Abstract
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder characterized by recurrent abdominal pain and abnormal bowel patterns. Alteration in gut flora, visceral hypersensitivity, and abnormal bowel motility are among numerous factors in the complex pathophysiology of IBS. Antibiotics have been used adjunctively to treat IBS for many years but are associated with various systemic side effects. Rifaximin is a nonabsorbable, broad-spectrum antimicrobial that inhibits bacterial RNA synthesis by binding the β-subunit of microbial RNA polymerase. It targets the gastrointestinal tract and works by reducing the quantity of gas-producing bacteria and altering the predominant species of bacteria present. In vivo animal studies suggest additional beneficial mechanisms of rifaximin, including reducing mucosal inflammation and visceral hypersensitivity. Clinical studies have demonstrated that rifaximin improves symptoms associated with IBS, such as bloating, flatulence, stool consistency, and abdominal pain, and has a side-effect profile similar to placebo. Although additional investigation into optimal dosing, treatment duration, and potential resistance is required, rifaximin presents as a safe and beneficial addition to the current management options for IBS.
Introduction
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder characterized by recurrent abdominal pain and abnormal bowel patterns for at least 3 months.Citation1 IBS is differentiated into constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), or mixed (IBS-M).Citation1 Symptoms often include changes in stool frequency and consistency, flatulence, and bloating.Citation2,Citation3 IBS is common in the US, with an estimated prevalence of up to 20%, and negatively impacts quality of life.Citation1,Citation3,Citation4 IBS confers a disproportionately high health care economic burden due to frequent doctor visits, repeated imaging and testing, and other diagnostic and surgical procedures.Citation4
Although the exact etiology remains unclear, important pathogenic factors are numerous: abnormal bowel motility, visceral hypersensitivity, neurotransmitter imbalance, infection, inflammation, brain–gut interaction, and psychosocial stress.Citation2 Nearly 10% of patients with an intestinal bacterial infection report postinfectious symptoms up to 10 years after the infectious event.Citation5 Recent studies have identified the role of bacteria in maintaining normal gut function, including protection against pathogens, metabolism, and absorption.Citation6 In addition, they demonstrated an increased quantity and alteration in the type and distribution of gut bacterial flora in patients with IBS.Citation7,Citation8 Previous studies have demonstrated less lactobacilli, coliforms, and bifidobacteria have been reported among IBS patients compared to healthy controls,Citation9 while previous studies have found no difference in the total quantity of bifidobacteria.Citation10 Varying bacterial load can correlate to subgroups of IBS, such as less Lactobacilli spp. in IBS-D and more Veillonella spp. in IBS-C.Citation10 Studies of fecal microbiota in IBS patients have been somewhat inconsistent and likely confounded by diet, obesity, age, gut transit, antibiotic use, and genetic factors.
Hydrogen and methane produced by the bacterial fermentation of unabsorbed carbohydrates in the gastrointestinal tract are excreted and detected by breath testing.Citation11 Breath testing is commonly used to diagnose lactose, fructose, or sorbitol malabsorption; small intestinal bacterial overgrowth (SIBO); and delayed gut transit.Citation11 A higher concentration of gas-producing bacteria and greater level of organic acid have been shown to correlate with severity of functional gastrointestinal symptoms, such as abdominal discomfort, distention, and flatulence.Citation12 SIBO, characterized by abnormally elevated levels of colonic bacteria (>105 colony-forming units/mL) in the small intestine, has