Abstract
Up to 93% of patients with hereditary angioedema (HAE) experience recurrent abdominal pain. Many of these patients, who often present to emergency departments, primary care physicians, general surgeons, or gastroenterologists, are misdiagnosed for years and undergo unnecessary testing and surgical procedures. Making the diagnosis of HAE can be challenging because symptoms and attack locations are often inconsistent from one episode to the next. Abdominal attacks are common and can occur without other attack locations. An early, accurate diagnosis is central to managing HAE. Unexplained abdominal pain, particularly when accompanied by swelling of the face and extremities, suggests the diagnosis of HAE. A family history and radiologic imaging demonstrating edematous bowel also support an HAE diagnosis. Once HAE is suspected, C4 and C1 esterase inhibitor (C1-INH) laboratory studies are usually diagnostic. Patients with HAE may benefit from recently approved specific treatments, including plasma-derived C1-INH or recombinant C1-INH, a bradykinin B2-receptor antagonist, or a kallikrein inhibitor as first-line therapy and solvent/detergent-treated or fresh frozen plasma as second-line therapy for acute episodes. Short-term or long-term prophylaxis with nanofiltered C1-INH or attenuated androgens will prevent or reduce the frequency and severity of episodes. Gastroenterologists can play a critical role in identifying and treating patients with HAE, and should have a high index of suspicion when encountering patients with recurrent, unexplained bouts of abdominal pain. Given the high rate of abdominal attacks in HAE, it is important for gastroenterologists to appropriately diagnose and promptly recognize and treat HAE, or refer patients with HAE to an allergist.
Introduction
Hereditary angioedema (HAE) is a potentially life-threatening disease that may go unrecognized or be misdiagnosed for an average of 8 years before the correct diagnosis is established.Citation1 Abdominal symptoms are extremely common, occurring in the majority (93%) of patients with HAE,Citation2 and may be the only manifestation of the disease. Patients with HAE may initially present with recurrent episodes of abdominal pain and distension, nausea, vomiting, and diarrhea.Citation3,Citation4
Because abdominal symptoms may precede by several years the episodes of subcutaneous tissue swelling that are characteristic of HAE, patients may undergo inappropriate surgical and medical treatment for any of a wide range of presumptive, incorrect diagnoses, including acute abdomen, biliary colic, hepatitis, regional enteritis, pancreatitis, cholecystitis, choledocholithiasis, nephrolithiasis, pyelonephritis, ruptured ovarian cyst, intestinal obstruction, duodenal ulcer, and ulcerative colitis.Citation3,Citation4 Patients who develop abdominal symptoms related to HAE are usually seen by gastroenterologists, emergency department physicians, primary care physicians, and general surgeons.Citation5 Given that the majority of patients with HAE experience abdominal attacks, it is important for gastroenterologists to appropriately diagnose and promptly recognize and treat HAE or refer patients with HAE to an allergist.Citation2 This review highlights HAE, its clinical presentation, and the role of the gastroenterologist in its diagnosis and management.
Disease burden
Patients with HAE who are seen in the emergency department often require hospitalization, considerably increasing the cost of care for each attack.Citation6 Review of a national database has shown that between 2006 and 2007 there were 5,040 emergency department visits by patients with HAE at a mean cost of $1,479 per visit, and with 41% of these visits requiring hospitalization.Citation6 Similarly, in a 4-year analysis of the epidemiology of HAE, there were 10,125 hospitalizations, with a mean length of stay of 5 days and mean charges of $22,728.Citation7 HAE is also associated with a high rate of morbidity, with many patients experiencing depression and poor health-related quality of life. HAE also negatively affects educational and career opportunities and reduces work productivity, compounding the substantial economic burden of HAE.Citation8
Types and pathophysiology
