Abstract
This case report provides data on unique challenges related to amoebiasis diagnostics and treatment in non-endemic regions. The presented case report is focused a 28-year-old male patient of Indian origin, temporarily living in Poland, who was diagnosed with an amoebic liver abscess. The patient presented with a range of non-specific symptoms including shortness of breath, chest pain, and fever. The differential diagnosis included cardio-pulmonary diseases, a range of tropical diseases such as malaria or typhoid fever, bacterial abscesses, and malignancies, necessitating a comprehensive, multi-modal diagnostic approach. This approach included an extensive review of patient history, physical examination, and various laboratory and imaging investigations. A further challenge in this case was the unavailability of standard cysticidal treatments in Poland, which required individualized therapeutic strategy. Despite these obstacles, the patient was successfully treated using an alternative regimen of intravenous metronidazole, ceftriaxone, doxycycline, chloroquine, and finally, trimethoprim/sulfamethoxazole (treatment with metronidazole was used as a base drug, due to the lack of typical cysticidal treatment, an alternative treatment was added: chloroquine is a recommended drug used in the treatment of pregnant patients, in addition, doxycycline showed in vitro activity against Entamoeba histolytica). This therapeutic journey underscored the value of adaptability in treatment protocols, particularly in regions where certain resources may not be readily available. This case report underlines the importance of broadening the differential diagnosis in non-endemic regions to include tropical diseases, particularly in the context of increasing global travel and migration. It also highlights the significance of employing comprehensive diagnostic strategies and adaptable treatment protocols in such scenarios. In addition, the report reiterates the need for global collaboration and education among healthcare providers to effectively manage tropical diseases, especially in non-endemic regions. Through its exploration of the complexities associated with diagnosing and managing amebiasis in a non-endemic region, this report offers valuable insights to clinicians worldwide.
Introduction
Tropical diseases, whilst less frequent in Europe due to temperate climates, pose a considerable challenge for medical professionals in these regions because of the relative unfamiliarity and potential diagnostic difficulties.Citation1 The global phenomenon of human migration, international travel, and the effects of climate change on the redistribution of vectors and pathogens necessitates constant vigilance and readiness to tackle such diseases.Citation2
Among the variety of tropical diseases, amoebiasis, caused by the protozoan Entamoeba histolytica, significantly contributes to morbidity and mortality in endemic regions, including India where the patient in focus originates.Citation3
Amoebiasis is a major health concern in many tropical and subtropical regions. In India, the prevalence of E. histolytica infection is high, with estimates suggesting millions of cases annually. This high burden underscores the potential for imported cases in non-endemic regions, highlighting the need for vigilance and preparedness among healthcare providers globally. According to the World Health Organization, amoebiasis ranks third among parasitic diseases in terms of deaths, after malaria and schistosomiasis.
In non-endemic regions like Poland, amoebiasis is generally uncommon, except for the colonization with non-pathogenic amoeba species more common among travelers or immigrants from endemic regions.Citation4,Citation5 Nonetheless, the potential under-recognition and under-diagnosis due to lack of local expertise, coupled with nonspecific symptomology, can lead to delayed management and potentially life-threatening complications.Citation6 Existing guidelines for managing tropical diseases in non-endemic settings recommend a high index of suspicion and thorough differential diagnosis, especially in patients with relevant travel or residential histories. The Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) provide detailed protocols for diagnosing and treating amoebiasis, emphasizing the importance of early identification and appropriate therapeutic strategies.
Amoebic liver abscess (ALA), the most frequent extraintestinal manifestation of E. histolytica infection, is often diagnosed based on clinical, ultrasonographic, or radiographic findings.Citation7 However, the overlap in presentation with other tropical diseases such as malaria, tuberculosis, or schistosomiasis, as well as non-tropical conditions including bacterial liver abscess and malignancies, imposes significant diagnostic challenges.Citation8 Moreover, non-invasive diagnostic tests like serology and stool antigen tests, while demonstrating high sensitivity and specificity in endemic regions, may present diminished specificity in non-endemic regions due to higher rates of seropositivity in healthy populations.Citation6 Additionally, for proper diagnostics, a suitably experienced laboratory is required, which may not be trained in the evaluation of preparations outside endemic areas. Such complexities may enhance the difficulty of diagnosing ALA in non-endemic settings like Poland.