also been associated with IBS symptoms, although a direct relationship has not been established and the evidence is conflicting.Citation13 The quantitative change in the bacterial concentration in the small bowel seen in SIBO and IBS disrupts normal gut function, including digestion and absorption. In SIBO, bacterial production of short-chain fatty acids leads to increased colonic motility but decreased motility in the proximal intestine. Gut dysmotility and distention from increased bacterial fermentation and gas production are common to the pathophysiology of both disorders.Citation14 A high proportion of IBS patients have an abnormal lactulose breath test (45%–84%) and symptom improvement following antibiotic treatment.Citation7,Citation15–Citation17 This suggests a strong overlap between bacterial overgrowth and IBS. However, the prevalence of SIBO in IBS patients varies significantly depending on the diagnostic criteria used (lactulose breath test, glucose hydrogen breath test, or jejunal aspirate test).Citation13 Additionally, bacterial infectious gastroenteritis is a significant risk factor for the development of IBS.Citation18
Treatment for IBS remains an ongoing clinical challenge. Current therapies include antispasmodics, prosecretory agents such as linaclotide and lubiprostone, peppermint oil, antidepressants, and psychotherapy.Citation1,Citation19 Prior studies have shown that broad-spectrum antibiotics, such as tetracycline, amoxicillin-clavulanate, metronidazole, neomycin, and fluoroquinolones, can improve bowel symptoms,Citation16,Citation17,Citation20 presumably through their ability to decrease bacterial overgrowth. In a prospective, randomized, controlled trial of 111 subjects, neomycin was more effective than placebo in improving a composite score calculated based on abdominal pain, diarrhea, and constipation symptoms; ≥50% improvement was determined to be by the clinical response.Citation16 In a retrospective study that evaluated the effect of antibiotics on IBS symptoms, initial treatment with neomycin and other antibiotics (excluding rifaximin) produced a clinical response, similarly defined as >50% symptom improvement, in only 38% and 44% of patients, respectively.Citation21 The symptoms rated included bloating, diarrhea, constipation, and abdominal pain. Retreatment with doxycycline, neomycin, or amoxicillin/clavulanate was ineffective in a majority of patients (75%) and demonstrated evidence of clinical resistance.Citation19 Also, readily absorbable oral antibiotics bear the relevant drawback of associated systemic side effects. This review discusses the clinical use of rifaximin, a nonabsorbable, gut-targeted antimicrobial, in the management of IBS.
Mechanism of action
Rifaximin (xifaxan; Salix Pharmaceuticals, Inc., Raleigh, NC, USA) is an oral semisynthetic derivative of rifamycin with antimicrobial activity against Gram-positive and Gram-negative aerobic and anaerobic organisms.Citation22 Pistiki et al demonstrated the in vitro effect of rifaximin using duodenal aspirates with quantitative cultures for diagnosing SIBO.Citation23 Minimal inhibitory concentrations and time-kill assays showed inhibition of small bowel flora associated with SIBO, including 85% of Escherichia coli, 44% of Klebsiella, 35% of Enterobacter, 82.6% of other Gram-negative species, 100% of Enterococcus faecalis, 100% of Enterococcus faecium, and 100% of Staphylococcus aureus. Rifaximin produced a >3log10 decrease after 24 hours of growth at concentrations of only 500 μg/mL, which is significantly less than the reported stool concentrations of 8,000 μg/mL. Treatment with rifaximin is also effective against enteric protozoal infections, including Cryptosporidium and Blastocystis.Citation24
