Abstract
Achalasia is a primary disorder of esophageal motility. It classically presents with dysphagia to both solids and liquids but may be accompanied by regurgitation and chest pain. The gold standard for the diagnosis of achalasia is esophageal motility testing with manometry, which often reveals aperistalsis of the esophageal body and incomplete lower esophageal sphincter relaxation. The diagnosis is aided by complimentary tests, such as esophagogastroduodenoscopy and contrast radiography. Esophagogastroduodenoscopy is indicated to rule out mimickers of the disease known as “pseudoachalasia” (eg, malignancy). Endoscopic appearance of a dilated esophagus with retained food or saliva and a puckered lower esophageal sphincter should raise suspicion for achalasia. Additionally, barium esophagography may reveal a dilated esophagus with a distal tapering giving it a “bird’s beak” appearance. Multiple therapeutic modalities aid in the management of achalasia, the decision of which depends on operative risk factors. Conventional treatments include medical therapy, botulinum toxin injection, pneumatic dilation, and Heller myotomy. The last two are defined as the most definitive treatment options. New emerging therapies include peroral endoscopic myotomy, placement of self-expanding metallic stents, and endoscopic sclerotherapy.
Introduction
Achalasia, a primary esophageal motility disorder, is classically characterized by impaired relaxation of the lower esophageal sphincter (LES) and loss of esophageal peristalsis.Citation1 The primary pathophysiologic disturbance is the loss of inhibitory interneurons in the myenteric plexus that are involved in facilitating LES relaxation for gastric accommodation of food boluses.Citation2 Consequently, the classic symptomatic presentation involves dysphagia to both solids and liquids associated with regurgitation of undigested food. Further associated symptoms include substernal chest pain with dysphagia, weight loss, and dyspepsia that often leads to a misdiagnosis of gastroesophageal reflux disease.Citation3,Citation4 The incidence of achalasia is one in 100,000 individuals annually with a prevalence of ten in 10,000. It occurs equally among women and men and is without racial predilection. The peak incidence is between 30 and 60 years of age.Citation5,Citation6
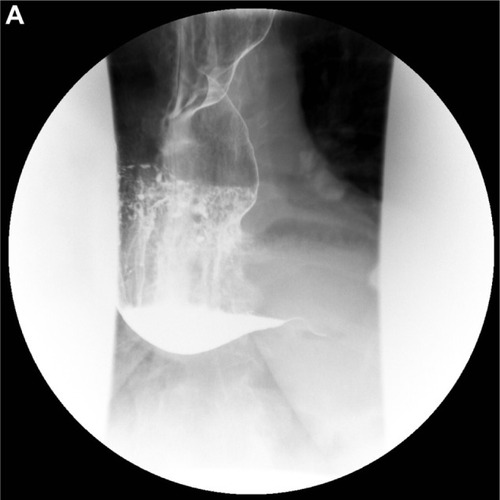
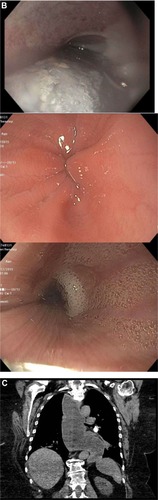
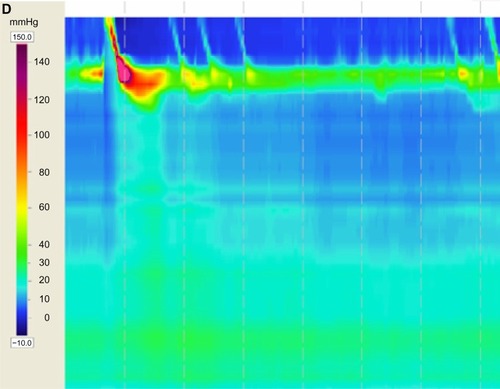
Various diagnostic modalities have been implemented that assist in the diagnosis of achalasia. It is characterized on radiographic barium swallow by aperistalsis resulting in poor emptying of barium, esophageal dilation, and minimal LES opening, resulting in a tapering of the barium column giving it a “bird’s beak” appearance (). Endoscopically, it is characterized by a dilated esophagus with retained saliva, undigested food particles, and liquid in the absence of attributing strictures or tumors (). Occasionally, patients are found to have a dilated esophagus when undergoing computed tomography of the chest (). Manometrically, it is characterized by incomplete relaxation of the LES and aperistalsis of the esophageal body ().Citation1
Figure 1 (A–D) Diagnostic tests for achalasia.
The options for treatment of achalasia are vast with many new emerging therapies. Although no current treatment option is a definitive cure, the aim is to reduce the hypertonicity of the LES in an effort to relieve symptoms, improve esophageal emptying, and prevent further esophageal dilation.Citation1 This is attempted via pharmacologic, endoscopic, or surgical means. The choice depends on the patient’s comorbidities and therefore their candidacy for operative intervention.
In addition to reviewing the pathogenesis and diagnostic workup of achalasia, the various treatment modalities and newest emerging therapies that are hoped to evolve the field and improve treatment efficacy will be discussed in depth.
Esophageal structure and motor innervation
The esophagus consists of four primary layers – the mucosa, submucosa, muscularis propria, and adventitia.Citation7 The muscularis propria consists of both a circular and longitudinal muscle layer. It gradually transitions from predominantly striated, skeletal muscle in the upper esophagus to predominantly smooth muscle in the lower esophagus. The esophagus terminates around the area of the diaphragmatic hiatus where there exists a 2–4 cm circular muscle layer, termed the lower esophageal sphincter.Citation7
Esophageal motor innervation occurs through the vagus nerve via the intrinsic enteric nervous system; namely, the myenteric or Auerbach’s plexus. The origin of neural innervation for the striated muscle of the proximal esophagus differs from that of the smooth muscle of the distal esophagus. The striated muscle of the proximal esophagus is predominantly innervated by somatic efferent fibers that have cell bodies originating from the nucleus ambiguous. They terminate on the motor end plate via cholinergic receptors.Citation8,Citation9 Alternatively, the smooth muscle of the distal esophagus is innervated by preganglionic neurons with cell bodies originating in the dorsal motor nucleus and terminating on the fibers of the myenteric plexus.Citation10,Citation11 The esophageal musculature (including the LES) is then innervated by postganglionic fibers. These consist of both excitatory neurons, which release acetylcholine resulting in esophageal and LES contraction, and inhibitory neurons, which release nitric oxide and vasoactive intestinal peptide resulting in relaxation.Citation12,Citation13 These neurons work together in a coordinated manner once a food or liquid bolus enters the esophagus to result in peristalsis that moves the bolus from the esophagus into the stomach.