Estimates of the incidence of HAE worldwide vary from one in 10,000 to one in 150,000 persons.Citation9,Citation10 In a retrospective review of patients with HAE, the median age at disease onset was 11.2 years, 93.3% of patients had recurrent abdominal pain, and women experienced a higher number of episodes per year than men.Citation2 HAE is caused by mutations in the C1 esterase inhibitor (C1-INH) gene, also known as the SERPING1 gene, which has been mapped to chromosome 11.Citation11 A known family history is present in 75% of cases, with an autosomal dominant inheritance pattern; in the remaining 25% of cases, the disease results from spontaneous mutations.Citation12–Citation14 Two types of HAE due to C1-INH deficiency have been characterized. Type I HAE accounts for 85% of cases and is due to mutations that result in decreased antigenic levels of functionally normal C1-INH. Type II HAE accounts for 15% of cases and is due to mutations that lead to levels of C1-INH that are normal but that have dysfunctional C1-INH proteins.Citation15 So far, more than 200 different mutations that cause HAE have been identified.Citation16,Citation17
A third type of HAE (HAE with normal C1-INH) has been identified, in which the level and activity of C1-INH are normal and there is no characteristic laboratory profile. The clinical presentation of this type of HAE is indistinguishable from types I and II; however, this third type tends to develop later in life. In some patients, this subtype is associated with a mutation in the coagulation factor XII gene, with subsequent increased levels of bradykinin. Estrogen exacerbates the severity of disease in patients with HAE who have normal C1-INH, and edema appears to be estrogen-dependent in a subset of patients.Citation18,Citation19
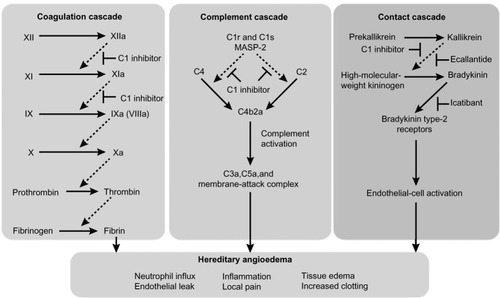
The underlying mechanism for HAE types I and II is functional impairment of C1-INH, a protease inhibitor that regulates complement activation (C1r, C1s, and mannose-binding lectin-associated serine protease [MASP]-1 and MASP-2), contact system activation (factor XII and kallikrein), and inactivation of several fibrinolytic (tissue plasminogen activator and plasmin) and coagulation (factor XI and thrombin) proteases.Citation16,Citation20,Citation21 Of the four systems regulated by C1-INH, regulation of plasma kallikrein, the enzyme that releases bradykinin from kininogen, is responsible for the development of angioedema. A deficiency in C1-INH leads to unregulated plasma kallikrein activity, with subsequent overproduction of bradykinin that enhances vasodilation and vascular permeability, causing extravasation of plasma into interstitial tissue, leading to angioedema.Citation18,Citation22 The kallikrein-kinin system has a central role in several other systems, including the clotting cascade, vasodilation, vascular permeability, and activation of the renin-angiotensin-aldosterone system.Citation21,Citation23,Citation24 shows the dysregulation of the coagulation, complement, and contact cascades in HAE.Citation25
Figure 1 Dysregulation of coagulation, complement, and contact cascades in hereditary angioedema.
Abbreviations: HAE, hereditary angioedema; MASP-2, mannose-binding lectin-associated serine protease 2.
Clinical presentation of type I and type II HAE
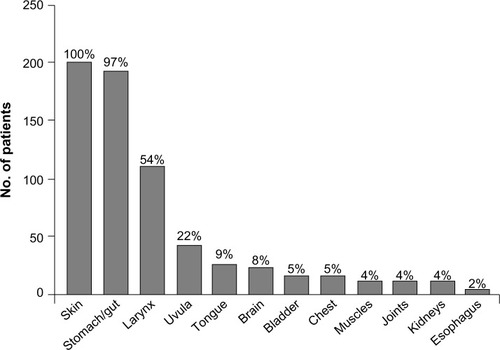
Precipitating factors for HAE episodes may include medications such as oral contraceptives or angiotensin-converting enzyme inhibitors, infection, trauma, hormonal changes, and surgical, endoscopic, or dental procedures.Citation18,Citation26,Citation27 Depending on the underlying cause of angioedema, an untreated attack will typically resolve within 1–5 days; however, episodes involving the abdomen or upper airways may require acute treatment to alleviate pain, nausea, and vomiting, in the case of abdominal angioedema, and to prevent death, in the case of respiratory symptoms.Citation5 The site of an HAE attack varies between patients and may also vary within the same patient.Citation28 The most common symptoms of HAE are recurrent abdominal symptoms, occurring in about 93% of patients, and diffuse skin edema, particularly in the extremities, occurring in 97.5% of patients.Citation2 The lips, eyelids, tongue, or genitalia may also be affected.Citation2,Citation28 The frequency and distribution of symptoms experienced by patients during an HAE attack are shown in .Citation2
Figure 2 Sites affected by angioedema in patients with clinical symptoms of hereditary angioedema.
