Abstract
Bariatric surgery is currently the most effective option for the treatment of morbid obesity and its associated comorbidities. Recent clinical and experimental findings have challenged the role of mechanical restriction and caloric malabsorption as the main mechanisms for weight loss and health benefits. Instead, other mechanisms including increased levels of satiety gut hormones, altered gut microbiota, changes in bile acid metabolism, and/or energy expenditure have been proposed as explanations for benefits of bariatric surgery. Beside the standard proximal Roux-en-Y gastric bypass and the biliopancreatic diversion with or without duodenal switch, where parts of the small intestine are excluded from contact with nutrients, resectional techniques like the sleeve gastrectomy (SG) have recently been added to the armory of bariatric surgeons. The variation of weight loss and glycemic control is vast between but also within different bariatric operations. We surveyed members of the Swiss Society for the Study of Morbid Obesity and Metabolic Disorders to assess the extent to which the phenotype of patients influences the choice of bariatric procedure. Swiss bariatric surgeons preferred Roux-en-Y gastric bypass and SG for patients with type 2 diabetes mellitus and patients with a body mass index >50 kg/m2, which is consistent with the literature. An SG was preferred in patients with a high anesthetic risk or previous laparotomy. The surgeons’ own experience was a major determinant as there is little evidence in the literature for this approach. Although trends will come and go, evidence-based medicine requires a rigorous examination of the proof to inform clinical practice.
Introduction
Previously, it was thought that caloric malabsorption and food restriction were important mechanisms determining the outcome of bariatric surgery. However, both preclinical and clinical studies revealed that complex physiological changes lead to body weight loss and beneficial metabolic changes after bariatric surgeries, such as Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). Mechanisms include altered levels of satiety gut hormones,Citation1,Citation2 alterations in energy expenditure,Citation3–Citation7 changes in bile acid levels,Citation8–Citation11 and modified gut microbiota.Citation12–Citation14 “Association” should, however, not be confused with “causation” as the measurable changes in circulating parameters after bariatric surgery do not necessarily infer a causal relationship with the benefits of surgery. It remains unclear whether these mechanisms alone or in combination with others are necessary or sufficient for reduced food intake or body weight loss.
There are substantial variations as regards metabolic efficacy between and within different surgical techniques, which can complicate clinical decision-making. This review focusses on the most common bariatric procedures and summarizes potential underlying physiological mechanisms. Moreover, we will report on a survey among clinicians in Switzerland as to which surgical technique they prefer in different scenarios. The findings are then presented within the framework of currently available evidence.
Common bariatric surgical techniques
Currently, RYGB and SG are the most commonly performed bariatric surgeries worldwide.Citation15 Gastric banding (GB) has lost a lot of its popularity within the last 10 years, whereas biliopancreatic diversion with or without duodenal switch remains the operation of choice in only a few international centers.Citation15 The latter two procedures will therefore not be discussed in detail in this review.
Currently, bariatric procedures are almost always performed laparoscopically.Citation15 For the RYGB, the stomach is divided into a proximal gastric pouch with a volume of about 15–30 mL and a distal gastric remnant (please see for preoperative anatomy ). Similarly, the jejunum is transected approximately 50 cm distal to the ligament of Treitz, creating a proximal and distal end of jejunum. An anastomosis between the distal end of the jejunum and the gastric pouch (gastrojejunostomy) creates the so-called alimentary limb. The proximal end of the jejunum, still continuous with the gastric remnant, constitutes the so-called biliopancreatic limb and is anastomosed with the small bowel approximately 150 cm distal from gastrojejunostomy leading to formation of the common channel. Thus, after completion of the standard Roux-en-Y reconstruction, there is an alimentary limb of 100–150 cm, a biliopancreatic limb of 50 cm, and a common channel of variable length, typically 300–500 cm ().Citation16
Figure 1 Schematic illustration of the preoperative (A) and postoperative anatomy of the gastrointestinal tract after a Roux-en-Y gastric bypass (B), and sleeve gastrectomy (C).
