term safety of tenapanor in patients with irritable bowel syndrome with constipation in the T3MPO-3 study. Neurogastroenterol Motil. 2023;35(11):e14658.