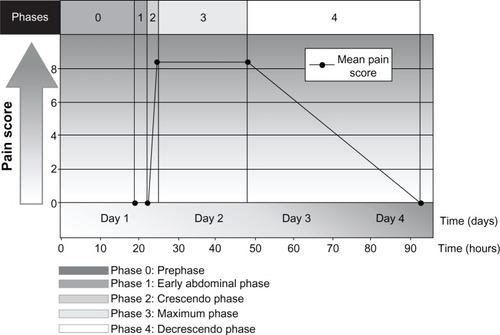
HAE may also present with episodic, recurrent, unexplained abdominal attacks that involve symptoms of tender, crampy pain and nausea or vomiting.Citation5,Citation29,Citation30 The abdominal pain experienced by patients with HAE may be severe and acute in onset, or may be recurrent and chronic and of moderate severity.Citation5,Citation29 The diarrhea is caused by edema of the gut that may result in extravascular fluid loss, leading to hypotension and shock in some patients.Citation29 In a prospective and retrospective analysis of 153 patients with HAE, pain caused by abdominal attacks was characterized by its intensity, quality, and time course, and was described as having four phases, as well as a prephase (phase 0) in which patients had extra-abdominal symptoms but not abdominal pain ().Citation30 Phase 1 was the time from the first abdominal complaint to the onset of crampy pain; phase 2 was the time between the initial crampy pain and its peak; phase 3 was the time of maximum symptoms; and phase 4 was the time from the beginning of symptom relief to complete resolution of symptoms.Citation30 The mean duration of phase 1 was 3.3 hours, during which patients had a wide range of symptoms, which included a feeling of satiety, abdominal distension, and nausea. All patients developed worsening crampy pain during phase 2, with the mean time to maximal pain being 2.4 hours. The median duration of the maximal pain phase was 23.5 hours, with crampy pain occurring in all patients, scored as severe to excruciating in 87% of patients and associated with vomiting and diarrhea in 78% and 65% of patients, respectively. The mean time to complete resolution of symptoms was 45.0 hours. Review of the character of the pain symptoms in these patients showed the presence of two different types of abdominal pain attack, ie, an upper gastrointestinal type and a lower gastrointestinal type. Patients with the upper gastrointestinal type of abdominal pain from HAE had extreme pain, nausea, vomiting, and hypotension in the absence of diarrhea; patients with the lower gastrointestinal type of abdominal attack had crampy pain and diarrhea, but no vomiting or hypotension. Other less frequent but potentially serious symptoms or complications in patients with HAE experiencing an abdominal attack have included circulatory collapse and shock due to hypovolemia, persistent noncrampy abdominal pain, dysuria, bloody diarrhea, intussusceptions, and tetany.Citation30
Figure 3 Phases and time course for typical abdominal pain attacks in patients with hereditary angioedema.
The abdominal symptoms of HAE are often similar to those of an acute abdomen, sometimes prompting exploratory abdominal surgery; as a result, approximately one third of patients with undiagnosed HAE undergo unnecessary surgery at the time of an abdominal attack.Citation31 Even after HAE has been diagnosed, distinguishing between an abdominal attack and a surgical emergency can be difficult.Citation31 In one case, a 46-year-old woman presented with recurrent abdominal pain and edema several years before developing intermittent, localized episodes of limb, trunk, neck, and facial swelling. She had undergone numerous surgical procedures for a variety of diagnoses, including pancreatitis, cholecystitis, common bile duct stone, ruptured ovarian cyst, renal stone, intestinal obstruction, pyelonephritis, duodenal ulcer, and ulcerative colitis before HAE was ultimately diagnosed.Citation3 In another case study, a 34-year-old woman who presented to the emergency department with severe abdominal cramping on several occasions was treated for presumed gastroenteritis; this patient, too, was later found to have HAE.Citation32 In a recent retrospective study, significant leukocytosis with neutrophilia and high hematocrit levels and no elevations in C-reactive protein levels were present in all patients with HAE during an acute gastrointestinal attack,Citation33 suggesting that such a laboratory profile may be a consideration in the differential diagnosis of HAE.
Upper airway edema with asphyxiation is a potentially fatal clinical presentation of HAE.Citation34 Although often referred to as “laryngeal edema,” upper airway edema often involves the mucosa of the mesopharynx and hypopharynx.Citation35 Interestingly, however, the formation of edema spares the mucosa of the nasal cavity and paranasal sinuses. In many cases, the exact anatomic location of swelling remains uncharted, because during attacks patients are only rarely seen by ear, nose, and throat specialists.Citation35 Upper airway edema may occur in approximately half of all patients with HAE at least once in their lives.Citation2 Upper airway edema has been associated with a high mortality rate, which has improved with appropriate diagnosis and treatment.Citation29
Diagnosis
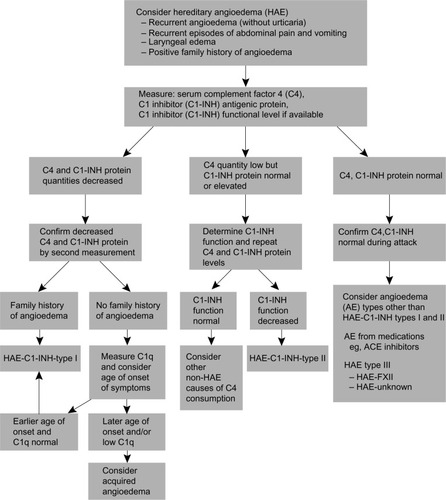