Rifaximin binds the β-subunit of microbial RNA polymerase, thereby inhibiting transcription and RNA synthesis.Citation25 Rifaximin acts locally within the gastrointestinal tract and has negligible absorption (<0.01% detected in plasma)Citation26 after oral administration.Citation27 Less than 0.01% of the unchanged medication is excreted in the urine.Citation28 Therefore, there is minimal risk of toxicity or systemic side effects.Citation22 However, the bioavailability of rifaximin varies throughout the gastrointestinal tract based on its hydrophobic properties and insolubility in water. Solubility of the drug increases 100-fold in the presence of bile acids, which suggests that its antimicrobial effect is primarily achieved in the small bowel and less so in the colon.Citation25
Xu et al hypothesized a multifactorial mechanism of rifaximin in the improvement of symptoms in IBS.Citation29 Using a rat model for visceral hyperalgesia, they measured visceromotor response to colorectal distension, gut permeability, and microbial DNA in the ileum and found that oral rifaximin decreased the overall quantity of bacteria and altered the bacterial composition in the ileum with a higher relative abundance of Lactobacillus species. Rifaximin decreased mucosal inflammation, measured by decreased levels of interleukins (IL-17 and IL-6) and tumor necrosis factor α (TNF-α) as well as visceral pain in response to chronic stress psychological.Citation29 In addition, neomycin did not decrease visceral pain in response to colon distension and did not prevent the elevation in IL-17, IL-6, or TNF-α.Citation26 The amount of fermentation and gas production is reduced mainly by lessening the bacterial load, particularly in the colon, which is a likely key component in reducing bloating, flatulence, and abdominal discomfort caused by bowel distention.Citation30 It is unclear whether changing the predominant species of gut flora may confer relief due to inadequate studies and inconclusive data regarding the effect of probiotics.Citation31 More recent studies also suggest that rifaximin may reverse chronic stress-induced mucosal inflammation and epithelial dysfunction, and the effect is accompanied by an increase in Lactobacillus and a decrease in segmented filamentous bacteria.Citation32
Rifaximin appears to impede the ability of enteric pathogens to adhere to epithelial cells and internalize, or invade, the host cells. Epithelial cells treated with rifaximin exhibited reduced enteroaggregative E. coli adherence as well as decreased attachment and translocation of Bacillus anthracis or Shigella sonnei.Citation33 IBS patients have been shown to have altered microbiota and an associated increase in mucosal inflammation and dysregulation of the host immune response. Also, there may be an impairment in the epithelial barrier and increased permeability.Citation34 In addition to reducing the harmful proinflammatory effects of bacterial mucosal invasion, rifaximin likely produces a direct antiinflammatory effect as well. Rifaximin acts as an agonist to human pregnane X receptor (PXR) in the gut intestinal epithelial cells, which regulates the expression of cytokines and chemokines via toll-like receptor (TLR)-4 and NF-kappa B (NF-κB) pathways. Rifaximin also suppresses the binding of NF-κB triggered by bacterial endotoxin through the activation of PXR and induces TGF-β, and thus, helps to maintain immune homeostasis.Citation35
Rifaximin use in IBS
In a randomized double-blind placebo-controlled trial of 124 patients with functional gastrointestinal symptoms, Sharara et al studied the efficacy of rifaximin 400 mg twice daily vs placebo for 10 days on IBS and non-IBS patients ().Citation36 All patients met inclusion criteria of at least 12 weeks of active symptoms, including bloating, excessive flatulence and chronic abdominal pain and discomfort, disturbances in bowel movements, feeling of incomplete evacuation, or abnormal stool consistency. Patient follow-up was for a total of 30 days (10 days in pretreatment baseline phase, 10 days in the treatment phase, and 10 days in the posttreatment phase). Patients recorded daily symptoms, including abdominal pain, distension, frequency of bowel movements, stool consistency, and feeling of incomplete evacuation. Rifaximin produced significant improvement in global symptom relief compared to placebo (41.3% vs 22.9%, P=0.03) in the subset of IBS-positive patients (40.5% vs 18.2%, P=0.04). Treatment with rifaximin was also associated with improvement in abdominal bloating, distension, and flatulence. Improvement in bloating score correlated with a decrease in hydrogen production on lactulose breath testing among responders to rifaximin (R=0.631), but no significant correlation was found between nonresponders (R=0.227).Citation36
