Abstract
Gastric antral vascular ectasia (GAVE), also known as “watermelon stomach”, is an uncommon condition, which can cause gastrointestinal bleeding due to rupture of blood vessels that line the stomach. The pathogenesis of GAVE remains unclear; however it is thought that hemodynamic changes, mechanical stress, and autoimmune factors all have a part to play. A range of conditions are also commonly associated with the syndrome, such as portal hypertensive gastropathy, liver cirrhosis, and autoimmune disorders. Less commonly, chronic renal failure, cardiac diseases, and bone marrow transplantation have coexisted with GAVE. The diagnosis is usually based on visualization of the tissue upon endoscopy; however, histology plays a role in uncertain cases. The typical “watermelon” appearance relates to the tissue having a striped appearance radiating out from the pylorus. Medical treatment has failed to show satisfactory results and surgery is usually considered as a last resort, due to its increased risk for complications and mortality. Lasers and argon plasma coagulation have been used recently, and been shown to be as effective as surgery and a safer option. We present three cases of gastric antral vascular ectasia treated at our institution with radiofrequency ablation and review the literature on treatment modalities for GAVE.
Introduction
Gastric antral vascular ectasia (GAVE), also known as “watermelon stomach”, is an uncommon cause of upper gastrointestinal bleeding and chronic iron deficiency anemia, often requiring long-term blood transfusion.Citation1 The pathogenesis of GAVE remains unclear, but it is thought that hemodynamic changes, mechanical stress, and autoimmune factors all have a part to play. A range of conditions are also commonly associated with the syndrome, including portal hypertensive gastropathy, liver cirrhosis, and autoimmune disorders. Less commonly, chronic renal failure, cardiac diseases, and bone marrow transplantation have coexisted with GAVE. The diagnosis is usually based on visualization of the tissue upon endoscopy; however, histology plays a role in uncertain cases. The typical “watermelon” appearance relates to the tissue having a striped appearance radiating out from the pylorus. Systemic interventions such as corticosteroids, hormonal therapy, and octreotide have failed to demonstrate changes in the abnormal mucosa, and patients continue to remain dependent on blood transfusions. Surgery, such as antrectomy, is a definitive treatment option, but is associated with high morbidity and mortality.Citation1 Endoscopic options for therapy include Nd:YAG laser ablation, cryotherapy, heater probe, and argon plasma coagulation (APC). Recently, APC has become one of the preferred modalities in the treatment of chronic radiation proctitis, and many centers have reported success with this treatment. Radiofrequency ablation (RFA) is a newer endoscopic technique that uses a catheter to deliver radiofrequency energy to ablate tissue, and in many centers has become the treatment of choice for early Barrett’s esophagus.Citation1 The HALO® RFA system uses two different types of probes with a closely spaced arrangement of electrodes that thermally ablate tissue. The depth (0.5–1 mm) is dependent on the power, density, and duration of contact. A generator connects to either a 360 degree HALO catheter or to a HALO-90 catheter to provide a circumferential or more focused ablation.Citation2 There have been some reports of this treatment being used for patients with GAVE who have failed other treatments like argon laser coagulation. We present three cases of GAVE treated with RFA and review the literature on its management. Institutional Review Board approval was not obtained as the Richmond University Medical Center review board does not require approval for case studies with three patients or less. Written informed consent for patient data and images was deemed not required for this study by the Richmond University Medical Center review board due to de-identified patient data and images.