In a normal functioning esophagus, inhibitory neurons are activated first to release nitric oxide, which relaxes the esophagus and allows for accommodation of the incoming food bolus. Following bolus front esophageal relaxation, there is a sequential activation of the excitatory neurons proximal to the bolus front to release acetylcholine, which then propels the food bolus forward.
Eventually, the peristaltic waves will propel the food bolus across the esophagogastric junction (EGJ) into the stomach. EGJ is a high-pressure area that is comprised of the LES, crural diaphragm, and proximal gastric cardia.Citation7 Normal resting lower esophageal pressure is 10–30 mmHg, which functions to prevent reflux of gastric contents back into the esophagus. When the food bolus reaches the LES, it relaxes to allow passage into the stomach. This is achieved via activation of the inhibitory neurons resulting in the release of nitric oxide.
Pathogenesis of achalasia
The primary pathophysiologic disturbance resulting in achalasia is the selective loss of inhibitory innervation from the myenteric plexus of the distal esophagus and LES.Citation14 The etiology for this process is unknown; however, genetics, infection, and autoimmune contributors have been discussed. The process may be an autoimmune phenomenon initiated by an indolent viral infection in a genetically predisposed host.Citation15 Ultrastructural studies of esophageal tissue have found inflammatory infiltrate in the myenteric plexus of patients with achalasia compared to no inflammatory infiltrate in patients without achalasia.Citation2,Citation16 Additionally, a few case–control studies have suggested an association with HLA class II antigens and the development of idiopathic achalasia.Citation17,Citation18 Furthermore, Ruiz-de-Leon et alCitation19 revealed that achalasia patients with these associated class II HLA antigens had a higher prevalence of circulating antimyenteric autoantibodies. This is strongly suggestive of an autoimmune component as the etiology. However, one should note that not all patients with achalasia have these associated HLA antigens.Citation20
The consequence of the myenteric plexus inflammation is the degeneration of inhibitory postganglionic neurons of the esophagus and LES.Citation21,Citation22 This results in unopposed cholinergic stimulation leading to impaired relaxation of the LES and hypercontractility of the distal esophagus.Citation7
Diagnosis of achalasia
The diagnosis of achalasia is typically suspected in a patient with dysphagia to both solids and liquids with associated regurgitation of undigested food. Complimentary tests include esophagogastroduodenoscopy and barium esophagram; however, definitive diagnosis should only be made following evaluation of esophageal motor function with manometric testing.Citation1 The following are the diagnostic modalities often employed in achalasia.
Esophageal manometry
Esophageal manometry is the gold standard for the diagnosis of achalasia. It functions to assess esophageal pressures along the length of a flexible catheter inserted into the esophagus. The classic manometric finding of aperistalsis of the esophageal body and incomplete LES relaxation without evidence of mechanical obstruction is strongly supportive toward the diagnosis of achalasia.Citation23 Other findings that include increased resting LES pressure and simultaneous nonpropagating contractions are also suggestive of the diagnosis, however, these are not required.Citation24
Manometry, as a diagnostic tool, has evolved significantly over the past decade from conventional catheters with pressure sensors spaced 3–5 cm apart utilizing solid-state technology or a water-perfused extrusion catheter to high resolution manometry where pressure sensors are placed 1 cm apart with either water-perfused extrusion or solid-state technologies.Citation1
High resolution manometry can display pressure data as esophageal topography plots (). Esophageal pressure topography gives the clinician the advantage of differentiating achalasia into three distinct subtypes that have important therapeutic outcome implications.Citation23 Subtype II has the most favorable prognosis, whereas subtype III is the most difficult to treat. The prognosis of subtype I is somewhere in between.Citation23,Citation25,Citation26
Barium esophagram
Esophageal dilation with a gradual taper down to the gastroesophageal junction giving a “bird’s beak” appearance is the classic description of achalasia on barium esophagram (). Additional findings include aperistalsis and poor emptying of barium.Citation1 Signs suggestive of late- or end-stage achalasia include tortuosity, angulation, and megaesophagus.Citation1
An additional role for contrast radiography is the assessment of esophageal emptying following treatment of achalasia. Symptom relief often does not parallel esophageal emptying. Therefore, objective assessment of treatment response can be helpful in identifying patients at risk of failed treatment. This is accomplished by timed barium esophagram where the barium column height is measured at 1 and 5 minutes after upright ingestion of a large barium bolus.Citation27 Subsequent studies have revealed the usefulness of timed barium esophagram for identifying patients at risk of failed treatment.Citation28,Citation29 Computed tomography can also be used to support an underlying diagnosis of achalasia and show the extent of disease, especially in those with sigmoid esophagus (); however, it is less sensitive and not commonly employed.