Poland’s robust health care infrastructure encompasses a range of specialties, including infectious diseases. Nevertheless, inherent diagnostic delays and hurdles in managing tropical diseases may exist due to the rarity of these conditions.Citation9 Presented case highlights the variety of diagnostic complexities associated with tropical diseases in a non-endemic region. The patient’s ambiguous symptomatology, the unavailability of standard treatments, and the necessity for differential diagnosis across a broad array of infectious diseases including influenza, RSV, COVID-19, syphilis, malaria, tuberculosis, schistosomiasis, HIV infection, HCV, and HBV, encapsulate the challenges of the healthcare professionals. It is, therefore, imperative to update knowledge on the management of such rare cases to augment our understanding and preparedness for managing tropical diseases in non-endemic settings. This ongoing exploration will enrich the existing body of medical knowledge and provide invaluable insights for future clinical practice.Citation10
A Case Report
A 28-year-old patient of Indian origin was admitted to the Infectious Diseases Department in Poland, Szczecin, with the presumptive diagnosis of an amoebic liver abscess in February 2023. Initially, patient was brought to the Emergency Department due to worsening symptoms such as shortness of breath and chest pain on the right side. From the additional symptoms - fever, cough – for which he was taking paracetamol. In the physical examination, a decrease in the heaving and the alveolar murmur over the lower and middle fields of the right lung. In the ultrasound of the right pleural cavity, 1200 mL of fluid was found. The abdominal ultrasonography showed a pathological mass in the liver with a diameter of about 12 cm. The patient has been staying in Poland since September 2022. He was working physically. In India, he has lived in the city, but drinking water comes from local wells - possible contamination, reported bathing in stagnant pools of water. When asked about diarrhea in an interview, said that he had diarrhea before leaving India, but declined any blood in the stool, or fever. Patient’s symptoms begun approximately a month prior to admission– and included pain in the right subcostal region and on the right side of the chest.
On admission to the Department, the patient was in generally stable, with severe pain in the projection of an enlarged liver, fully conscious. On examination key abnormalities included Alveolar murmur over the lung, muffled over the lower lobe of the right lung, single crackles on both sides. Abdomen soft, slightly bloated, slightly tense, tender in the projection of the liver, no peripheral edema. Tender liver, enlarged about 3–4 cm below the costal arch.
Key laboratory abnormalities on diagnoses included, neutrophil count increase of 19.2 thousand/ul [2.0–6.9, microcytic anemia: Hb (hemoglobin) 12.1 g/dl [14.0–17.0], features of liver insufficiency - high INR (international normalized ratio): 2.38 [0.8–1.2], hypoalbuminemia 3.0 g/dl [3.2–4.8], low cholinesterase 1987 U/L [7000–19,000], high activity of cholestatic enzymes; ALP (alcaic phosphatase): 336 U/L [46–116]; GGTP (Gamma Glutamyl Transferase/Transpeptidase): 114 U/L [0–73]. Additionally, inflammatory parameters indicative of sepsis were also elevated (CRP (C-reactive protein): 323.57 mg/L [< 10.0], procalcitonin: 18.00 ng/mL [< 0.5 ng/mL], high lactate level 7.94 mmol/L [0.50–2.20], d-dimers 3343.0 FEU ug/L [< 500.0]).
The treatment implemented included intravenous metronidazole, ceftriaxone, and symptomatic and hepatoprotective treatment. The implemented antibiotic therapy was chosen due to the suspicion of “classic” etiology of liver abscesses. Additional tests excluded active influenza, RSV, COVID-19, were excluded using molecular assays. Syphilis, venereal research disease laboratory (VDRL) test proved negative. For exclusion of malaria, enzyme-linked immunosorbent assay, thick drop and thin smear were performed. For tuberculosis IGRA test was performed and acid-fast bacilli test was performed from sputum. For schistosomiasis, HIV infection, HCV, HBV, serology examination was performed. Blood and urine cultures were found without bacterial growth. Entamoeba histolytica antigen test in feces was negative.
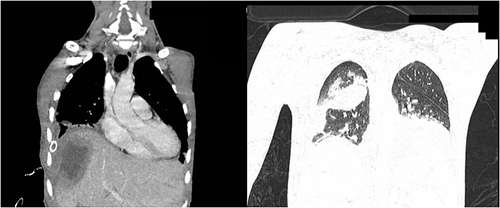
In the first days of treatment, increasing shortness of breath and pain on the right side of the chest were observed. A chest CT (computed tomography) scan performed 3 days after admission revealed a thick-walled fluid lesion measuring 11.5×6.3 cm in the right lobe of the liver, the walls of which undergo progressive enhancement in the subsequent phases of the examination (). In the right pleura, a large amount of fluid, which fills the entire volume of the right half of the chest, caused compression and atelectasis of the right lung and displacement of the mediastinal organs to the left ().
Figure 1 Computed tomography of the chest, abdomen and pelvis: right pleura, was filled with large amount of fluid, causing compression and atelectasis of the right lung and displacement of the mediastinal organs to the left.