Table 1 Studies of rifaximin in patients with IBS
Meyrat et al evaluated the efficacy of rifaximin among IBS patients in daily clinical practice as well as assessed the prevalence of positive lactulose hydrogen breath test. They treated 106 of 150 (71%) IBS patients with a positive breath test using 200 mg of rifaximin four times per day for 14 days. Patients rated their symptoms at baseline, week 4, and week 14. The 11-point Likert scale was used to measure the severity of symptoms and overall well-being (0 – no symptoms or no change in the overall well-being and 10 – most severe symptoms and severe reduction in the overall well-being). IBS patients with a positive breath test reported more severe bloating (5.5% vs 4.6%, P=0.028) and diarrhea (2.9% vs 2.2%, P=0.026) at baseline compared to IBS patients with a negative lactulose breath test. They found a significant improvement in the following IBS-related symptoms, comparing scores at baseline with scores after 4 weeks of rifaximin treatment: bloating (5.5% vs 3.6%, P<0.01), flatulence (5.0% vs 4.1%, P=0.01), diarrhea (2.9% vs 2.0%, P<0.01), abdominal pain (4.8% vs 3.3%, P<0.01), and overall well-being (3.9% vs 2.7%, P<0.01). In a cohort undergoing repeat breath testing, a negative test at 4 weeks was reported in 55 of 64 (86%) patients. Interestingly, those patients who remained positive for lactulose hydrogen breath test reported no change in symptoms between week 0 and week 4.Citation15
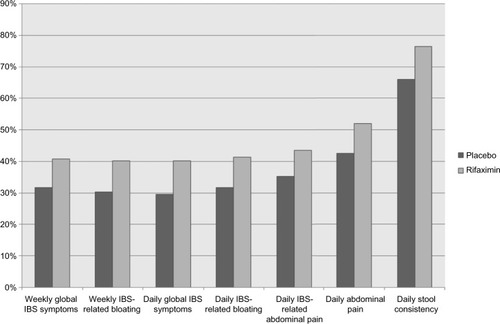
More recently, Pimentel et al described two identically designed, Phase III, double-blind, randomized, multicenter, placebo-controlled trials (targeted, nonsystemic antibiotic rifaximin gut-selective evaluation of treatment for non-c irritable bowel syndrome [TARGET], [TARGET] 1 and TARGET 2) of 1,260 IBS patients, diagnosed by Rome II criteria, without constipation. The study compared rifaximin 550 mg three times a day vs placebo for 14 days. Patients were assessed for an additional 10-week period after treatment. The primary outcome measured was relief of global IBS symptoms determined by a weekly yes or no response to the question, “In regard to all your symptoms of IBS, as compared with the way you felt before you started the study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms”? Secondary end points were self-reported symptomatic relief of bloating and abdominal pain (). More patients in the rifaximin group related adequate improvement of global IBS symptoms (40.8% vs 31.2%, P=0.01, in TARGET 1; 40.6% vs 32.2%, P=0.03, in TARGET 2; and 40.7% vs 31.7%, P<0.001, combined). Treatment with rifaximin also resulted in significantly higher relief of IBS-related bloating for >2 weeks after treatment (39.5% vs 28.7%, P=0.005, in TARGET 1; 41.0% vs 31.9%, P=0.02, in TARGET 2; and 40.2% vs 30.3%, P<0.001, combined). A significantly higher proportion of patients in the rifaximin group also reported greater improvement in abdominal pain or discomfort and loose or watery stools (46.6% vs 38.5%, P=0.04, in TARGET 1 and 46.7% vs 36.3%, P=0.008, in TARGET 2). The incidence of adverse events (AEs) was similar among both groups (1.6% vs 2.4%), and no case of Clostridium difficile-associated diarrhea or ischemic colitis was reported.Citation37
Figure 1 Percentage of patients with relief of symptoms during the primary evaluation period (weeks 3–6).
Abbreviation: IBS, irritable bowel syndrome.