The diagnosis of HAE is often delayed due to low awareness of the disease; however, unacceptable delays in diagnosis have been reported even when a family history of HAE is known.Citation36 Gastroenterologists should consider a diagnosis of angioedema in all patients presenting with episodic, recurrent, unexplained abdominal attacks that involve symptoms of tender, crampy pain, and nausea or vomiting ().Citation26,Citation30,Citation32 Between abdominal attacks, the patient with HAE is completely asymptomatic, unlike patients with functional bowel disorders. Analysis of the history should attempt to identify triggers for the symptoms, such as medications, allergens, trauma, or infection, and to identify either a personal or family history of HAE. Although the presence of a family history is helpful in making the diagnosis, the absence of a family history does not rule out the condition, because 25% of cases are the result of spontaneous mutations.Citation13 During an acute episode, the physical examination may show marked abdominal distension; possible cutaneous swelling; diffuse or localized tenderness, sometimes with rebound; and shifting dullness with possible ascites.Citation5 Urticaria and pruritus are not present and are seen only in allergic forms of angioedema,Citation5 although in some patients, erythema marginatum precedes acute events and may be misdiagnosed as urticaria.Citation37,Citation38
Table 1 Differential diagnosis of intestinal angioedema
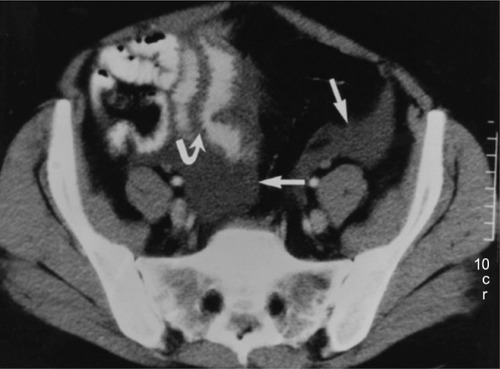
Imaging studies for the diagnosis of HAE involving the abdomen include plain radiographs, ultrasonography, contrast-enhanced computed tomography (CT), and magnetic resonance imaging of the abdomen.Citation39 An abdominal CT scan in a patient with HAE may show thickening of the bowel wall and mucosa and an increase in enhancement when a contrast agent is used ().Citation39 An abdominal CT scan may also show more layers of the small bowel than are normally visible and more prominent mesenteric vessels and ascites. Abdominal ultrasonography is useful for confirming the accumulation of fluid in the peritoneal cavity during an attack and for early detection of adverse effects of therapy,Citation40,Citation41 and may be less expensive and more readily available than CT. A CT scan or ultrasonography is usually sufficient to diagnose angioedema of the abdomen,Citation27,Citation42 and magnetic resonance imaging may be necessary with manifestations of potential cerebral edema.Citation43
Figure 4 Abdominal computed tomography scan of patient with hereditary angioedema showing thickening of the small bowel (stacked-coin appearance) due to angioedema.
Capsule endoscopy can provide rapid visual confirmation of bowel occlusion due to HAECitation44 and may be a useful diagnostic tool. When HAE is suspected or known, proper prophylactic measures must be taken to prevent induction or exacerbation of potentially life-threatening oropharyngeal edema.Citation5
Laboratory evaluation for HAE should include measurement of C4 complement, concentration of C1-INH, and functional measurement of C1-INH (see ). C4 levels are typically less than 30% of mean normal levels, which usually range from 0.15 to 0.65 g/L in untreated HAE.Citation45 Because C4 levels have a high sensitivity and negative predictive value for the disease, they are considered to be a good screening test for HAE.Citation45,Citation46 C1-INH antigen levels are low in type I HAE and are normal or high in type II HAE.Citation15
If either the C1-INH concentration or functions are low in an individual with low C4 levels, the tests should be repeated before making the diagnosis, to minimize false positive results.Citation45 Given the high negative predictive value of C4, the diagnosis of HAE should be questioned in individuals with normal C4 and low C1-INH concentration or function.Citation15,Citation45,Citation46 Normal C4 levels and normal C1-INH concentration and function in patients with a clinical presentation consistent with HAE should prompt an evaluation for HAE with normal C1-INH.Citation47,Citation48 A thorough patient history, along with imaging and laboratory evaluations, is critical to successfully making a differential diagnosis of acute HAE and initiating prompt and appropriate treatment ().Citation49,Citation50
Figure 5 Diagnostic algorithm for hereditary angioedema.
Abbreviations: AE, adverse event; ACE, angiotensin-converting enzyme; C1-INH, C1 esterase inhibitor; C1q, complement component 1, q subcomponent; HAE, hereditary angioedema.
Treatment
Current awareness of treatment
Once a patient is diagnosed with HAE, the gastroenterologist should involve an allergist or immunologist to guide the long-term management of the patient.Citation5 Patients should be well trained for self-administration of medications and should be advised to wear a medical bracelet or carry a wallet card detailing their treatment plan in the event of an acute HAE attack.Citation51,Citation52 Several evidence-based management guidelines and recommendations have been developed for the acute and prophylactic treatment of angioedema due to C1-INH deficiency.Citation47,Citation53–Citation55 Management guidelines from the Hereditary Angioedema International Working Group are shown in .Citation54
Table 2 Hereditary Angioedema International Working Group consensus guidelines for the management of hereditary angioedema
Acute treatment
Assessment and maintenance of the airway should be the first consideration in the setting of acute episodes of HAE because angioedema involving the airway can be potentially life-threatening. Physicians should consider intubation at the first signs of airway compromise because laryngeal edema may worsen during the attack, making endotracheal intubation difficult.Citation56 Gastrointestinal attacks can be painful and debilitating, and in extreme cases (eg, hypovolemia) can be life-threatening. Although disfiguring and painful, cutaneous attacks are not associated with serious complications.