Endoscopy
Most patients presenting with dysphagia require esophagogastroduodenoscopy primarily to rule out a mechanical obstruction due to cancer that can mimic achalasia both clinically and manometrically. This is termed “pseudoachalasia.”Citation30–Citation32 Similar to achalasia, mechanical obstruction can manometrically result in impaired LES relaxation and aperistalsis or spastic contractions of the esophageal body.Citation33
Endoscopic findings of achalasia range from a grossly normal appearance to a tortuous, dilated sigmoid esophagus with retained food and saliva ().Citation1 Oftentimes, the gastroesophageal junction will have a puckered appearance. Intubation of the stomach may give mild resistance; however, a strong resistance should raise suspicion of pseudoachalasia.Citation1
Of note, patients with achalasia can develop candidiasis secondary to esophageal stasis. Endoscopic evidence of candidiasis in the setting of immunocompetence suggests an esophageal motility disorder.Citation7
Clinical management of achalasia
At the moment, there are no therapies that will reverse the loss of the inhibitory interneurons of the myenteric plexus and restore normal esophageal motility. Current treatment options aim to reduce the hypertonicity of the LES to improve esophageal emptying by gravity. This is attempted via oral pharmacologic therapy, endoscopic therapy, or surgery. The choice of treatment is dependent on patient age, comorbidities, severity, patient preference, and locally available expertise.Citation34 Currently, pneumatic dilation (PD) and surgical myotomy are considered the most effective therapies. Other options are employed in those who are not candidates for these more effective therapies.Citation1 Regardless of the intervention, LES hypertonicity returns over time requiring repeat intervention. The decision on treatment options is based on several factors, including operative candidacy and durability ().
Table 1 Pros and cons of conventional treatments for achalasia
Oral pharmacologic therapy
Oral pharmacologic therapies are considered the least effective treatment option for achalasia.Citation35 The clinical response to these agents is short lived and their side effect profile is unfavorable. Additionally, they do not provide complete alleviation of symptoms and therefore they are usually reserved for patients who are poor candidates for more effective therapies (PD, surgical myotomy) or in those who have failed botulinum toxin injections (BTIs).Citation1 The aim of these medications is to induce smooth muscle relaxation of the LES thereby facilitating passage of esophageal contents into the stomach. The two most commonly employed medications are calcium channel blockers and long-acting nitrates. Sildenafil, a phosphodiesterase-5-inhibitor, is another option.Citation36 Other less commonly used medications include anticholinergics, β-adrenergic agonists, and theophylline.
Endoscopic pharmacologic therapy
Botulinum toxin, a potent inhibitor of acetylcholine release from the presynaptic terminals by cleaving the SNAP-25 protein, is a useful treatment strategy for patients who are unable to tolerate more invasive therapies, such as PD or surgical myotomy. The objective of this therapy is to block unopposed cholinergic stimulation caused by the selective loss of inhibitory interneurons that release neurotransmitters to relax the LES.Citation37
The technique involves injecting up to 100 units of toxin with a sclero-needle just proximal to the squamo–columnar junction evenly distributed over four quadrants. Doses greater than 100 units are shown to be no more effective.Citation38 Complications related to the procedure are rare and typically involve chest pain seen in 16%–25%. Rare, more serious, complications include mediastinitis and an allergic reaction to an egg-based protein. Additionally, repeated BTIs can lead to subsequent submucosal fibrosis making future surgical myotomy difficult.Citation39–Citation41 Therefore, BTI should be reserved for patients in whom PD and surgical myotomy are precluded due to operative risk factors.Citation42
The response rate in the first month of treatment is high at 80%–90%; however, the therapeutic effect wanes rapidly over time such that ∼50% of patients are symptomatic at 1 year.Citation38,Citation43,Citation44 Thus, repeat treatments every 6–12 months are often required. Factors predictive of a favorable and prolonged response to BTI are older age (>40 years old), a type II manometric pattern, and a decreased basal LES pressure following treatment.Citation44
Pneumatic dilation
PD, a procedure employing air pressures to disrupt or fracture the LES circular muscle fibers, is the most effective nonsurgical option in the treatment of achalasia.Citation1,Citation5 Currently, the most widely used balloon dilator is the Rigiflex, a nonradiopaque graded size polyethylene balloon. Another less commonly employed and essentially abandoned balloon dilator is the Witzel dilator that comes in only one size (4.0 cm balloon). This has fallen out of favor over the past years.
With Rigiflex dilators, the procedure can be performed under radiologic guidance (fluoroscopy); although balloon positioning under direct endoscopic guidance can also be employed.Citation45,Citation46 The dilators are available in three diameters (3.0, 3.5, and 4.0 cm). There are two different strategies for performing PD that include a single dilation approach and a graded dilation approach. When employing the graded approach, relief of symptoms is possible in 50%–93% of patients.Citation5,Citation35,Citation47,Citation48 One cross-sectional study demonstrated that symptomatic response to a single PD was 62% at 6 months and 28% at 2 years compared to 90% at 6 months and 44% at 6 years in the graded PD cohort.Citation47
Graded PD is performed by an initial dilation at 3.0 cm, then 3.5 cm, and finishing at 4.0 cm with 4–6 weeks in between dilations. Reassessment of symptoms and LES pressure can be performed between each session to determine the necessity of subsequent treatments. Additionally, the rate of perforation may be lower with the graded dilation approach.Citation1
It is estimated that a third of patients treated with PD will experience symptom relapse within 4–6 years.Citation1 Predictive factors of a poor clinical response to treatment include age <40 years,Citation49–Citation51 male sex,Citation52 LES pressure after dilation greater than 10–15 mmHg,Citation53 and continued symptoms after one or two treatments.Citation52–Citation55 Additionally, males younger than 45 years of age may not be as responsive to the serial approach. This is possibly due to thicker LES musculature. In these patients, it is recommended to either start with PD at 3.5 cm or proceed straight to surgical myotomy as the initial step in management.Citation1 Of note, patients with a type II high resolution manometry pattern have been shown to have better outcomes.Citation25
All patients being considered for PD must also be appropriate surgical candidates as esophageal perforation is a known complication of this procedure. The rate of perforation is ∼1.9% but is shown to range from 0% to 5%.Citation47,Citation56 Although there are no factors predictive of perforation, it is believed to most commonly occur during the initial dilation and is thought to be related to improper balloon positioning.Citation57 Small, asymptomatic perforations can be managed conservatively with parenteral nutrition, antibiotics, and stent placement.Citation58 However, for large perforations with mediastinal contamination, surgical repair through thoracostomy is required.Citation1 An additional complication includes gastroesophageal reflux disease (GERD), which is seen in 15%–35% of patients following PD. Therefore, starting a proton pump inhibitor following PD in those with preexisting GERD is recommended.Citation59
Surgical myotomy