In another study by Pimentel et al, the potential use of rifaximin was extended to IBS-C. This double-blind, randomized, placebo-controlled trial compared the adjunctive use of rifaximin with neomycin vs neomycin alone in 31 patients aged 18–65 years who fulfilled Rome II criteria for IBS-C. Inclusion criteria were less than three complete and spontaneous bowel movements per week and breath methane >3 ppm. Exclusion criteria included use of antibiotic or probiotic within 30 days and use of narcotics, proton pump inhibitors, tricyclic antidepressants, or other bowel-altering medications. Patients were treated with 500 mg neomycin twice daily and 550 mg rifaximin three times a day for 14 days or neomycin alone. Weekly symptom questionnaires were filled out using a visual analog scale (0 – no symptoms and 100 – severe symptoms) to assess abdominal pain, constipation, bloating, urgency, incomplete evacuation, straining, and diarrhea. They reported a significant improvement in the primary outcome, severity of constipation (28.6 mm vs 61.2 mm, P=0.0042), as well as secondary outcomes (straining and bloating) with the adjunctive use of rifaximin. This therapeutic gain continued up to 4 weeks post treatment. However, they did not find an improvement in abdominal pain. Lower methane levels following treatment were associated with improved symptoms. Both groups had a similar proportion of subjects with methane levels ≤3 ppm after treatment: ten out of 15 subjects receiving adjuvant rifaximin and eleven out of 16 subjects receiving neomycin alone. In the neomycin and rifaximin groups, subjects with methane ≤3 ppm after treatment reported significantly lower constipation severity than subjects with persistent methane (P=0.02).Citation38 It is important to mention that Salix Pharmaceuticals, the manufacturer of rifaximin, supported this trial.
Given the chronicity of IBS and probable necessity of patients requiring repeated rifaximin treatments, Pimentel et al reported that >75% of subjects who initially responded to rifaximin also responded to further retreatment with no significant reduction in benefit with successive treatments. Furthermore, there was no change in the duration of benefit (median time between treatments) of successive retreatments.Citation39 Yu et al studied orocecal transit time using scintigraphy and concluded that the abnormal lactulose breath test in IBS patients can be explained by variation in gut transit time and not necessarily SIBO.Citation40
Additional clinical use
Rifaximin is currently used for a variety of gastrointestinal-related conditions such as infectious diarrhea, hepatic encephalopathy, SIBO, inflammatory bowel disease, and diverticular disease.Citation22 However, approval by the US Food and Drug Administration is limited to traveler’s diarrhea caused by non-invasive E. coli in patients aged 12 years or older and prevention of hepatic encephalopathy in adults.Citation41 The proposed mechanism by which rifaximin, like other antibiotics, improves hepatic encephalopathy is by decreasing the amount of ammonia-producing bacteria in the gastrointestinal tract. Studies have shown that treatment with rifaximin successfully decreases the frequency of hepatic encephalopathy episodes and hospitalizations.Citation26 In a meta-analysis of 19 randomized controlled trials encompassing 1,370 patients, treatment with rifaximin was significantly more effective than placebo in the secondary prevention of hepatic encephalopathy and in promoting recovery from hepatic encephalopathy. More importantly, rifaximin was shown to reduce mortality (relative risk: 0.68, 95% CI 0.48–0.97).Citation42
More recently, investigators have been exploring new potential applications for rifaximin, particularly in gastrointestinal-related conditions. There is some preliminary data that rifaximin, both as monotherapy and adjuvant therapy, can be effective in producing clinical improvement and remission in patients with inflammatory bowel disease.Citation43 The use of rifaximin to treat C. difficile-associated diarrhea has also shown promising results. Several small, uncontrolled studies have reported efficacy between 64% and 79% in patients with recurrent disease unresponsive to first-line therapy.Citation43 In a retrospective review of 32 patients with recurrent C. difficile infection having undergone prior antibiotic courses with a mean of 4.4, 17 of the 32 patients (53%) had no relapse 3 months post treatment with rifaximin.Citation44
Utilizing the beneficial properties such as lack of absorption and broad spectrum of activity, the clinical application of rifaximin has been extended beyond the gastrointestinal tract. Topical formulations of rifaximin to treat skin infections, periodontal disease, and bacterial vaginosis are currently being developed and studied.Citation45
Safety