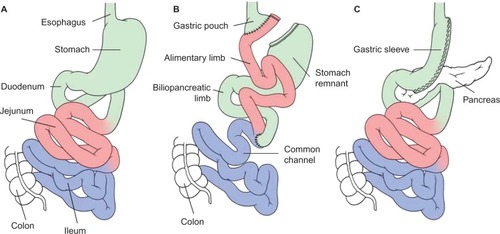
For the SG, the stomach is stapled along the big curvature creating a gastric tube of a volume of around 100–200 mL leading to an accelerated gastric emptying ().Citation17
Underlying physiological mechanisms of bariatric surgery
For decades, the body-weight-lowering effects of bariatric procedures were mainly attributed to mechanical restriction and caloric malabsorption. However, an increasing body of evidence questions the contribution of these anatomical and mechanical explanations. Recent findings suggest the importance of physiological mechanisms including changes in gastrointestinal hormone levels,Citation1,Citation2,Citation18,Citation19 bile acid metabolism,Citation8–Citation11 gut microbiota,Citation13,Citation14,Citation20,Citation21 and energy expenditure,Citation3–Citation7 among others. Key findings pertaining to these mechanisms will be briefly introduced with a special focus on the RYGB procedure, which is the most commonly performed and studied bariatric surgery worldwide. When possible and appropriate, reference will also be made to the SG.
Changes in gut hormones
The most consistent findings after RYGB in patients and animal models of RYGB are the increased secretion of satiety gut hormones after RYGB surgery.Citation1,Citation2,Citation18,Citation19,Citation22,Citation23 Several studies focused on altered levels of gut hormones that are known to affect food intake and to modulate nutrient metabolism.Citation24–Citation26 Insights into the role of gut hormones in the control of eating behavior has led to their therapeutic exploitation as possible antiobesity drugs.Citation27
Glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) responses are elevated postprandially after RYGB and SG.Citation2,Citation26,Citation28–Citation30 Circulating GLP-1 and PYY are released by the enteroendocrine L-cells when in direct contact with nutrients or bile acids in the gastrointestinal tract.Citation31,Citation32 When RYGB patients are divided into “good and poor responders”, those with more weight loss had significantly higher postprandial GLP-1 and PYY responses.Citation33 To examine causation of the elevated gut hormone response, blockade of these responses with somatostatin analogs resulted in increased food intake in three groups, including RYGB patients, rats after RYGB, and patients after esophagectomy with gastric conduit reconstruction (which mimics SG in both anatomy and physiology).Citation2,Citation34,Citation35 However, although blockade of all postprandial gut hormones led to doubling of food intake, the amount of calories consumed was still lower than preoperative values, suggesting that other mechanisms may also play an important role.Citation2,Citation36
The underlying mechanisms of an increase in postprandial GLP-1 and PYY L-cell secretion after RYGB surgery remain controversial. One explanation is the exposure of the distal gut lumen to higher concentrations of undigested nutrients as distal parts of the small intestine exhibit a higher mucosal L-cell density.Citation37–Citation39 Alternatively, increased L-cell secretions may also be due to enhanced stimulation of L-cells by bile acids or even gut microbial interactions.Citation31,Citation32
However, measurable changes in circulating parameters after bariatric surgery do not necessarily mean that these hormones have a causal role.Citation40 In other words, it is not yet clear whether hormonal changes alone or in combination with other mechanisms are necessary or sufficient for reduced food intake or body weight. Most efforts trying to establish a causal role for single hormones in RYGB-mediated effects have failed so far. For example, inhibition of a single gut hormone appears insufficient as the efficacy of RYGB is unaffected in GLP-1 receptor-knockout mice.Citation41 Moreover, central blockade of GLP-1 receptors as well as blockade of PYY receptors also did not affect food intake in either sham-operated or RYGB-operated rats.Citation41 Thus, there remains a certain level of uncertainty regarding the causal role of gut hormones for the observed changes in eating behavior after bariatric surgery, and it becomes evident that many different – partly unknown – factors may underlie the effects of RYGB surgery.