Due to direct life threat the patient underwent pleural drainage immediately following the transfusion of Fresh Frozen Plasma to supplement for due to coagulation factors). During this procedure, 3000 mL of brown purulent content () was obtained, with decreasing quantities in the following days. The content obtained from the drainage was tested for the Entamoeba histolytica antigen - positive result. In addition, the diagnostics was performed with the serology of amoebiasis (Immunoglobulin G antibody positive with the 43.7 NTU [<9] activity). Intravenous doxycycline and then chloroquine were also introduced for empiric treatment. There was no availability of cysticidal preparations in Poland.
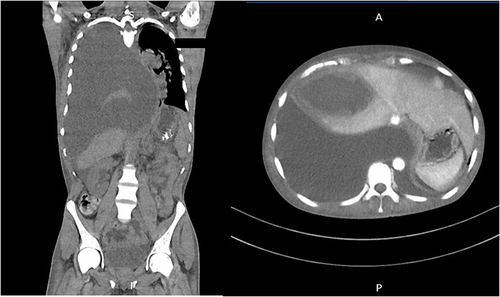
A follow-up CT scan 7 days after the procedure showed that the abscess in the right lobe of the liver - in segments 5 and 8 - was reduced to 6.7×5 cm. Described abscess formed a 1.2 cm wide projection directed towards the anterior chest wall. In the anterior part of the right dome of the diaphragm, an opening was visible through which the liver abscess with communication with the pleural cavity. Reduction of fluid amount in the right pleura were seen - the fluid layer surrounding the dorsal lung segments was 2 cm. In the location of the upper part of the right oblique interlobar fissure, an encapsulated fluid of 4.6×3 cm is visible. Right lung partially expanded ().
Figure 3 Computed tomography of the chest with a reduction in the amount of purulent content and partial visualization of the right lung.
After the examination, the liver abscess was punctured again under ultrasound guidance - 140 mL of purulent content was obtained.
In the following days, the treatment was gradually reduced, and the amount of fluid in the right pleural cavity decreased in control radiological examinations of the chest. Antibiotic therapy was changed to trimethoprim/sulfamethoxazole, with good results and tolerability. At the time of discharge, laboratory tests show normal inflammatory parameters, better red blood cells values (no anemia or thrombocytopenia), and improving liver function. The patient denied pain, shortness of breath, cough and declared willingness to return to India upon discharge.
Discussion
This intricate case encapsulates a wider issue experienced by non-endemic regions when confronted with tropical diseases.Citation4 The patient’s initial condition – an Indian immigrant who had resided in Poland for several months, presented with symptoms including fever, shortness of breath, cough, and discomfort in the right subcostal region which posed a complex diagnostic puzzle. In these cases, the clinical picture is often fuzzy due to the overlap of symptoms with other prevalent infectious and non-infectious diseases.Citation11 Given the broad range of possible etiologies, the differential diagnosis process required rigorous exploration.
The diagnostic challenge in this case was amplified by the nonspecific nature of the symptoms, common to amoebic liver abscess – the most frequent extraintestinal manifestation of amoebiasis.Citation11 However, these symptoms also may suggest a multitude of other conditions, including bacterial abscess, malignancies, and other tropical diseases such as malaria and tuberculosis.Citation7 The conundrum was further intensified by the patient’s symptoms having gradually developed over a month, without an accompanying history of typical amoebiasis indicators like bloody diarrhea.Citation7
This case thus accentuates the inherent difficulty in diagnosing tropical diseases in non-endemic regions.Citation12 Given that amoebiasis is rare in Poland, physicians may not immediately consider it as a potential diagnosis, leading to possible delays in intervention and increased morbidity. Healthcare providers need to maintain a high index of suspicion for tropical diseases, especially in patients with relevant travel or residential histories.Citation12
Furthermore, the diagnostic methods commonly employed in endemic regions, such as serological tests and stool antigen assays for amoebiasis, showed limitations in this context.Citation6,Citation9,Citation11 Here, the stool antigen test yielded a negative result, while the serological testing showed a positive outcome, further complicating the diagnostic process. Such contradictions emphasize the necessity for comprehensive diagnostic strategies that incorporate not only laboratory results but also clinical and epidemiological aspects, particularly in non-endemic regions.Citation11
The therapeutic challenges this case brought to light are also noteworthy. Due to the rarity of amoebiasis in Poland, the country did not have ready access to standard cysticidal preparations such as paromomycin, iodoquinol, or diloxanide furoate. This shortage required the use of alternative treatments, such as metronidazole, doxycycline, and chloroquine, followed by trimethoprim/sulfamethoxazole.Citation13 Although not the typical treatment protocol for amoebiasis, it seemed to yield positive results, as seen in the patient’s progressive recovery. However, this case also faced several other limitations and challenges. There were potential delays in diagnosis due to the rarity of amoebiasis in Poland, leading to a possible under-recognition and under-diagnosis of the disease. Such delays could have significant impacts on patient outcomes, emphasizing the need for healthcare providers in non-endemic regions to maintain a high index of suspicion for tropical diseases, particularly in patients with relevant travel or residential histories. Another significant challenge was the access to specialized laboratory facilities. Non-invasive diagnostic tests like serology and stool antigen tests, while demonstrating high sensitivity and specificity in endemic regions, may present diminished specificity in non-endemic regions due to higher rates of seropositivity in healthy populations.