According to several recent evidence-based recommendations, the first-line treatment option for acute episodes of HAE is a C1-INH replacement product.Citation54,Citation55,Citation57 Two plasma-derived nanofiltered C1-INH products and one recombinant C1-INH product are currently available for the treatment of acute episodes of HAECitation58 (). Berinert® P (CSL Behring, Kankakee, IL, USA) is a plasma-derived nanofiltered C1-INH approved in Europe for self-administration to treat all acute attacks and for preprocedure prevention of attacks, and in the US for treatment of acute facial, laryngeal, and abdominal attacks of HAE.Citation54,Citation58,Citation59 Cinryze® (ViroPharma Biologics, Exton, PA, USA) is another nanofiltered, plasma-derived C1-INH that is approved in Europe for treating all acute attacks of HAE and for short-term and long-term prophylaxis, and in the US for routine prophylaxis.Citation54,Citation60 Cetor® (Sanquin Blood Supply Foundation, Amsterdam, the Netherlands), another C1-INH, was approved in 1997 in a few European countries but has never been tested in clinical trials.Citation54,Citation61
Table 3 Specific agents for hereditary angioedema (HAE)
Ruconest® (conestat alfa; Pharming Group NV, Leiden, the Netherlands) is a human recombinant C1-INH derived from the milk of transgenic rabbits with the same inhibitory profile as plasma-derived C1-INH.Citation62,Citation63 This recombinant C1-INH is effective in relieving symptoms of acute angioedema and is approved in Europe for the acute treatment of HAE.Citation62 Ruconest is undergoing clinical trials in the US and at the time of this writing was under review by the US Food and Drug Administration for the treatment of acute attacks of HAE.Citation64
Ecallantide (Kalbitor®; Dyax Corp, Burlington, MA, USA) is a recombinant protein, synthesized in yeast, that is a potent, specific, and reversible inhibitor of plasma kallikrein.Citation65,Citation66 Ecallantide binds to plasma kallikrein and directly inhibits conversion of high-molecular-weight kininogen to bradykinin.Citation65 Because elevated levels of bradykinin are essential for the development of symptoms of HAE,Citation65 inhibition of bradykinin release is an important mechanism for treating attacks of HAE. Ecallantide is administered subcutaneously and is rapidly distributed throughout the vascular compartment.Citation22,Citation67 Ecallantide is approved in the US for the treatment of acute attacks of HAE.Citation65
Icatibant (Firazyr®; Shire Orphan Therapies, Inc, Lexington, MA, USA) is a selective, competitive bradykinin B2 receptor antagonist that acts as a bradykinin inhibitor and is administered by subcutaneous injection in the abdominal area.Citation68,Citation69 Icatibant is approved for the acute treatment of HAE in Europe and in the US.Citation68–Citation70
According to Hereditary Angioedema International Working Group consensus recommendations, patients with HAE should have access to any one of these medications on demand for use during any type of HAE attack and should be trained to self-administer the medication.Citation54 However, if laryngeal symptoms persist following treatment, patients should report to a hospital immediately.Citation54
Second-line therapies in the absence of first-line agents or if the patient cannot tolerate them include solvent/detergent-treated plasma or fresh frozen plasma.Citation57,Citation71 No controlled clinical trials have been conducted on the efficacy of plasma for the treatment of HAE; however, reports of successful resolution of acute exacerbations have been published in case studies.Citation72,Citation73 Disease transmission with plasma products remains a concern, and fresh frozen plasma may exacerbate angioedema.Citation57
Prophylaxis
Although acute events can be unpredictable, certain triggers for acute HAE, such as environmental allergens, medications, surgeries, or childbirth, can be managed effectively.Citation5,Citation53,Citation71 Prophylactic therapy for HAE is warranted for both short-term use prior to precipitating events such as dental or surgical procedures and for long-term use in high-risk patients, ie, those with frequent or severe episodes and those whose symptoms cannot be controlled with on-demand therapy (see ).