Rifaximin has been shown to be safe and well tolerated with no more increased risk of AEs than placebo.Citation37,Citation46 A post hoc analysis of Phase IIb and Phase III trials performed in the US and Canada demonstrated comparable rates of drug-related AEs (12.1% vs 10.7%) between rifaximin (n=1,103) and placebo (n=829).Citation46 The safety assessments included overall AEs, serious AEs, events resulting in study discontinuation, gastrointestinal-associated AEs, and infection-related AEs and were not substantially different with the use of rifaximin. The majority of AEs were mild–moderate and most commonly gastrointestinal related with similar occurrences in the rifaximin and placebo groups (nausea: 4.4% vs 3.7%, abdominal pain: 3.7% vs 4.7%, diarrhea: 3.4% vs 3.1%, and vomiting: 2.0% vs 1.4%). There was no increased risk of infection, including C. difficile. A different study of 106 patients undergoing rifaximin treatment reported infrequent side effects of headache (3%), dry skin (1%), and nausea without vomiting (1%). However, this was not a placebo-controlled trial, and it is unclear if these were drug-related side effects.Citation15 In the larger study by Pimentel et al, a safety analysis included 1,258 subjects (624 in the rifaximin group and 634 in the placebo group). The risk of serious AEs was less in the rifaximin group than in the placebo group (1.6% vs 2.4%), and there were no cases of C. difficile, ischemic colitis, or death.Citation37 Rifaximin is not known to have significant drug interactions; however, it should be used with caution in patients with Child-Pugh class C cirrhosis.Citation26
A recent study compared generic and branded formulations of rifaximin and found significant differences in systemic bioavailability. Plasma drug concentrations were higher after administration of generic rifaximin compared to branded formulation that contains the polymorph rifaximin-α, which has limited bioavailability. This variance was attributed to drug polymorphisms and inherent differences between crystalline and amorphous forms of the compounds.Citation47 It is important to emphasize these differences as different formulations are produced and the potential for dosing and the development of systemic effects changes.
Conclusion
There is cumulative data that rifaximin is a safe and effective addition to the current armamentarium of treatment for IBS. Rifaximin has been shown to improve global IBS symptoms, such as bloating, flatulence abdominal pain, and stool consistency. As a nonabsorbable antimicrobial drug, it targets the gastrointestinal tract with minimal systemic effects. The American College of Gastroenterology issued an evidence-based statement that rifaximin is effective in reducing total IBS symptoms as well as bloating in IBS-D; however, the level of evidence supporting this statement is moderate, and the authors emphasize that further research is needed.Citation19
A large proportion of IBS patients have been shown to have SIBO based on a positive lactulose hydrogen breath testing, and this may correlate with severity of symptoms.Citation15 Additionally, studies have suggested an association between IBS and SIBO, with antibiotics such as rifaximin or neomycin resulting in improvement.Citation16 However, the relationship appears to be inconsistent with dramatic heterogeneity between different types of tests.Citation13,Citation40 Interestingly, in non-IBS patients presenting with bloating and flatulence and positive lactulose hydrogen breath testing, treatment with rifaximin was only successful (defined as normalization of breath test) in 42% of subjects.Citation48 This finding supports the concept of a multifaceted benefit of rifaximin in treating IBS beyond simply SIBO eradication.
Thus far, there is a variation in medication dose and length of treatment between all mentioned clinical trials. Further investigation is needed to establish a standard dose and duration. Although rifaximin is superior to placebo, the symptom–response curve suggests some loss of efficacy toward the end of the 10-week follow-up periods.Citation30 Given the chronicity of IBS, additional studies to date are needed to determine if a similar effect can be seen with subsequent therapy. Additionally, it is important to recognize the significant cost of rifaximin treatment. Costing approximately $21.6 per day (at a dose of 400 mg three times per day), it is comparatively more expensive than other antibiotics, for instance, $3.6 for a month of neomycin.Citation49 Cost–benefit analysis of rifaximin use for the treatment of hepatic encephalopathy, but not for IBS, has noted rifaximin as being the most effective yet most expensive therapy, and recommended rifaximin salvage therapy after other less-expensive measures fail as a more cost-effective alternative.Citation49 Future studies analyzing the cost–benefit of rifaximin for the treatment of IBS would be helpful in guiding clinical management. Other important outcomes such as risk of C. difficile infection or antibiotic resistance will need to be further assessed. Although future studies will be required to determine the long-term safety and efficacy of rifaximin, it emerges as a safe and beneficial addition to the current management option for IBS.