Bile acids
Levels of systemic bile acid concentrations are decreased in obese individuals.Citation42 Bile acids act through various receptors, including the FXR and the TGR5, to influence nutrient metabolism and energy expenditure. RYGB normalizes postprandial plasma bile acid responses,Citation42 whereas systemic bile acid levels increase massively and may therefore be responsible for some RYGB-induced effects.Citation8–Citation10,Citation43 Increased bile acids may also explain the increased secretion of GLP-1 and PYY after RYGB via TGR5 receptors located in the luminal membrane of L-cells.Citation44 However, TGR5 stimulation would require a higher bile acid concentration in the intestinal lumen, which has not yet been shown.Citation45 A causal contribution of bile acids to increased L-cell secretion after RYGB has also not yet been tested, and specific antagonists of TGR5 are currently not available. Experiments with TGR5 knockout mice undergoing RYGB are still awaited.
As bile acids increase energy expenditure in skeletal muscle and in brown adipose tissue,Citation46 and because circulating bile acids are strongly correlated with postprandial energy expenditure in lean individuals,Citation47 altered bile acid levels may contribute to changes in postprandial energy expenditure after RYGB.
In SG-operated mice, bile acid signaling via the FXR has been suggested to be a key determinant of body weight loss and glucose homeostasis.Citation11 So far, similar studies have not yet been performed after RYGB.
Gut microbiota
Numerous studies in recent years have shown that metabolic processes mediated by the gut microbiome play an important role in whole-body metabolism in health and disease.Citation12–Citation14,Citation48–Citation50 The observation that fecal transfer of gut microbiota from obese donors can induce body weight gain in lean recipients has led to intensive research activities in recent years to investigate the role of the gut microbiome on the observed metabolic effects after bariatric surgery.Citation50 Changes in gut microbiota seem to directly contribute to reduced body weight and obesity after RYGB as transfer of gut microbiota from RYGB-treated mice to nonoperated, germ-free mice resulted in body weight loss and decreased fat mass in the recipient animals.Citation13 In humans, variations of gut microbiota after RYGB are associated with changes in the expression of various genes in white adipose tissue, indicating a direct link between these two processes.Citation21,Citation51,Citation52 Furthermore, postbariatric changes of the gut microbiome occur independently of body mass index (BMI) and result in altered levels of fecal and circulating metabolites compared with obese controls.Citation14 By colonizing germ-free mice with stools from the patients, the RYGB-altered microbiota promoted reduced fat deposition in recipient mice.Citation14
An interaction between the gut microbiota and bile acid metabolism is probable as bile acids have known antibacterial effects. In addition, gut microbiota is known to be able to chemically modify endogenous bile acids to a number of different chemical species with different affinities at different bile acid receptors.Citation53
Energy expenditure
Changes in energy expenditure after RYGB in humans are controversial.Citation6,Citation7,Citation54,Citation55 Thivel et alCitation56 recently reviewed the available data on energy expenditure after bariatric surgery and concluded that the total energy expenditure in humans often decreases postoperatively due to the decrease in fat-free and fat mass, but that postprandial diet-induced thermogenesis is often increased. A few human studies suggest an increase in total energy expenditure after RYGB.Citation6,Citation7 Discrepancy in findings may be explained by the short duration of energy expenditure measurements and by the subsequent extrapolation of results to a 24-hour period; hence, true effects may have been missed. The necessary control groups were also not always included in the studies.