Surgical myotomy, a technique involving the division of the circular muscle fibers of the LES, was initially performed via an open thoracotomy and laparotomy approach. Studies at the time revealed good response with 60%–94% of patients achieving symptomatic improvement when followed over 1–36 years.Citation35 However, over time, this approach was replaced with more minimally invasive techniques; first with thoracoscopy which was then replaced with laparoscopic myotomy because of improved morbidity and faster recovery time.Citation60 Additionally, these minimally invasive techniques can be performed with similar efficacy to the open approach; 94% versus 84%, respectively.Citation35
There are no randomized control trials comparing the different approaches of surgical myotomy. All published data in this area are prospective or retrospective cohort and case–control studies. A systematic review analyzing surgical techniques in 4,871 patients reported patient symptom improvement after all surgical myotomies. This included 84.5% of those who underwent the open transabdominal approach, 83.3% of those with the open transthoracic approach, 77.6% of those with the thoracoscopic approach, and 89.3% of those who had a laparoscopic myotomy.Citation48 A subset of the analysis comparing studies with laparoscopic Heller myotomy (LHM) (3,086) and the thoracoscopic approach (211) showed better symptomatic improvement with the laparoscopic approach compared to the thoracoscopic approach (89.3% vs 77.6%, P=0.048).Citation48
GERD is a known and frequent complication following surgical myotomy thereby making intraoperative fundoplication a consideration to remedy it. The incidence of postoperative reflux symptoms is reported to be lower for the laparoscopic than for the thoracoscopic approach (28.3% vs 14.9%, P=0.03) and open transthoracic approach (24.6% vs 14.9%, P=0.04).Citation48 Reflux may be less if fundoplication is added to myotomy (41.5% without fundoplication vs 14.5% with fundoplication, P=001).Citation48 A randomized controlled trial comparing myotomy with or without fundoplication reported that performing intraoperative fundoplication was associated with a lower incidence of postoperative reflux.Citation61 Therefore, in the guidelines published by the Society of American Gastrointestinal and Endoscopic Surgeons, it is recommended to perform fundoplication in patients who undergo myotomy.Citation62 Additionally, the rate of postoperative dysphagia is shown to be independent of whether or not a fundoplication was performed after myotomy.Citation48 Of note, Rawlings et alCitation63 demonstrated in a randomized control trial comparing anterior Dor with posterior Toupet fundoplications that both provide similar outcomes in terms of postoperative reflux following LHM.
Of note, robotic surgery is an emerging minimally invasive alternative to LHM. It is a computer-assisted device under the remote control of a surgeon. It has the benefit of providing a magnified surgical field that allows for more precise movements thereby reducing complications.Citation64
Esophagectomy
“End-stage” achalasia, characterized by a dilated and tortuous esophagus (megaesophagus or sigmoid esophagus), is often unresponsive to conventional treatments for achalasia. Although PD is ineffective, surgical myotomy should still be considered the initial treatment before consideration of the more morbid esophagectomy. Two recent studies involving patients with megaesophagus revealed that surgical myotomy resulted in improvement of symptoms ranging from 72% to 92%.Citation65,Citation66 When symptoms are unresponsive to myotomy, esophagectomy should be the next step for consideration.Citation67 Observational studies do show symptomatic response of up to 80% with esophagectomy; however, its association with a greater morbidity and mortality make it a last resort treatment modality.Citation68
Advances in treatment for achalasia
Peroral endoscopic myotomy
Peroral endoscopic myotomy (POEM), a minimally invasive endoscopic technique, is one of the most recent advances in the treatment of achalasia (). It was first described in a case series published in 1980 by Ortega et alCitation69 where two 1 cm long myotomies were performed through the mucosa at a depth of 3 mm. However, this was not further studied until 2007 when Pasricha et alCitation70 demonstrated endoscopic myotomy in a porcine model. Then, in 2010, Inoue et alCitation71 published a prospective trial of 17 patients undergoing endoscopic myotomy that revealed significant reduction in the index of dysphagia symptoms (10 to 1.3, P=0.0003) as well as resting LES pressure (52.4 to 19.9 mmHg, P=0.0001). No serious complications were reported. It was at this point where endoscopic myotomy became adopted into clinical practice.Citation72 In 2012, von Renteln et alCitation73 published another prospective trial. It included 16 patients and illustrated symptomatic response in 94% after 3 months. Furthermore, resting LES pressure was reduced from 27.3 to 11.8 mmHg (P<0.001). In 2014, Bhayani et alCitation74 conducted a prospective observational study that compared 64 patients treated by LHM and 37 by POEM. It showed that mean operative time and length of stay were significantly higher in the LHM cohort but complication rates were similar. Patient symptoms, manometry, and postoperative esophageal acid exposure revealed similar outcomes among the two groups.
Table 2 Studies evaluating the efficacy of perioral endoscopic myotomy (POEM)
The preparation for POEM begins with a liquid diet 1–5 days prior to the procedure to minimize residual food in the esophagus.Citation75 The first step in the procedure involves injection of 10 mL of saline solution with contrast (methylene blue or indigo carmine) to the central esophagus 10–16 cm proximal to the squamo–columnar junction.Citation72 The purpose of this is to expand the submucosa so it is easily accessed for dissection and tunnel formation. This allows safer access to the circular muscle layer. Following this, a 2 cm incision is made to gain access into the submucosal space. Then, a submucosal tunnel is dissected through the EGJ and 2–3 cm into the gastric cardia.Citation76 Once access is made to the circular muscle layer of the LES, the myotomy is usually extended to 6 cm into the esophagus and 2 cm below the EGJ.Citation20
Serious adverse events are rare with POEM. They occur at a rate of <0.1% with the most common serious event being perforation.Citation77 Another, albeit less serious, complication following POEM is GERD. In carefully selected patients, some studies have shown short-term postoperative clinical symptoms of GERD following POEM is 10.9%, comparable to that of LHM.Citation78
Self-expanding metal stents
The use of esophageal self-expanding metal stent (SEMS) has long been beneficial in the treatment of esophageal malignancies, esophageal perforations, and anastomotic leaks. There have been a few studies published in the recent past that have described the utility of SEMS in benign esophageal disease, particularly achalasia ().Citation79,Citation80
Table 3 Studies evaluating the efficacy of self-expanding metal stent (SEMS)
In 2009, Zhao et alCitation81 published a prospective observational study that concluded temporary SEMS to be a safe and effective modality in the management of achalasia with very good long-term symptomatic response rates. It involved 75 patients with achalasia who were treated with a temporary 30 mm diameter SEMS. They were placed under fluoroscopic guidance and removed via gastroscopy 4–5 days later. Follow-up was arranged at 6 months, 1 year, 3–5 years, 5–8 years, 8–10 years, and >10 years. Clinical response was 100% at 1 month and 83.3% at follow-up past 10 years. Complications were few but included migration (5.3%), chest pain (38.7%), and reflux (20%). There were no perforations or 30-day mortality.