Case report 1
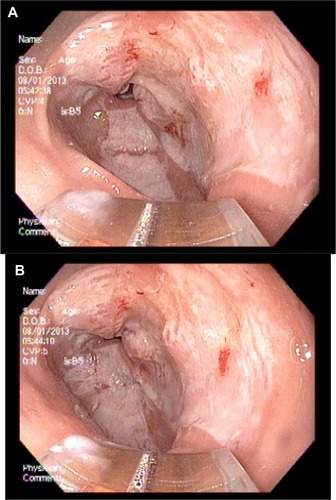
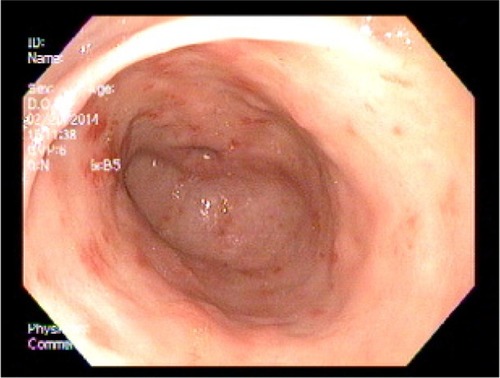
A 51-year-old female patient with a history of gastroesophageal reflux disease, dysphagia, and iron deficiency anemia underwent an upper and lower endoscopy. The patient was found to have findings consistent with gastric antral vascular ectasia (). At that time, no definitive therapy was performed, the patient was continued on supplemental iron, and her hemoglobin/hematocrit levels were checked periodically. Her hemoglobin progressively decreased over the next 2 months, and prior to her repeat endoscopy her hemoglobin level was at 9 g/dL. Six months after the initial endoscopy was performed, the patient underwent a repeat upper endoscopy with RFA to the GAVE (). The antrum showed early GAVE, which was more prominent than previously seen. The HALO-90 ultra ablator was attached, and the GAVE lesions were cauterized using the 4 cm paddle starting at the “12 o’clock position” going circumferentially in both directions. This technique was performed for half of the area. The instrument was withdrawn and the paddle was attached at the “6 o’clock” position, and the remaining posterior vascular ectatic lesions were cauterized. Over the course of the next year, the patient’s hemoglobin started to increase and on follow-up her hemoglobin was 11.4 g/dL. The patient has done well since the initial therapeutic intervention, and has not required any further sessions of RFA. Three years after the therapeutic intervention was performed, her repeat upper endoscopy shows almost complete healing of the mucosa ().
Figure 1 Gastric antral vascular ectasia on initial upper endoscopy.
Case report 2
A 79-year-old female patient with anemia (hemoglobin 8.4 g/dL) underwent an upper endoscopy and was found to have longitudinal strips of submucosal vascular ectasia leading toward the pylorus, consistent with GAVE. The GAVE lesions were cauterized using cold probe cautery. The patient was monitored over the next few years and did well, with a stable hemoglobin level approximately 8.6–9.8 g/dL. Three years after the initial cauterization, the patient presented with a decrease in her hemoglobin level, and underwent a repeat upper endoscopy. The patient was found to have a lesion in the antrum that was oozing blood, and she again underwent cauterization using the BiCap gold probe until the bleeding had stopped with coaptive coagulation. The patient did well after the procedure, and again maintained a hemoglobin level approximately 8.5–10 g/dL. Over the next few years, the patient required multiple sessions of cauterization (total of six), and her hemoglobin continued to fluctuate. After a total of six sessions, the patient underwent RFA of the GAVE. Her hemoglobin level prior to the therapy was 8.7 g/dL, and during a 1-year follow-up period her hemoglobin levels have been maintained at 10.2–11.5 g/dL. It has been 3 years since the endoscopic intervention with RFA was performed, and the patient has done very well, requiring no additional treatment. Hemoglobin levels have normalized; however, she continues to be followed.