Author contributions
Ron Schey is the guarantor of this article. Natalya Iorio reviewed the medical literature and contributed to drafting the manuscript. Zubair Malik contributed to drafting the manuscript. Ron Schey initiated and contributed to drafting the manuscript and final edits. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All authors approved the final draft submitted.
Disclosure
The authors report no funding and no conflicts of interest in this work.
References
- BrandtLJCheyWDFoxx-OrensteinAEAn evidence-based position statement on the management of irritable bowel syndromeAm J Gastroenterol2009104Suppl 1S1S3519521341
- HorwitzBJFisherRSThe irritable bowel syndromeN Engl J Med2001344241846185011407347
- OldenKWDiagnosis of irritable bowel syndromeGastroenterology200212261701171412016433
- AgarwalNSpiegelBMThe effect of irritable bowel syndrome on health-related quality of life and health care expendituresGastroenterol Clin North Am2011401111921333898
- Schwille-KiuntkeJEnckPZendlerCPostinfectious irritable bowel syndrome: follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or CampylobacterNeurogastroenterol Motil20112311e479e48821883703
- BolinoCMBercikPPathogenic factors involved in the development of irritable bowel syndrome: focus on a microbial roleInfect Dis Clin North Am2010244961975ix20937460
- EspositoIde LeoneADi GregorioGBreath test for differential diagnosis between small intestinal bacterial overgrowth and irritable bowel disease: an observation on non-absorbable antibioticsWorld J Gastroenterol200713456016602118023092
- KassinenAKrogius-KurikkaLMakivuokkoHThe fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjectsGastroenterology20071331243317631127
- BalsariACeccarelliADubiniFFesceEPoliGThe fecal microbial population in the irritable bowel syndromeMicrobiologica1982531851947121297
- MalinenERinttilaTKajanderKAnalysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCRAm J Gastroenterol2005100237338215667495
- RanaSVMalikABreath tests and irritable bowel syndromeWorld J Gastroenterol201420247587760124976698
- TanaCUmesakiYImaokaAHandaTKanazawaMFukudoSAltered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndromeNeurogastroenterol Motil2010225512519e114e11519903265
- FordACSpiegelBMTalleyNJMoayyediPSmall intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysisClin Gastroenterol Hepatol20097121279128619602448
- GhoshalUCSrivastavaDIrritable bowel syndrome and small intestinal bacterial overgrowth: meaningful association or unnecessary hypeWorld J Gastroenterol201420102482249124627585
- MeyratPSafroneevaESchoepferAMRifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 monthsAliment Pharmacol Ther20123611–121084109323066911
- PimentelMChowEJLinHCNormalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. A double-blind, randomized, placebo-controlled studyAm J Gastroenterol200398241241912591062
- PimentelMChatterjeeSChowEJParkSKongYNeomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled studyDig Dis Sci20065181297130116832617
- FutagamiSItohTSakamotoCSystematic review with meta-analysis: post-infectious functional dyspepsiaAliment Pharmacol Ther201541217718825348873
- FordACMoayyediPLacyBEAmerican College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipationAm J Gastroenterol2014109Suppl 1S2S26 quiz S725091148
- BasseriRJWeitsmanSBarlowGMPimentelMAntibiotics for the treatment of irritable bowel syndromeGastroenterol Hepatol201177455493
- YangJLeeHRLowKChatterjeeSPimentelMRifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBSDig Dis Sci200853116917417520365
- ScarpignatoCPelosiniIRifaximin, a poorly absorbed antibiotic: pharmacology and clinical potentialChemotherapy200551Suppl 1366615855748
- PistikiAGalaniIPylerisEBarbatzasCPimentelMGiamarellos-BourboulisEJIn vitro activity of rifaximin against isolates from patients with small intestinal bacterial overgrowthInt J Antimicrob Agents201443323624124461710