The successful implementation of this alternative treatment strategy underlines the significance of flexibility and adaptability in managing tropical diseases in non-endemic areas. It serves as an affirmation of the fact that physicians’ deep understanding of varied therapeutic modalities can facilitate successful patient outcomes, even amidst resource constraints.
In essence, this case demonstrates the pertinence of a multifaceted approach to managing tropical diseases in non-endemic regions, which involves thorough diagnosis, unconventional but effective treatment strategies, and constant monitoring of the patient’s progress. It also underscores the necessity of increasing awareness and education among healthcare professionals about such diseases.
Conclusion
In conclusion, this case illustrates the diagnostic and therapeutic complexities involved in managing tropical diseases, like amoebiasis, in non-endemic regions. It calls for healthcare professionals to maintain a high level of clinical vigilance for tropical diseases, particularly in patients with pertinent travel or residential histories. The application of unconventional yet effective treatment strategies underscores the necessity for flexibility and adaptability in managing such conditions. Ultimately, this case contributes to a wider understanding of tropical diseases in non-endemic areas, offering invaluable insights for future similar presentations. The significance of global collaboration, education, and knowledge-sharing among healthcare professionals is paramount to effectively manage tropical diseases, particularly in non-endemic regions where expertise may be limited.
Ethics Approval and Consent to Participate
Ethics approval was not required. Institutional approval to publish the case details was obtained.
Consent for Publication
Patient’s written, informed consent for publication included case details as well as publication of images.
Disclosure
Dr Miłosz Parczewski reports personal fees from ViiV/GSK, Gilead, Janssen, and MSD, outside the submitted work. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Hotez PJ, Bottazzi ME, Franco-Paredes C, et al. The neglected tropical diseases of latin america and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2:e300. doi:10.1371/JOURNAL.PNTD.0000300
- Tatem AJ, Rogers DJ, Hay SI. Global transport networks and infectious disease spread. Adv Parasitol. 2006;62:293–343.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi:10.1016/S0140-6736(12)61728-0
- Hotez PJ, Gurwith M. Europe’s neglected infections of poverty. Int J Infect Dis. 2011;15:e611–e619. doi:10.1016/J.IJID.2011.05.006
- Nowak P, Mastalska K, Loster J, et al. Entamoeba histolytica - pathogenic protozoan of the large intestine in humans. J Clin Microbiol Bioch Technol. 2015;1:7. doi:10.17352/jcmbt.000003
- Fotedar R, Stark D, Beebe N, et al. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev. 2007;20:511–532. doi:10.1128/CMR.00004-07
- Stanley SL. Amoebiasis. Lancet. 2003;361:1025–1034. doi:10.1016/S0140-6736(03)12830-9
- Hughes MA, Petri WA. Amebic liver abscess. Infect Dis Clin North Am. 2000;14:565–582. doi:10.1016/S0891-5520(05)70121-5
- Solaymani-Mohammadi S, Rezaian M, Babaei Z, et al. Comparison of a stool antigen detection kit and PCR for diagnosis of Entamoeba histolytica and Entamoeba dispar infections in asymptomatic cyst passers in Iran. J Clin Microbiol. 2006;44:2258–2261. doi:10.1128/JCM.00530-06
- Ali IKM, Solaymani-Mohammadi S, Akhter J, et al. Tissue invasion by Entamoeba histolytica: evidence of genetic selection and/or DNA reorganization events in organ tropism. PLoS Negl Trop Dis. 2008;2:219. doi:10.1371/journal.pntd.0000219
- Hooshyar H, Rostamkhani P. Accurate laboratory diagnosis of human intestinal and extra-intestinal amoebiasis. Gastroenterol Hepatol Bed Bench. 2022;15:343. doi:10.22037/ghfbb.v15i4.2496
- Desmet S, Henckaerts L, Ombelet S, et al. Imported diseases in travellers presenting to the emergency department after a stay in a malaria-endemic country: a retrospective observational study. Trop Dis Travel Med Vaccines. 2023;9:1–9. doi:10.1186/s40794-023-00190-0
- Intestinal Entamoeba histolytica amebiasis. Available from: https://www.uptodate.com/contents/intestinal-entamoeba-histolytica-amebiasis. Accessed July 30, 2023.