Case report 3
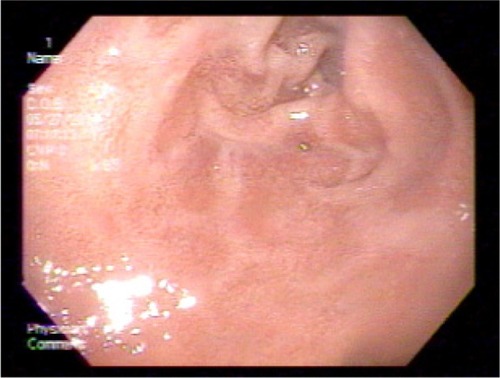
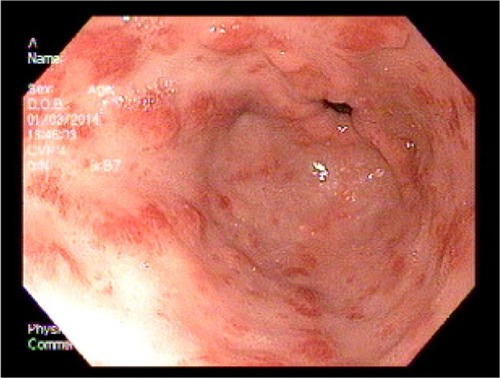
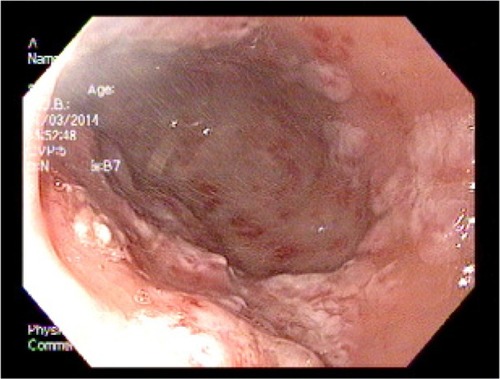
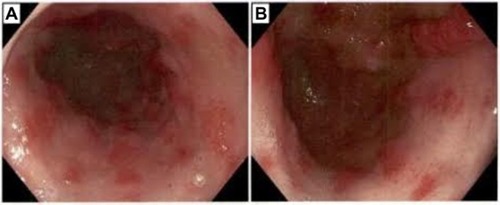
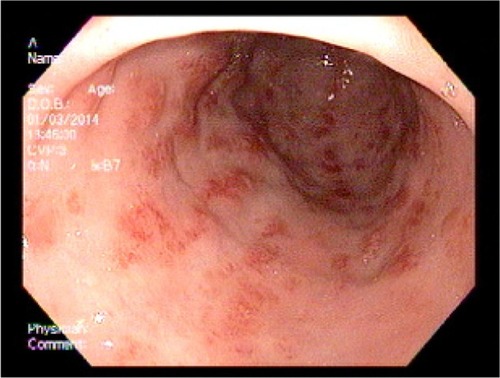
An 83-year-old female patient with a history of GAVE requiring multiple transfusions in the past presented with symptomatic anemia due to acute blood loss (hemoglobin 8.8 g/dL). On upper endoscopy, the patient was found to have mild gastritis in the fundus and body, and multiple vascular ectasia in the antrum consistent with GAVE ( and ). The patient underwent cauterization with the gold probe electro hemostasis catheter until the bleeding had stopped (). The patient did well after the procedure, and maintained a hemoglobin level in the range of 8.7–9.2 g/dL over the next 13 months. However, the patient returned after 14 months with anemia secondary to an upper gastrointestinal bleed. The antrum was noted to have multiple GAVE, and the patient underwent RFA with the HALO-90 catheter (). The patient has done very well since the procedure, and at 2-year follow-up her hemoglobin level is 12.3 g/dL.
Figure 4 Upper endoscopy showing the typical longitudinal red stripes in the antrum radiating out from the pylorus, consistent with gastric antral vascular ectasia.