Cheng et al,Citation79 in 2010, published another prospective observational study comparing the efficacy of 20, 25, and 30 mm diameter temporary SEMS. It involved a total of 90 patients with follow-up arranged at 6 months and 1, 3–5, 5–8, 8–10, and >10 years. The study found that patients who received the 30 mm diameter SEMS had the best clinical response and lowest incidence of stent migration. It found that the clinical symptomatic remission rate with 30 mm SEMS at >10 years was 83.3%. This is comparable to the long-term clinical response of those who underwent LHM. In 2010, Li et al,Citation82 along with the same authors above at the same institution, published a prospective study comparing 30 mm SEMS with PD. It found that the clinical remission rate in those treated with PD was 0% at 10 years compared with 83.3% at 10 years in those treated with a 30 mm SEMS.
Although the results of the earlier studies are promising, they were published at a single institution and therefore their generalizability is questionable. Further investigation with randomized controlled trials will still be needed before they become accepted into clinical practice.
Endoscopic sclerotherapy
Two recent studies have been published describing the use of a sclerosing agent, ethanolamine oleate, in the treatment of achalasia ().Citation83,Citation84 The theory prompting its investigation was based on its necrotizing effect on the applied muscle.
Table 4 Studies evaluating the efficacy of endoscopic sclerotherapy
In 2013, Moreto et alCitation83 published a study involving 103 patients who received endoscopic sclerotherapy every 2 weeks until dysphagia resolved. It reported a symptom remission rate of 90% at 50-month follow-up. In 2014, Niknam et alCitation84 performed a study involving 31 patients who received three treatments of ethanolamine oleate injections at 2-week intervals. They found that the mean symptom score at 12 months was significantly reduced compared with preinjection scores. It is important to note that, if the use of this procedure is entertained, it should be considered only in those with refractory achalasia who are not candidates for PD or surgical myotomy because there is a thought that the LES fibrosis that ensues might make invasive therapies more difficult.Citation85
Comparison of therapeutic modalities
PD versus BTI
Multiple randomized controlled trials have been conducted comparing the efficacy of PD and BTI. In 2006, Leyden et alCitation86 published a systematic review of seven randomized controlled trials totaling 178 patients. It found similar outcomes at a short-term follow-up of 4 weeks from the initial procedure. However, three studies within the review recorded a 12-month follow-up that discovered a significant difference in response. It found 55 of 75 (73.3%) PD subjects were in symptomatic remission compared to 27 of 72 (37.5%) BTI subjects (relative risk 1.88, P=0.0002). Similar results were elucidated by a systematic review published by Wang et alCitation87 in 2009. Therefore, current evidence supports that PD is more effective than BTI in achieving long-term symptomatic response.
PD versus BTI-PD
In 2006, Mikaeli et alCitation88 performed a randomized controlled trial comparing PD alone versus BTI before PD. At 1-year follow-up, it found remission rates were slighter higher in the BTI-PD group (77%) compared with the group that received PD alone (62%). In 2009, Zhu et alCitation89 published a randomized controlled trial that compared PD alone versus BTI alone versus PD with BTI 15 days later. All subjects were followed for 2 years. At 2 years, clinical remission was achieved in 13.7% treated with BTI alone, 35.7% in those treated with PD alone, and 56.6% in those who received combination therapy. However, despite the earlier evidence, given the concern for fibrosis induced by BT, in clinical practice BT is not combined with pre- or post-PD in those who are candidates for definitive therapy with either PD alone or surgical myotomy.
PD versus LHM
In their systematic review, Campos et alCitation48 compared the efficacy of LHM and PD. It totaled 3,086 patients treated with LHM and 1,065 treated with PD. The study demonstrated significantly improved symptomatic relief in those treated with LHM compared to those treated with PD at 12 months (89.3% vs 68.2%) and past 36 months (89.3% vs 56.3%).
More recently, in 2011, BoeckxstaensCitation90 conducted a randomized controlled trial comparing LHM to PD. In all, 95 patients were randomized to receive PD and 106 patients were randomized to undergo LHM with a mean follow-up time of 43 months. The primary outcome was therapeutic success (as defined by a drop in Eckardt score to <3). It found a comparable success rate for both treatments at 1 year (90% in PD vs 93% in LHM) and at 2 years (86% in PD vs 90% in LHM).
A recent meta-analysis published by Weber et alCitation91 in 2012 found that LHM has greater durability than PD. It included 36 studies with 3,211 patients in the PD group and 1,526 patients in the LHM group. In those treated with PD, it found the mean 5-year remission rate to be 61.9% and mean 10-year remission rate to be 47.9%. In those treated with LHM, it found the mean 5- and 10-year remission rates were 76.1% and 79.6%, respectively.Citation91 It also found a perforation rate for those treated with LHM to be double that of those treated with PD (4.8% vs 2.4%; P<0.05).
Therefore, based on the available evidence, success rates for PD and LHM are believed to be comparable although LHM may be associated with greater long-term durability, especially in younger males.