Compared to the human studies, data in rats and mice are more consistent, where RYGB seems to prevent or at least reduce the usual adaptive response (also known as “starvation response”), ie, body weight loss in RYGB rats is not associated with the same decrease in reduced energy expenditure that parallels weight loss by food restriction.Citation3–Citation5
RYGB and SG often produce similar changes in metabolism,Citation57,Citation58 but data indicate that changes in energy expenditure may not be an important contributor to body weight loss after SG.Citation59,Citation60 Furthermore, both clinical and animal studies have reported at least regain of body weight after SG, which could be explained by differences in postoperative energy expenditure between RYGB and SG.Citation60,Citation61
The physiological mechanisms underlying altered energy expenditure after RYGB are only incompletely understood, although it seems evident that neither an increased spontaneous physical activity nor a general increase in body temperature due to postoperative inflammation plays a role.Citation3 Instead, potential mechanisms include changes in gut hormone levels,Citation3 altered brown adipose tissue activity,Citation62 altered energy efficiency of skeletal muscle, or a higher energy requirement of hypertrophied small intestine.Citation3,Citation4,Citation31,Citation32,Citation63
Survey
Surgical and nonsurgical members (n=177) of the Swiss Society for the Study of Morbid Obesity and Metabolic Disorders representing 52 bariatric centers in Switzerland were formally invited by email to participate in the survey and to complete a nonvalidated, self-designed questionnaire. All responses that were received within 3 weeks after the initial contact were included in the analysis. In the questionnaire, participants were asked to indicate which of the following clinical conditions impacts their surgical treatment of choice to which degree (0 – no impact, 1 – minor impact, 3 – major impact):
Difficult to treat type 2 diabetes mellitus (T2DM), eg, high dosages of insulin or high HbA1c levels
BMI >50 kg/m2
Concomitant hiatal herniation
Open abdominal surgery in patients’ previous medical history
Increased anesthetic risk, ie, American Society of Anesthesiologists (ASA) >III.
About 79% of participants regarded the presence of severe T2DM as major and 21% as minor importance for their choice of surgical technique. Of these, the majority reported a preference for RYGB (n=20) over a BPD (n=12) or SG (n=7). None of the participants indicated GB as the operation of choice in this situation. A BMI >50 kg/m2 was considered to be of major importance by 66% of participants, whereas 33% regarded it as being of minor importance. In this situation, the majority of participants reported that they prefer an SG as their first option (n=19) over an RYGB (n=10) or a BPD (n=9). Again, none of the participants suggested GB.
In total, 33% participants reported that the presence of a hiatal hernia has major impact on their choice of surgical technique, whereas 58% consider the presence of hiatal herniation as less important and 8% as not important at all for their decision. Of these, the majority of responders considered an RYGB as their preferred option (n=18), whereas only five and three participants, respectively, consider an SG or BPD in this situation. None of the participants considered a GB for patients with hiatal herniation.
Previous open abdominal surgery was regarded to be of minor importance for decision-making by 63% of participants, whereas 33% attach no impact and 4% attach major impact to this clinical condition. However, the majority of responders preferred an SG (n=16), whereas eight participants considered an RYGB and only one participant a BPD as potential options. In addition, two colleagues considered a GB as a potential option.
Finally, an increased anesthetic risk indicated by an ASA score >III was given major importance by 42% and minor importance by 46% of the participants. Interestingly, 13% of the participants consider anesthetic risk as not important at all. In patients with increased anesthetic risk (ASA >III), most participants chose SG (n=16), whereas fewer participants considered an RYGB (n=8), a GB (n=2), or a BPD operation (n=1) as the preferred option.
Current evidence to support results of the survey
Type 2 diabetes mellitus
Bariatric surgery has been shown to induce and maintain normoglycemia in obese patients suffering from T2DM more effectively than any present medical therapy alone for 5 years.Citation64–Citation67 The results obtained from our survey suggest that bariatric surgeons consider severe T2DM as an important factor when it comes to the choice of bariatric procedure. However, the question of whether one single bariatric procedure is superior to all other procedures in patients with T2DM is challenging as many factors need to be considered.