Discussion
GAVE is an uncommon cause of chronic gastrointestinal bleeding and anemia. On endoscopy, it has a characteristic appearance, demonstrating prominent erythematous streaks protruding out from the pylorus to the antrum, often referred to as “watermelon stomach”.Citation3 The pathogenesis of GAVE remains unclear; however, it is thought that hemodynamic changes, mechanical stress, and autoimmune factors all have a part to play. A range of conditions are commonly associated with the syndrome, including portal hypertensive gastropathy, liver cirrhosis, chronic kidney diseases, and autoimmune and systemic disorders. APC has been the first-line treatment for patients with GAVE and shown promising results; however, approximately two-thirds of patients continue to require blood transfusions.Citation4
Results from our case series suggest that RFA is a safe and effective treatment for recurrent bleeding from GAVE. A range of medical and surgical treatments have been used for symptomatic GAVE, with varied results. Examples of available treatment options can range from less invasive modalities such as corticosteroids, hormonal therapy, and octreotide to more invasive procedures including laser therapy, electrocoagulation, APC, RFA, and even surgery in the most refractory cases. Endoscopic thermal therapy is currently the first-line treatment, with APC being the most favored due to its fair success rate, low complication rate, and low costs.Citation5
Medical treatment
Hormonal therapy
Randomized controlled trails have demonstrated that use of estrogen alone has no benefit in the resolution of gastrointestinal bleeding.Citation6 Subsequently, Van Cutsem et al reported a randomized, crossover trial, concluding that estrogen–progesterone used together can achieve resolution of bleeding from gastrointestinal vascular abnormalities.Citation7 Tran et al reported an open pilot study of six patients with GAVE, demonstrating complete resolution of bleeding in four patients, and a decrease in the need for blood transfusions in all six patients using ethinyl estradiol 30 μg and norethisterone 1.5 mg.Citation8 Additionally, there have been isolated case reports suggesting that dual therapy with estrogen and progesterone can be effective in the treatment of GAVE.Citation9–Citation12 Although resolution of bleeding was achieved, no endoscopic changes were noted in any patients,Citation11,Citation12 suggesting that bleeding would reappear on discontinuing the therapy. Hormonal therapy for a prolonged period should be used with caution. In the open pilot study reported by Tran et al, menorrhagia and gynecomastia was described in three patients.Citation8 Like other pharmacological agents, hormonal therapy can be used as a substitute in patients (especially post-menopausal women) with dispersed lesions who are not suitable for endoscopic therapy or surgery.
Corticosteroids
Corticosteroids have been used in patients not suitable for endoscopic treatment or surgery. On review of the literature, eleven patients were found who were treated with oral steroids alone. Over a 3-year period, six of these patients had stopped bleeding and were transfusion-independent. One patient developed hyperglycemia and had to be switched to an alternative agent, and the remaining four patients showed no success with steroid use and required endoscopic intervention or surgery.Citation9,Citation13–Citation20
Octreotide
Octreotide, a somatostatin analog, is another agent that has been used in patients not suitable for endoscopic treatment or surgery. Unlike published trials on hormones, most of the data regarding octreotide as a treatment option for GAVE have come from isolated case reports or case series only. To date, there are 18 published cases of the use of octreotide.Citation21–Citation29 The mean follow-up time in these cases was 20.1 (range: 0.1–64) months, and the doses of octreotide ranged from 100 μg subcutaneously twice daily to 500 μg subcutaneously twice daily. In these reports, it was noted that seven of the 18 patients had a significant reduction in transfusion requirements after treatment. A further nine of the 18 patients had a moderate reduction in transfusion requirements. The effect was equivocal in one patient,Citation28 and treatment completely failed in another.Citation24 Nardone et al reported a case series of 17 patients with vascular irregularities of the gastrointestinal tract refractory to endoscopic treatment. Three of the 17 patients were cirrhotics with GAVE. Resolution of bleeding was seen in one patient, and the other two patients had a decrease in transfusion requirements over a 2-year period.Citation28 The success seen in these patients with underlying cirrhosis may be due to the fact that octreotide causes a decrease in portal pressure. In addition, octreotide has a low rate of side effects. However, due to the lack of controlled trials and the small number of case reports/series, further studies are required to evaluate its usefulness in the treatment of GAVE.