Treatment algorithm
A generally agreed upon stepwise treatment algorithm for achalasia is illustrated in . Many factors are considered in the selection of the appropriate therapy. They include age, sex, comorbidities and surgical risk, type of achalasia, and available expertise.Citation92
In general, patients with achalasia who are deemed good surgical candidates should be referred for consideration of PD or LHM. Although evidence has shown both therapies to be equally effective in the short term, LHM is the most durable form of treatment for long-term symptom remission, especially in younger male patients.Citation1,Citation91
One recent study determined outcomes in the treatment of achalasia based on manometric subtype. For PD, it reported response rates of 96% in patients with type II achalasia, 56% for type I, and 29% for type III.Citation93 Therefore, PD seems to be most effective in the treatment of type II achalasia. Although PD is shown to induce long-term symptomatic remission, multiple sessions are often required due to symptom recurrence. PD is considered a failed treatment when there is lack of symptom improvement after 2–3 sessions or following the use of the largest diameter balloon. In these situations, referral to surgery for consideration of surgical myotomy is most appropriate.Citation72 Furthermore, it is appropriate to consider PD in patients who have failed initial treatment with surgical myotomy and who have recurrence of symptoms.
Surgical myotomy is shown to be an effective therapy for inducing long-term symptomatic remission.Citation91 In patients who are good operative candidates, it is recommended in adolescents and young adults (especially male),Citation94 patients with achalasia type III,Citation93 patients with pulmonary symptoms, and those who have not responded to two to three sessions of PD or following the use the largest diameter balloon.Citation72
BTI is considered first-line therapy in patients with advanced age, short life expectancy, and significant comorbidities who are high risk of PD and surgical myotomy.Citation42 Oral pharmacologic therapies with nitrates, calcium channel blockers, and phosphodiesterase-5-inhibitors (sildenafil) are generally recommended for patients who cannot undergo more definitive treatment with PD/LHM or in whom BTI has failed. They theoretically function to reduce pressure at the LES but the efficacy is very poor. In general, BTI and oral pharmacologic therapies should only be considered in high-risk patients or as a stepping stone to more definitive therapies.Citation95
POEM is an emerging therapy for patients with achalasia but at this time there is no general consensus as to where it will fall within the achalasia treatment algorithm. Randomized controlled trials will have to be undertaken before it becomes widely accepted into clinical practice. However, the preliminary reports are promising for its role as a potential initial therapy in select group of patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- VaeziMFPandolfinoJEVelaMFACG clinical guideline: diagnosis and management of achalasiaAm J Gastroenterol2013108812381249 quiz 125023877351
- ClarkSBRiceTWTubbsRRRichterJEGoldblumJRThe nature of the myenteric infiltrate in achalasia: an immunohistochemical analysisAm J Surg Pathol20002481153115810935657
- RichterJEThe diagnosis and misdiagnosis of achalasia: it does not have to be so difficultClin Gastroenterol Hepatol20119121010101121699819
- KessingBFBredenoordAJSmoutAJErroneous diagnosis of gastroesophageal reflux disease in achalasiaClin Gastroenterol Hepatol20119121020102421683804
- VaeziMFRichterJEDiagnosis and management of achalasia. American College of Gastroenterology Practice Parameter CommitteeAm J Gastroenterol199994123406341210606295
- FrancisDLKatzkaDAAchalasia: update on the disease and its treatmentGastroenterology2010139236937420600038
- PandolfinoJEGawronAJAchalasia: a systematic reviewJAMA2015313181841185225965233
- BiegerDHopkinsDAViscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguusJ Comp Neurol198726245465623667964
- ToyamaTYokoyamaINishiKEffects of hexamethonium and other ganglionic blocking agents on electrical activity of the esophagus induced by vagal stimulation in the dogEur J Pharmacol19753116371236195
- CollmanPITremblayLDiamantNEThe central vagal efferent supply to the esophagus and lower esophageal sphincter of the catGastroenterology19931045143014388387041
- GoyalRKRattanSNature of the vagal inhibitory innervation to the lower esophageal sphincterJ Clin Invest197555511191126164484
- GoyalRKRattanSSaidSIVIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neuronesNature198028857893783806107863
- YamatoSSpechlerSJGoyalRKRole of nitric oxide in esophageal peristalsis in the opossumGastroenterology199210311972041612326
- GoldblumJRWhyteRIOrringerMBAppelmanHDAchalasia. A morphologic study of 42 resected specimensAm J Surg Pathol19941843273378141427
- BoeckxstaensGEAchalasia: virus-induced euthanasia of neurons?Am J Gastroenterol200810371610161218557706
- RaymondLLachBShamjiFMInflammatory aetiology of primary oesophageal achalasia: an immunohistochemical and ultrastructural study of Auerbach’s plexusHistopathology199935544545310583560
- WongRKMaydonovitchCLMetzSJBakerJRJrSignificant DQw1 association in achalasiaDig Dis Sci19893433493522920639
- VerneGNHahnABPineauBCHoffmanBJWojciechowskiBWWuWCAssociation of HLA-DR and -DQ alleles with idiopathic achalasiaGastroenterology19991171263110381906
- Ruiz-de-LeonAMendozaJSevilla-MantillaCMyenteric antiplexus antibodies and class II HLA in achalasiaDig Dis Sci2002471151911837716
- AtesFVaeziMFThe pathogenesis and management of achalasia: current status and future directionsGut Liver20159444946326087861