In patients with suspected HAE, endoscopy of the gastrointestinal tract or oropharynx is not recommended due to the risk of inducing a potentially life-threatening laryngeal attack.Citation5 If warranted for additional clinical reasons, prophylactic measures to protect the patient against laryngeal swelling should be initiated.Citation5
Attenuated androgens and nanofiltered C1-INH are the only treatments for the prophylaxis of HAE approved by the US Food and Drug Administration. Attenuated androgens such as danazol, oxandrolone, and stanozololCitation50 work by increasing the levels of aminopeptidase P, which inactivates kinins and thereby increases production of C1-INH in the liver, and contributes to the protective effects of androgens against attacks of angioedema.Citation74,Citation75 Androgens can be used effectively and safely for the short-term prophylaxis of HAE.Citation76 Long-term prophylaxis with androgens is associated with numerous adverse events, including weight gain, lipid abnormalities, liver cysts, hepatic adenomas and carcinomas, myopathies, hematuria, headaches, abnormal menses, hair loss or gain, increased or decreased libido, and anxiety.Citation50,Citation76 The side effects of attenuated androgens can be minimized by titrating down to the minimal effective dose.Citation77
Owing to the risk of adverse events in patients taking attenuated androgens, currently the preferred therapy for both short-term and long-term HAE prophylaxis is nanofiltered C1-INH.Citation78 In a randomized trial evaluating the efficacy of C1-INH for the prophylaxis of HAE attacks, patients treated with C1-INH had an average decrease of 6.47 attacks (P<0.001) compared with patients receiving placebo over the 12-week evaluation period.Citation78 Treatment with C1-INH significantly reduced the frequency, severity, and duration of attacks, the number of rescue treatments of C1-INH needed, and the number of days of swelling.Citation78
Antifibrinolytic agents are not commonly used in the US. Agents such as tranexamic acid and epsilon-aminocaproic acid are chemically synthesized and exert their action in HAE by inhibiting conversion of plasminogen to plasmin.Citation50 These agents have been used for short-term and long-term prophylaxis,Citation57,Citation75 although they are the least effective of the available therapeutic modalities and not recommended based on recent data.Citation57 The dosing recommendation for tranexamic acid is 25 mg 2–3 times a day, not to exceed 6 g/day for the goal. Although prophylaxis reduces the occurrence of edematous episodes, it should be emphasized that life-threatening attacks may nevertheless occur.Citation50
Conclusion
Gastroenterologists are likely to be consulted when patients with HAE are hospitalized for acute HAE attacks involving abdominal symptoms, and may also see patients with HAE in the clinic when they are referred from the emergency department after an attack, usually during asymptomatic periods. The consequences of a missed diagnosis of HAE are considerable, with undiagnosed patients likely visiting the emergency department and undergoing hospitalization and even unnecessary surgical procedures. Furthermore, inadequately treated attacks may progress, resulting in needless significant additional morbidity and even mortality. However, because abdominal pain is often associated with HAE, a high index of suspicion should be maintained for this diagnosis, and a family history should be elicited and appropriate imaging and laboratory studies obtained. In this way, gastroenterologists can promptly diagnose and treat HAE and proceed to make the appropriate referral to an allergist, who can manage this potentially life-threatening disease with current treatment.
Disclosure
In developing this manuscript, writing and editorial assistance with copyediting, formatting, and the creation of tables and figures was provided by Connexion Healthcare (Newtown, PA, USA). The manuscript was financially supported by Dyax Corp (Burlington, MA, USA).
References
- LunnMLSantosCBCraigTJIs there a need for clinical guidelines in the United States for the diagnosis of hereditary angioedema and the screening of family members of affected patients?Ann Allergy Asthma Immunol2010104321121420377110
- BorkKMengGStaubachPHardtJHereditary angioedema: new findings concerning symptoms, affected organs, and courseAm J Med2006119326727416490473
- FellerEJSpiroHMKatzLAHereditary angioneurotic oedema: an unusual cause of recurring abdominal painGut197011129839885511819
- NzeakoUCLonghurstHJMany faces of angioedema: focus on the diagnosis and management of abdominal manifestations of hereditary angioedemaEur J Gastroenterol Hepatol201224435336122410711
- NzeakoUCDiagnosis and management of angioedema with abdominal involvement: a gastroenterology perspectiveWorld J Gastroenterol201016394913492120954277
- ZilberbergMDNathansonBHJacobsenTTillotsonGDescriptive epidemiology of hereditary angioedema emergency department visits in the United States, 2006–2007Allergy Asthma Proc201132539039422195693
- ZilberbergMDNathansonBHJacobsenTTillotsonGDescriptive epidemiology of hereditary angioedema hospitalizations in the United States, 2004–2007Allergy Asthma Proc201132324825421549036