Tranexamic acid
There have been a number of cases reporting on treatment of vascular abnormalities of the gastrointestinal tract with the antifibrinolytic agent tranexamic acid. However, there are only two reports of tranexamic acid being used in the treatment of GAVE. Park et al reported on a patient with alcoholic cirrhosis and GAVE, who demonstrated a reduction in blood loss with the use of tranexamic acid.Citation30 Since then, McCormick et al have reported on a patient with cirrhosis and GAVE who required 130 units of packed red blood cell products prior to use of tranexamic acid. The bleeding did not respond to portal decompression with transjugular intrahepatic portosystemic shunt or beta-blockers. Following treatment with oral tranexamic acid, the patient did not require further blood transfusion over a follow-up period of 30 months.Citation30 Despite these promising results, controlled trials are required to establish the value of tranexamic acid in the treatment of GAVE. Further, tranexamic acid has been linked to a number of side effects, including pulmonary embolism and central venous retinopathy.Citation31
Other agents
A number of other agents have been studied in single case reports, including calcitonin,Citation18 cyproheptadine,Citation32 alpha-interferon,Citation33 and thalidomide.Citation34–Citation36 Among the agents studied, cyproheptadine stood out because it resolved the gastrointestinal bleeding and caused remodeling of the gastric mucosa.Citation32
Endoscopic treatment
Nd:YAG laser
The Nd:YAG laser penetrates to a depth of 5 mm, is well absorbed by tissue, and is a non-contact method.Citation37–Citation39 Gostout et al reported a study of 45 patients treated by endoscopic Nd:YAG laser coagulation for the treatment of GAVE. Complete resolution of visible disease was noted after laser therapy (a median of one treatment, range: 1–4) in four patients (13%) and >90% resolution in 24 patients (80%). During a follow-up period of 2 years (range: 1 month to 6 years), hemoglobin levels normalized in 87% of patients, and there were no major complications reported.Citation40 Liberski et al reported a study of 15 patients who were followed for a period of 2–8 years from the time of initial diagnosis. Both endoscopic and hematological improvement was noted in this study.Citation14 Bjorkman and Buchi reported a small study comparing the use of the Nd:YAG laser with that of the argon laser. This study demonstrated that use of the Nd:YAG laser resulted in fewer sessions needed to eliminate the lesions and a lower recurrence rate when compared with the argon laser.Citation41 The Nd:YAG laser is expensive and has a lot of complications, so its use has decreased over time.
Argon plasma coagulation
APC uses high frequency energy transmitted to tissue by ionized gas. In APC, the laser current is conducted through argon gas to the tissue from the tip of a monopolar electrode. The coagulation depth is variable (from 0.8 mm to 3.0 mm) according to the generator settings, gas flow rate, time applied, and distance from the tissue. It has frequently been used in the treatment of radiation proctopathy due to its superficial penetration. Wahab et al reported a study of 125 patients with vascular ectasias, including six with GAVE. These six patients had complete resolution of their lesions with a mean of 2.8 sessions.Citation14 There have been a number of other case series and reports supporting the use of APC as the most useful and widespread treatment modality for GAVE. APC is less expensive compared with lasers, and is more widely available.
Bipolar electrocoagulation and heater probe
These are widely available and relatively inexpensive compared with lasers. They are contact probes and are useful for delivering focal, directed therapy to actively bleeding mucosa. Char formation at the probe tip is a complication that can sometimes be experienced. Binmoeller and Katon reported a patient with GAVE who underwent four sessions of BiCap, resulting in almost complete resolution of visible disease and no recurrence.Citation4 These treatment modalities are dependent on the energy setting (1, 3, 5, 7, and 9 watts), duration (2, 6, 10, and 14 seconds), and force applied (0, 50, and 100 g).Citation42
Cryotherapy
Cryotherapy is non-contact method of tissue ablation by application of extreme cold temperatures to a targeted area. It has the benefit of uniform treatment of larger surface areas and ease of targeted application. Cho et al reported a study assessing the efficacy and safety of cryotherapy in 12 patients with GAVE. Six of the 12 patients demonstrated a complete response to treatment and six demonstrated a partial response. An increased mean hemoglobin level of 9.9–11.3 g/dL was noted. Of interest, in 32 of the 36 cryotherapy sessions performed (89%), more than 90% of GAVE lesions were treated. No immediate cryotherapy-related complications were noted in this study.Citation43 One of the risks of cryotherapy is over distention perforation of the bowel.Citation44 Cryotherapy requires the use of liquid nitrogen or carbon dioxide, which is difficult to perform in smaller practices. The unit is less mobile compared to RFA, and the depth of ablation is greater, increasing the risk of bowel perforation.