- AmentaMDalle NogareERColombaCIntestinal protozoa in HIV-infected patients: effect of rifaximin in Cryptosporidium parvum and Blastocystis hominis infectionsJ Chemother199911539139510632386
- UmezawaHMizunoSYamazakiHNittaKInhibition of DNA-dependent RNA synthesis by rifamycinsJ Antibiot19682132342364876998
- RivkinAGimSRifaximin: new therapeutic indication and future directionsClin Ther201133781282721741091
- LaterzaLIaniroGScoleriIRifaximin for the treatment of diarrhoea-predominant irritable bowel syndromeExpert Opin Pharmacother201516460761525641072
- DescombeJJDubourgDPicardMPalazziniEPharmacokinetic study of rifaximin after oral administration in healthy volunteersInt J Clin Pharmacol Res199414251567836025
- XuDGaoJGillillandM3rdRifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in ratsGastroenterology2014146248449624161699
- ScheyRRaoSSThe role of rifaximin therapy in patients with irritable bowel syndrome without constipationExpert Rev Gastroenterol Hepatol20115446146421780893
- BrennerDMMoellerMJCheyWDSchoenfeldPSThe utility of probiotics in the treatment of irritable bowel syndrome: a systematic reviewAm J Gastroenterol2009104410331049 quiz 5019277023
- GaoJGillillandMG3rdOwyangCRifaximin, gut microbes and mucosal inflammation: unraveling a complex relationshipGut Microbes20145457157525244596
- BrownELXueQJiangZDXuYDupontHLPretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profilesAntimicrob Agents Chemother201054138839619858255
- LeeKNLeeOYIntestinal microbiota in pathophysiology and management of irritable bowel syndromeWorld J Gastroenterol201420278886889725083061
- MencarelliARengaBPalladinoGInhibition of NF-kappaB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cellsEur J Pharmacol20116681–231732421806984
- ShararaAIAounEAbdul-BakiHMounzerRSidaniSElhajjIA randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulenceAm J Gastroenterol2006101232633316454838
- PimentelMLemboACheyWDRifaximin therapy for patients with irritable bowel syndrome without constipationN Engl J Med20113641223221208106
- PimentelMChangCChuaKSAntibiotic treatment of constipation-predominant irritable bowel syndromeDig Dis Sci20145961278128524788320
- PimentelMMoralesWChuaKEffects of rifaximin treatment and retreatment in nonconstipated IBS subjectsDig Dis Sci20115672067207221559740
- YuDCheesemanFVannerSCombined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBSGut201160333434021112950
- CottreauJBakerSFDuPontHLGareyKWRifaximin: a nonsystemic rifamycin antibiotic for gastrointestinal infectionsExpert Rev Anti Infect Ther20108774776020586560
- KimerNKragAMollerSBendtsenFGluudLLSystematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathyAliment Pharmacol Ther201440212313224849268
- GuslandiMRifaximin in the treatment of inflammatory bowel diseaseWorld J Gastroenterol201117424643464622180705
- MattilaEArkkilaPMattilaPSTarkkaETissariPAnttilaVJRifaximin in the treatment of recurrent Clostridium difficile infectionAliment Pharmacol Ther201337112212823095030
- PelosiniIScarpignatoCRifaximin, a peculiar rifamycin derivative: established and potential clinical use outside the gastrointestinal tractChemotherapy200551Suppl 112213015855757
- SchoenfeldPPimentelMChangLSafety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trialsAliment Pharmacol Ther201439101161116824697851
- BlandizziCViscomiGCMarzoAScarpignatoCIs generic rifaximin still a poorly absorbed antibiotic? A comparison of branded and generic formulations in healthy volunteersPharmacol Res201485394424836868
- BoltinDPeretsTTShpornERifaximin for small intestinal bacterial overgrowth in patients without irritable bowel syndromeAnn Clin Microbiol Antimicrob2014134925319626
- HuangEEsrailianESpiegelBMThe cost-effectiveness and budget impact of competing therapies in hepatic encephalopathy – a decision analysisAliment Pharmacol Ther20072681147116117894657
- PimentelMParkSMirochaJKaneSVKongYThe effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trialAnn Intern Med2006145855756317043337