- GockelIBohlJREckardtVFJungingerTReduction of interstitial cells of Cajal (ICC) associated with neuronal nitric oxide synthase (n-NOS) in patients with achalasiaAm J Gastroenterol2008103485686418070236
- GockelIBohlJRDoostkamSEckardtVFJungingerTSpectrum of histopathologic findings in patients with achalasia reflects different etiologiesJ Gastroenterol Hepatol200621472773316677160
- PandolfinoJEKahrilasPJAmerican gastroenterologicalAAGA technical review on the clinical use of esophageal manometryGastroenterology2005128120922415633138
- SpechlerSJCastellDOClassification of oesophageal motility abnormalitiesGut200149114515111413123
- SalvadorRCostantiniMZaninottoGThe preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasiaJ Gastrointest Surg201014111635164520830530
- PratapNReddyDNCan achalasia subtyping by high-resolution manometry predict the therapeutic outcome of pneumatic balloon dilatation?: author’s replyJ Neurogastroenterol Motil201117220521603005
- de OliveiraJMBirgissonSDoinoffCTimed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasiaAJR Am J Roentgenol199716924734799242756
- VaeziMFBakerMERichterJEAssessment of esophageal emptying post-pneumatic dilation: use of the timed barium esophagramAm J Gastroenterol19999471802180710406238
- AnderssonMLundellLKosticSEvaluation of the response to treatment in patients with idiopathic achalasia by the timed barium esophagogram: results from a randomized clinical trialDis Esophagus200922326427319431219
- TuckerHJSnapeWJJrCohenSAchalasia secondary to carcinoma: manometric and clinical featuresAnn Intern Med1978893315318686541
- DoddsWJStewartETKishkSMKahrilasPJHoganWJRadiologic amyl nitrite test for distinguishing pseudoachalasia from idiopathic achalasiaAJR Am J Roentgenol1986146121232866701
- KahrilasPJKishkSMHelmJFDoddsWJHarigJMHoganWJComparison of pseudoachalasia and achalasiaAm J Med19878234394463548347
- SchererJRKwiatekMASoperNJPandolfinoJEKahrilasPJFunctional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasiaJ Gastrointest Surg200913122219222519672666
- VelaMFManagement strategies for achalasiaNeurogastroenterol Motil20142691215122125167952
- VaeziMFRichterJECurrent therapies for achalasia: comparison and efficacyJ Clin Gastroenterol199827121359706766
- BortolottiMMariCLopilatoCPorrazzoGMiglioliMEffects of sildenafil on esophageal motility of patients with idiopathic achalasiaGastroenterology2000118225325710648452
- HoogerwerfWAPasrichaPJPharmacologic therapy in treating achalasiaGastrointest Endosc Clin N Am2001112311324vii11319064
- AnneseVBassottiGCocciaGA multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. GISMAD Achalasia Study GroupGut200046559760010764700
- PattiMGFeoCVArceritoMEffects of previous treatment on results of laparoscopic Heller myotomy for achalasiaDig Dis Sci199944112270227610573373
- HorganSHuddaKEubanksTMcAllisterJPellegriniCADoes botulinum toxin injection make esophagomyotomy a more difficult operation?Surg Endosc199913657657910347294
- SmithCDStivalAHowellDLSwaffordVEndoscopic therapy for achalasia before Heller myotomy results in worse outcomes than heller myotomy aloneAnn Surg20062435579584 discussion 584–58616632991
- KumarARSchnoll-SussmanFHKatzPOBotulinum toxin and pneumatic dilation in the treatment of achalasiaTec Gastrointest Endosc20141611019
- VaeziMFRichterJEWilcoxCMBotulinum toxin versus pneumatic dilatation in the treatment of achalasia: a randomised trialGut19994422312399895383
- PasrichaPJRaiRRavichWJHendrixTRKallooANBotulinum toxin for achalasia: long-term outcome and predictors of responseGastroenterology19961105141014158613045
- LambrozaASchumanRWPneumatic dilation for achalasia without fluoroscopic guidance: safety and efficacyAm J Gastroenterol1995908122612297639219
- ThomasVHarishKSunilkumarKPneumatic dilation of achalasia cardia under direct endoscopy: the debate continuesGastrointest Endosc200663473416564896
- VelaMFRichterJEKhandwalaFThe long-term efficacy of pneumatic dilatation and Heller myotomy for the treatment of achalasiaClin Gastroenterol Hepatol20064558058716630776
- CamposGMVittinghoffERablCEndoscopic and surgical treatments for achalasia: a systematic review and meta-analysisAnn Surg20092491455719106675
- TusetJALujanMHuguetJMCanellesPMedinaEEndoscopic pneumatic balloon dilation in primary achalasia: predictive factors, complications, and long-term follow-upDis Esophagus2009221747919021691
- EckardtAJEckardtVFCurrent clinical approach to achalasiaWorld J Gastroenterol200915323969397519705490
- GockelIJungingerTBernhardGEckardtVFHeller myotomy for failed pneumatic dilation in achalasia: how effective is it?Ann Surg2004239337137715075654
- FarhoomandKConnorJTRichterJEAchkarEVaeziMFPredictors of outcome of pneumatic dilation in achalasiaClin Gastroenterol Hepatol20042538939415118976
- EckardtVFAignherrCBernhardGPredictors of outcome in patients with achalasia treated by pneumatic dilationGastroenterology19921036173217381451966
- EckardtVFGockelIBernhardGPneumatic dilation for achalasia: late results of a prospective follow up investigationGut200453562963315082578
- DagliUKuranSSavasNFactors predicting outcome of balloon dilatation in achalasiaDig Dis Sci20095461237124218975085
- EckardtVFKanzlerGWestermeierTComplications and their impact after pneumatic dilation for achalasia: prospective long-term follow-up studyGastrointest Endosc19974553493539165313
- MetmanEHLagasseJPd’AlterocheLPiconLScottoBBarbieuxJPRisk factors for immediate complications after progressive pneumatic dilation for achalasiaAm J Gastroenterol19999451179118510235189
- VanuytselTLerutTCoosemansWConservative management of esophageal perforations during pneumatic dilation for idiopathic esophageal achalasiaClin Gastroenterol Hepatol201210214214922064041
- RichterJEUpdate on the management of achalasia: balloons, surgery and drugsExpert Rev Gastroenterol Hepatol20082343544519072391