Radiofrequency ablation
RFA is a new endoscopic therapy that may be a valuable alternative in the treatment of GAVE. The HALO RFA system uses two different types of probes with a closely spaced arrangement of electrodes, which thermally ablate tissue. The depth (0.5–1 mm) is dependent on the power, density, and duration of contact. A generator connects to either a 360 degree HALO catheter or a HALO-90 catheter to provide a circumferential or more focused ablation.Citation2 Dray et al reported an open-label, retrospective, case series evaluating the effectiveness and safety of RFA for the treatment of GAVE. Twenty-four patients were enrolled in the study and underwent a mean of 1.8±0.8 RFA sessions. In all 23 transfusion-dependent patients, the number of red blood cell packs decreased from a mean of 10.6±12.1 during the 6 months prior to RFA to a mean of 2.5±5.9 during the 6 months after RFA. Fifteen patients were reported to have been weaned off transfusions completely.Citation3 McGorisk et al reported an open-label prospective cohort study of patients with GAVE refractory to APC. Twenty-one patients were enrolled in the study, and underwent endoscopic RFA to the gastric antrum using the HALO-90 ultra ablation catheter until transfusion independence was achieved or a maximum of four sessions were performed. Six months after completion of RFA therapy, 18 of the 21 patients (86%) were transfusion-independent. The mean hemoglobin increased from 7.8 to 10.2 g/dL, and there were two adverse events reported (minor acute bleeding and superficial ulceration), both of which resolved without intervention.Citation1
There are multiple advantages of using RFA with the HALO-90 ablation catheter for the treatment of GAVE. RFA covers a broader surface area compared with APC and electrocautery. Applying pressure to the probe results in some coaptive coagulation before the application of power to ablate the tissue, leading to a more superficial burn in less time. This helps shorten the treatment duration even in extensive disease. Squamous re-epithelialization, which occurs after RFA, minimizes bleeding, fibrosis, and stricture formation.Citation45–Citation47 This is due to the superficial depth of ablation (0.5–1 mm).
These studies show that RFA could prove to be a useful method for refractory GAVE. Our experience indicates that it could even be used as the primary treatment modality for GAVE. However, it is still considered a newer method, and we need more experience and longer follow-up times before recommending it as a primary treatment.
Surgical treatment
Surgical intervention, most commonly antrectomy, is a definitive therapy for GAVE but its use is associated with significant morbidity and mortality. Earlier reports have shown a 7.4% operative mortality.Citation44 From review of previous cases, and the newer endoscopic treatment options available, antrectomy should be restricted to those who remain refractory to medical and endoscopic treatment.
Conclusion
RFA is an emerging technique that has been used in the treatment of Barrett’s esophagus for the last few years. Recent reports of its use in GAVE have been promising, and we believe that in the future RFA may become the preferred treatment. RFA is becoming more widely available, and it is easy to perform with minimal risks. However, more reports are needed with longer follow-up before it can be routinely recommended for GAVE. Two of our patients had been treated with other endoscopic thermal therapies prior to undergoing RFA (cases 2 and 3). The third patient had RFA as a primary treatment (case 1). All three patients have shown good results.
Disclosure
The authors report no conflicts of interest in this work.