- LumryWRCastaldoAJVernonMKBlausteinMBWilsonDAHornPTThe humanistic burden of hereditary angioedema: impact on health-related quality of life, productivity, and depressionAllergy Asthma Proc201031540741420929608
- FrankMMUrticaria and angioedemaGoldmanLBennettJCCecil Textbook of Medicine21st edPhiladelphia, PA, USAWB Saunders2000
- TalaveraALarraonaJLRamosJLHereditary angioedema: an infrequent cause of abdominal pain with ascitesAm J Gastroenterol19959034714747872288
- CicardiMJohnstonDTHereditary and acquired complement component 1 esterase inhibitor deficiency: a review for the hematologistActa Haematol2012127420822022456031
- CaballeroTBaezaMLCabanasRConsensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part II. Treatment, follow-up, and special situationsJ Investig Allergol Clin Immunol2011216422441
- AgostoniACicardiMHereditary and acquired C1-inhibitor deficiency: biological and clinical characteristics in 235 patientsMedicine (Baltimore)19927142062151518394
- GooiJHCHereditary angioedema: a clinical review for the general physicianDevon, UKPriory Lodge Education Limited Available from: http://www.priory.com/medicine/angioedema.htmAccessed January 17, 2014
- LonghurstHCicardiMHereditary angio-oedemaLancet2012379981447448122305226
- CugnoMZanichelliAFoieniFCacciaSCicardiMC1-inhibitor deficiency and angioedema: molecular mechanisms and clinical progressTrends Mol Med2009152697819162547
- KalmarLHegedusTFarkasHNagyMTordaiAHAEdb: a novel interactive, locus-specific mutation database for the C1 inhibitor geneHum Mutat20052511515580551
- CaballeroTBaezaMLCabanasRConsensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part I. Classification, epidemiology, pathophysiology, genetics, clinical symptoms, and diagnosisJ Investig Allergol Clin Immunol2011215333347
- ZurawBBorkKBinkleyKEHereditary angioedema with normal C1 inhibitor function: consensus of an international expert panelAllergy Asthma Proc201233Suppl 1S145S15623394603
- AntoniuSATherapeutic approaches in hereditary angioedemaClin Rev Allergy Immunol201141111412221279474
- DavisAEIIILuFMejiaPC1 inhibitor, a multi-functional serine protease inhibitorThromb Haemost2010104588689320806108
- LevyJHO’DonnellPSThe therapeutic potential of a kallikrein inhibitor for treating hereditary angioedemaExpert Opin Investig Drugs200615910771090
- BryantJWShariat-MadarZHuman plasma kallikrein-kinin system: physiological and biochemical parametersCardiovasc Hematol Agents Med Chem20097323425019689262
- KaplanAPKinins, airway obstruction, and anaphylaxisChem Immunol Allergy201095678420519882
- MorganBPHereditary angioedema: therapies new and oldN Engl J Med2010363658158320818894
- BorkKFischerBDewaldGRecurrent episodes of skin angioedema and severe attacks of abdominal pain induced by oral contraceptives or hormone replacement therapyAm J Med2003114429429812681457
- AgostoniACicardiMCugnoMZingaleLCGioffréDNussbergerJAngioedema due to angiotensin-converting enzyme inhibitorsImmunopharmacology1999441–2212510604520
- KaplanAPEnzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapyJ Allergy Clin Immunol2010126591892520889195
- FrankMMGelfandJAAtkinsonJPHereditary angioedema: the clinical syndrome and its managementAnn Intern Med19768455805931275365
- BorkKStaubachPEckardtAJHardtJSymptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiencyAm J Gastroenterol2006101361962716464219
- AgostoniAAygoren-PursunEBinkleyKEHereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyondJ Allergy Clin Immunol2004114Suppl 3S51S13115356535
- LocascioEJMahlerSAArnoldTCIntestinal angioedema misdiagnosed as recurrent episodes of gastroenteritisWest J Emerg Med201011439139421079716
- OhsawaINagamachiSSuzukiHLeukocytosis and high hematocrit levels during abdominal attacks of hereditary angioedemaBMC Gastroenterol20131312323915279
- BorkKSiedleckiKBoschSSchopfREKreuzWAsphyxiation by laryngeal edema in patients with hereditary angioedemaMayo Clin Proc200075434935410761488
- FarkasHManagement of upper airway edema caused by hereditary angioedemaAllergy Asthma Clin Immunol2010611920667122
- ZanichelliAMagerlMLonghurstHFabienVMaurerMHereditary angioedema with C1 inhibitor deficiency: delay in diagnosis in EuropeAllergy Asthma Clin Immunol2013912923937903
- FarkasHHarmatGFáyAErythema marginatum preceding an acute oedematous attack of hereditary angioneurotic oedemaActa Derm Venereol200181537637711800154
- ZurawBLHereditary angioedemaN Engl J Med2008359101027103618768946
- De BackerAIDe SchepperAMVandevenneJESchoetersPMichielsenPStevensWJCT of angioedema of the small bowelAJR Am J Roentgenol2001176364965211222198
- FarkasHHarmatGKaposiPNUltrasonography in the diagnosis and monitoring of ascites in acute abdominal attacks of hereditary angioneurotic oedemaEur J Gastroenterol Hepatol200113101225123011711780
- PedrosaMCaballeroTGómez-TraseiraCOlveiraALópez-SerranoCUsefulness of abdominal ultrasonography in the follow-up of patients with hereditary C1-inhibitor deficiencyAnn Allergy Asthma Immunol2009102648348619558006
- DinkelHPMaroskeJSchrodLSonographic appearances of the abdominal manifestations of hereditary angioedemaPediatr Radiol200131429629811321752