- AliAPellegriniCALaparoscopic myotomy: technique and efficacy in treating achalasiaGastrointest Endosc Clin N Am2001112347358vii11319066
- RichardsWOTorquatiAHolzmanMDHeller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trialAnn Surg20042403405412 discussion 412–41515319712
- StefanidisDRichardsonWFarrellTMSAGES guidelines for the surgical treatment of esophageal achalasiaSurg Endosc201226229631122044977
- RawlingsASoperNJOelschlagerBLaparoscopic Dor versus Toupet fundoplication following Heller myotomy for achalasia: results of a multicenter, prospective, randomized-controlled trialSurg Endosc2012261182621789646
- MelvinWSNeedlemanBJKrauseKRWolfRKMichlerREEllisonECComputer-assisted robotic heller myotomy: initial case reportJ Laparoendosc Adv Surg Tech A200111425125311569517
- SweetMPNipomnickIGasperWJThe outcome of laparoscopic Heller myotomy for achalasia is not influenced by the degree of esophageal dilatationJ Gastrointest Surg200812115916517710504
- MineoTCAmbrogiVLong-term results and quality of life after surgery for oesophageal achalasia: one surgeon’s experienceEur J Cardiothorac Surg20042561089109615145014
- GlatzSMRichardsonJDEsophagectomy for end stage achalasiaJ Gastrointest Surg20071191134113717623258
- KadakiaSCWongRKPneumatic balloon dilation for esophageal achalasiaGastrointest Endosc Clin N Am2001112325346vii11319065
- OrtegaJAMadureriVPerezLEndoscopic myotomy in the treatment of achalasiaGastrointest Endosc19802618107358270
- PasrichaPJHawariRAhmedISubmucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasiaEndoscopy200739976176417703382
- InoueHMinamiHKobayashiYPeroral endoscopic myotomy (POEM) for esophageal achalasiaEndoscopy201042426527120354937
- Lujan-SanchisMSuarez-CallolPMonzo-GallegoAManagement of primary achalasia: the role of endoscopyWorld J Gastrointest Endosc20157659360526078828
- von RentelnDInoueHMinamiHPeroral endoscopic myotomy for the treatment of achalasia: a prospective single center studyAm J Gastroenterol2012107341141722068665
- BhayaniNHKurianAADunstCMSharataAMRiederESwanstromLLA comparative study on comprehensive, objective outcomes of laparoscopic Heller myotomy with per-oral endoscopic myotomy (POEM) for achalasiaAnn Surg201425961098110324169175
- StavropoulosSNModayilRJFriedelDSavidesTThe International Per Oral Endoscopic Myotomy Survey (IPOEMS): a snapshot of the global POEM experienceSurg Endosc20132793322333823549760
- BecharaRIkedaHInoueHPeroral endoscopic myotomy: an evolving treatment for achalasiaNat Rev Gastroenterol Hepatol201512741042626035678
- StavropoulosSNDesiletsDJFuchsKHPeroral endoscopic myotomy white paper summarySurg Endosc20142872005201924935204
- TalukdarRInoueHReddyDNEfficacy of peroral endoscopic myotomy (POEM) in the treatment of achalasia: a systematic review and meta-analysisSurg Endosc201529113030304625539695
- ChengYSMaFLiYDTemporary self-expanding metallic stents for achalasia: a prospective study with a long-term follow-upWorld J Gastroenterol201016405111511720976849
- SharmaPKozarekRPractice Parameters Committee of American College of GRole of esophageal stents in benign and malignant diseasesAm J Gastroenterol20101052258273 quiz 27420029413
- ZhaoJGLiYDChengYSLong-term safety and outcome of a temporary self-expanding metallic stent for achalasia: a prospective study with a 13-year single-center experienceEur Radiol20091981973198019296113
- LiYDChengYSLiMHChenNWChenWXZhaoJGTemporary self-expanding metallic stents and pneumatic dilation for the treatment of achalasia: a prospective study with a long-term follow-upDis Esophagus201023536136720353447
- MoretoMOjembarrenaEBarturenACasadoITreatment of achalasia by injection of sclerosant substances: a long-term reportDig Dis Sci201358378879623179151
- NiknamRMikaeliJMehrabiNEthanolamine oleate in resistant idiopathic achalasia: a novel therapyEur J Gastroenterol Hepatol201123121111111521971376
- RichterJEEsophageal motility disorder achalasiaCurr Opin Otolaryngol Head Neck Surg201321653554224136218
- LeydenJEMossACMacMathunaPEndoscopic pneumatic dilation versus botulinum toxin injection in the management of primary achalasiaCochrane Database Syst Rev20064CD00504617054234
- WangLLiYMLiLMeta-analysis of randomized and controlled treatment trials for achalasiaDig Dis Sci200954112303231119107596
- MikaeliJBishehsariFMontazeriGInjection of botulinum toxin before pneumatic dilatation in achalasia treatment: a randomized-controlled trialAliment Pharmacol Ther200624698398916948810
- ZhuQLiuJYangCClinical study on combined therapy of botulinum toxin injection and small balloon dilation in patients with esophageal achalasiaDig Surg200926649349820090338
- BoeckxstaensGThe European experience of achalasia treatmentGastroenterol Hepatol (N Y)20117960961122299000
- WeberCEDavisCSKramerHJGibbsJTRoblesLFisichellaPMMedium and long-term outcomes after pneumatic dilation or laparoscopic Heller myotomy for achalasia: a meta-analysisSurg Laparosc Endosc Percutan Tech201222428929622874676
- PandolfinoJEKwiatekMANealisTBulsiewiczWPostJKahrilasPJAchalasia: a new clinically relevant classification by high-resolution manometryGastroenterology200813551526153318722376
- RohofWOSalvadorRAnneseVOutcomes of treatment for achalasia depend on manometric subtypeGastroenterology20131444718725 quiz e713–e71423277105
- GhoshalUCRanganMA review of factors predicting outcome of pneumatic dilation in patients with achalasia cardiaJ Neurogastroenterol Motil201117191321369487
- BoeckxstaensGEZaninottoGRichterJEAchalasiaLancet20143839911839323871090
- PerssonJJohnssonEKosticSLundellLSmedhUTreatment of achalasia with laparoscopic myotomy or pneumatic dilatation: long-term results of a prospective, randomized studyWorld J Surg201539371372025409838