References
- McGoriskTKrishnanKKeeferLKomanduriSRadiofrequency ablation for refractory gastric antral vascular ectasia (with video)Gastrointest Endosc20137858458823660565
- AkiyamaJTriadafilopoulosGEndoscopic ablation therapy of Barrett’s esophagusMinerva Gastroenterol Dietol20105640542021139540
- DrayXRepiciAGonzalezPRadiofrequency ablation for the treatment of gastric antral vascular ectasiaEndoscopy20144696396925111135
- SebastianSO’MorainCABuckleyMJReview article: current therapeutic options for gastric antral vascular ectasiaAliment Pharmacol Ther20031815716512869075
- ShibukawaGIrisawaASakamotoNGastric antral vascular ectasia (GAVE) associated with systemic sclerosis: relapse after endoscopic treatment by argon plasma coagulationIntern Med20074627928317379994
- VasePEstrogen treatment of hereditary hemorrhagic telangiectasia. A double-blind controlled clinical trialActa Med Scand19812093933967018182
- Van CutsemERutgertsPvan TrappenGTreatment of bleeding gastrointestinal vascular malformations with oestrogen progesteroneLancet19903359539551970032
- TranAVilleneuveJPBilodeauMTreatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with oestrogen-progesterone in cirrhotic patients: an open pilot studyAm J Gastroenterol199494202211
- MossSFGhoshPThomasDMJacksonJECalamJGastric antral vascular ectasia; maintenance treatment with oestrogen-progesteroneGut1992337157171612493
- HermanCGofinEHarsmanYWatermelon stomach. An unusual cause of recurrent upper gastrointestinal tract bleeding in the uremic patient: efficacy of treatment with oestrogen progesterone therapyNephrol Dial Transpl199611871874
- SchonbroodtNHorsmannYHoangPVascular gastric lesions, CREST syndrome and primary biliary cirrhosis: efficacy of oestrogen progesterone treatmentGastroenterol Clin Biol1994186496517875423
- ManningRJOestrogen progesterone treatment of diffuse antral vascular ectasiaAm J Gastroenterol1995901541567801925
- JabbariMCherryRLoughJODalyDSKinnearDGGoreskyCAGastric antral vascular ectasia: the watermelon stomachGastroenterology198487116511706332757
- LiberskiSMMcGarrityTJHartleRJVaranoVReynoldsDThe watermelon stomach: long-term outcome in patients with Nd:YAG laser therapyGastrointest Endosc1990363994022210286
- CalamJWalkerRJAntral vascular lesion, achlorhydria and chronic gastrointestinal blood loss: response to steroidsDigest Dis Sci1980252362396966212
- BowmickBKWatermelon stomach treated with oral corticosteroidJ R Soc Med19938652
- KrugerRRyanMEDicksonKBNunezJFDiffuse vascular ectasia of the gastric antrumAm J Gastroenterol1987824214263578221
- KishiKKinishitaYKitajimaNTwo cases of gastric antral vascular ectasia – response to medical treatmentGastroenterol Jpn199926757762
- CalesPVoigtJJPayenJLDiffuse vascular ectasia of the antrum, duodenum and jejunum in a patient with nodular regenerative hyperplasia: lack of response to portosystemic shunt or surgeryGut1993345585618491407
- RawlinsonDBareCDLinBPAntral vascular ectasia – the watermelon stomachMed J Aust19861447097113724602
- TorsoliAAnnibaleBViscardiADelle FaveGTreatment of bleeding due to diffuse angiodysplasia of the small intestine with somatostatin analogueEur J Gastroenterol Hepatol19913785787
- RossiniFPArrigoniAPennazioMOctreotide in the treatment of bleeding due to angiodysplasia of the small intestineAm J Gastroenterol199388142414278362842
- AndersenMRAasebyJSomatostatin in the treatment of gastrointestinal bleeding caused by angiodysplasiaScand J Gastroenterol199631103710398898427