- HoxhaMMetaDKaloTHereditary angioedema as a potential cause of cerebral edemaOtorhinolaryngologia – Head and Neck Surgery2013513134
- ZingaleLCZanichelliADeliliersDLRondonottiEDe FranchisRCicardiMSuccessful resolution of bowel obstruction in a patient with hereditary angioedemaEur J Gastroenterol Hepatol200820658358718467921
- GompelsMMLockRJMorganJEOsborneJBrownAVirgoPFA multicentre evaluation of the diagnostic efficiency of serological investigations for C1 inhibitor deficiencyJ Clin Pathol200255214514711865013
- GompelsMMLockRJAbinunMC1 inhibitor deficiency: consensus documentClin Exp Immunol2005139337939415730382
- BowenTHereditary Angioedema Consensus 2010Allergy Asthma Clin Immunol2010611320667116
- BorkKDiagnosis and treatment of hereditary angioedema with normal C1 inhibitorAllergy Asthma Clin Immunol2010611520667118
- EidelmanFJHereditary angioedema: New therapeutic options for a potentially deadly disorderBMC Blood Disord201010320470390
- BowenTCicardiMFarkasH2010 international consensus algorithm for the diagnosis, therapy and management of hereditary angioedemaAllergy Asthma Clin Immunol2010612420667127
- CaballeroTSala-CunillACancianMCurrent status of implementation of self-administration training in various regions of Europe, Canada and the USA in the management of hereditary angioedemaInt Arch Allergy Immunol2013161Suppl 1101623689239
- RiedlMACreating a comprehensive treatment plan for hereditary angioedemaImmunol Allergy Clin N Am2013334471485
- CraigTAygören-PürsünEBorkKWAO guideline for the management of hereditary angioedemaWorld Allergy Organ J201251218219923282420
- CicardiMBorkKCaballeroTEvidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working GroupAllergy201267214715722126399
- BanerjiACurrent treatment of hereditary angioedema: an update on clinical studiesAllergy Asthma Proc201031539840620929607
- TeminoVMPeeblesRSJrThe spectrum and treatment of angioedemaAm J Med2008121428228618374684
- ZurawBLBernsteinJALangDMAmerican Academy of Allergy, Asthma and ImmunologyAmerican College of Allergy, Asthma and ImmunologyA focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedemaJ Allergy Clin Immunol201313161491149323726531
- EpsteinTGBernsteinJACurrent and emerging management options for hereditary angioedema in the USDrugs200868182561257319093699
- Berinert [C1 esterase inhibitor (human)] package insertKankakee, IL, USACSL Behring2011
- Cinryze (C1 esterase inhibitor) package insertExton, PA, USAViroPharma Biologics2012
- Cetor (C1 esterase inhibitor) 500 U powder and solvent for solution for injection (package leaflet)Amsterdam, The NetherlandsSanquin Blood Supply Foundation2003
- VargaLFarkasHrhC1INH: a new drug for the treatment of attacks in hereditary angioedema caused by C1-inhibitor deficiencyExpert Rev Clin Immunol20117214315321426252
- Ruconest (Rhucin in non-European territories)Recombinant human C1 esterase inhibitorPharmingLeiden, the Netherlands2013 Available from: http://www.pharming.com/index.php?act=prodAccessed February 17, 2014
- SantarusSantarus and Pharming announce FDA acceptance for review of Ruconest (recombinant human C1 esterase inhibitor) Biologics License Application6182013 Available from: http://www.businesswire.com/news/home/20130617006581/en/Santarus-Pharming-Announce-FDA-Acceptance-Review-RUCONEST#.UtlxG50o7IUAccessed January 17, 2014Accessed October 8, 2013
- LunnMBantaEEcallantide for the treatment of hereditary angiodema in adultsClin Med Insights Cardiol20115495421695090
- Kalbitor (ecallantide) package insertBurlington, MA, USADyax Corp2012
- LevyRJLumryWRMcNeilDLEDEMA4: a phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedemaAnn Allergy Asthma Immunol2010104652352920568386
- DuboisEACohenAFIcatibantBr J Clin Pharmacol201069542542620573077
- Firazyr (icatibant) package insertLexington, MAShire Orphan Therapies, Inc2011
- SchmidtPWHirschlMMTrautingerFTreatment of angiotensin-converting enzyme inhibitor-related angioedema with the bradykinin B2 receptor antagonist icatibantJ Am Acad Dermatol201063591391420554347
- PremattaMGibbsJGPrattELStoughtonTRCraigTJFresh frozen plasma for the treatment of hereditary angioedemaAnn Allergy Asthma Immunol200798438338817458436
- LonghurstHJEmergency treatment of acute attacks in hereditary angioedema due to C1 inhibitor deficiency: what is the evidence?Int J Clin Pract200559559459915857357
- PekdemirMErselMAksayEYanturaliSAkturkAKiyanSEffective treatment of hereditary angioedema with fresh frozen plasma in an emergency departmentJ Emerg Med200733213717917692764
- DrouetCDesormeauxARobillardJMetallopeptidase activities in hereditary angioedema: effect of androgen prophylaxis on plasma aminopeptidase PJ Allergy Clin Immunol2008121242943318158172
- BorkKCurrent management options for hereditary angioedemaCurr Allergy Asthma Rep201212427328022729959
- FrankMMUpdate on preventive therapy (prophylaxis) of hereditary angioedemaAllergy Asthma Proc2011321172121262094
- SloaneDELeeCWShefferALHereditary angioedema: Safety of long-term stanozolol therapyJ Allergy Clin Immunol2007120365465817765757
- ZurawBLBussePJWhiteMNanofiltered C1 inhibitor concentrate for treatment of hereditary angioedemaN Engl J Med2010363651352220818886