- BarbaraGDe GiorgioRSalvioliBStanghelliniVCorinaldesiRUnsuccessful octreotide treatment of the watermelon stomachJ Clin Gastroenterol1998263453469649027
- BowersMMcNultyOMayneEOctreotide in the treatment of gastrointestinal bleeding caused by angiodysplasia in two patients with von Willebrand’s diseaseBr J Haematol200010852452710759709
- OrsiPGuatti-ZulianiCOkolicsanyiLLong-acting octreotide is effective in controlling rebleeding angiodysplasia of the gastrointestinal tractDig Liver Dis20013333033411432511
- NordquistLTWallachPMOctreotide for gastrointestinal bleeding of obscure origin in an anticoagulated patientDig Dis Sci2002471514151512141810
- CoppolaADe StefanoVTufanoALong-lasting intestinal bleeding in an old patient with multiple mucosal vascular abnormalities and Glanzmann’s thrombasthenia: 3-year pharmacological managementJ Intern Med200225227127512270009
- BlichMFruchterOEdelsteinSEdouteYSomatostatin therapy ameliorates chronic and refractory gastrointestinal bleeding caused by diffuse angiodysplasia in a patient on anticoagulation therapyScand J Gastroenterol20033880180312889571
- ParkRMDaneshBJZUpadhyayRGastric antral vascular ectasia (watermelon stomach) therapeutic optionsPostgrad Med J1990667207232235802
- WooKSTseLKWooJLMassive pulmonary embolism after tranexamic acid antifibrinolytic therapyBr J Clin Pract1989434654662611113
- Pina CabralJEPontesJMTosteMWatermelon stomach: treatment with a serotonin antagonistAm J Gastroenterol1991869279282058646
- DisdierPSchleinitzNPerreardMDramatic improvement of watermelon stomach with alpha-interferonAm J Gastroenterol199590100910107771396
- Perez-EncinasMRabunal MartinezMJBello LopezJLIs thalidomide effective for the treatment of gastrointestinal bleeding in hereditary hemorrhagic telangiectasia?Haematologica200287ELT3412161379
- ShurafaMKambojGThalidomide for the treatment of bleeding angiodysplasiasAm J Gastroenterol20039822122212526972
- BauditzJSchachschalGWedelSLochsHThalidomide for treatment of severe intestinal bleedingGut20045360961215016759
- SwaroopVSGostoutCJEndoscopic treatment of chronic radiation proctopathyJ Clin Gastroenterol19982736409706767
- WilsonSARexDKEndoscopic treatment of chronic radiation proctopathyCurr Opin Gastroenterol20062253654016891886
- PatelAPathakRDeshpandeVPatelSHWickremesinghePCVadadaDRadiofrequency ablation using BarRx for the endoscopic treatment of radiation proctopathy: a series of three casesClin Exp Gastroenterol2014745346025525377
- GoustoutCJViggianoTRAhlquistDAWangKKLarsonMVBalmRThe clinical and endoscopic spectrum of the watermelon stomachJ Clin Gastroenterol1992152562631479175
- BjorkmanDJBuchiKNEndoscopic laser therapy of the watermelon stomachLaser Surg Med199212478481
- LaineLDetermination of the optimal technique for bipolar electro-coagulation treatment. An experimental evaluation of the BICAP and gold probesGastroenterology19911001071121983812
- ChoSZanatiSYongEEndoscopic cryotherapy for the management of gastric antral vascular ectasiaGastrointest Endosc20086889590218640673
- HouJKAbudayyehSShaibYTreatment of chronic radiation proctitis with cryoablationGastrointest Endosc20117338338921295650
- SharmaVKWangKKOverholtBFBalloon-based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1-year follow-up of 100 patientsGastrointest Endosc20076518519517258973
- GanzRAUtleyDSSternRAJacksonJBattsKPTerminPComplete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagusGastrointest Endosc2004601002101015605025
- DunkinBJMartinezJBejaranoPAThin-layer ablation of human esophageal epithelium using a bipolar radiofrequency balloon deviceSurg Endosc20062012513016333533