The best evidence for T2DM remission is available for RYGB and SG. In the study of Schauer et al,Citation66,Citation67 both bariatric surgeries improved glycemic control; however, the number of patients with normal HbA1c without antidiabetic medication was significantly higher 3 years after RYGB than after SG (28 vs 16, P>0.01).Citation66 RYGB was also more effective than SG in terms of HbA1c reduction and partial remission of T2DM.Citation68 Other randomized controlled trials showed glycemic control to a similar extent for both surgical procedures.Citation57,Citation69–Citation73
Jimenez et alCitation74 performed continuous glucose monitoring after ingestion of a standardized liquid mixed meal. They observed an earlier and higher peak of serum glucose levels in RYGB patients. Moreover, RYGB patients spent more time in a hypoglycemic state than patients after SG.Citation74 Both high glucose peaks as well as the fast changes from hyper- to hypoglycemic state are considered undesirable, as such an abnormal postprandial glucose profile may be associated with higher rates of relapse of diabetes over time. However, this has not been shown so far. In contrast, recent data suggest that relapse of diabetes might be higher after SG than after RYGB.Citation66
Interestingly, 50% of all bariatric surgeons in our survey considered BPD in case of severe metabolic disorder. However, BPD is only performed by a few surgeons due to an increased risk for surgical complications and long-term side effects such as diarrhea or micronutrient deficiencies.Citation75 Although the study of Mingrone et alCitation65 was primarily designed to compare medical with surgical treatment, it revealed that the remission rate of T2DM was higher and that relapse of hyperglycemia was lower 5 years after BPD than after RYGB.Citation65
In contrast to RYGB and SG, GB has lost much of its initial attraction, although a randomized controlled trial by Dixon et alCitation76 showed that 73% of recently diagnosed patients with T2DM achieve diabetic remission 2 years after their GB operation.
In summary, the evidence that bariatric surgery alone or in combination with best medical care is superior for the treatment of T2DM when compared to best medical care alone is undisputed.Citation77 RYGB and SG seem to offer the best risk–benefit ratio, as other procedures such as BPD and GB are characterized in some setting by high complication rates and/or high numbers of reoperations.Citation30
Severe obesity
Morbidly obese patients loose more weight after RYGB and SG operations than after laparoscopic adjustable GB, irrespective of their initial BMI.Citation78–Citation81 A multivariate regression analysis of the Bariatric Outcomes Longitudinal Database including 31 443 laparoscopic adjustable GB, 40 352 RYGB, and 2 194 SG patients demonstrated that the type of bariatric surgery is the most important predictor of postoperative weight loss. Weight loss was highest after RYGB across all levels of body weight and significantly lower after GB. SG was underrepresented in this study, and thus comparison with RYGB was not possible.Citation78 BPD was also not included in this analysis.
A systematic review including studies with >100 patients published before 2005 revealed that banded RYGB, a modification of RYGB with placement of a nonadjustable or adjustable band around the gastric pouch, and BPD showed the best results in terms of weight loss 3 years after surgery. The lack of long-term follow-up data and data of SG are important limitations of this study.Citation82
SG, RYGB, and BPD are more effective in inducing and maintaining weight loss in patients with BMI >50 kg/m2, which is consistent with the results of our survey. Despite the fact that the existing evidence suggests a greater body weight loss after RYGB than after SG, the majority of bariatric surgeons in our survey favored SG for superobese patients, possibly because of technical considerations. The creation of gastrojejunostomy, which has to be performed for RYGB, can be challenging in this subgroup due to a large left liver lobeCitation83,Citation84 or accentuated visceral fat.Citation85 Moreover, an SG can still be converted into an RYGB, if primary weight loss is insufficient or weight regain occurs.Citation83 However, surgeons seem to place more importance on perceived safety and the avoidance of possible postsurgical side effects as one important guiding factor for the choice of the procedures.
Concomitant hiatal hernia
The prevalence of gastroesophageal reflux disease (GERD) in patients with morbid obesity is as high as 45%.Citation86 GERD in morbidly obese patients is often associated or explained by the presence of a hiatal hernia.Citation87 Hiatal hernias can be expected in up to 38.5% of patients undergoing bariatric surgery.Citation88 The effect of different bariatric procedures on the resolution of GERD was investigated by Pallati et al,Citation89 who found that the Savary–Miller GERD score improved after all procedures, with the best results being obtained after RYGB (56% of patients) when compared to GB (46%) and SG (41%). GERD symptoms in some SG patients deteriorated, whereas RYGB and Nissen fundoplication were found to be equally effective for the treatment of GERD in obese patients with hiatal hernia.Citation90 Nearly all the participants of our survey considered hiatal hernia before deciding on the surgical technique.
Previous laparotomy
As every previous laparotomy increases the risk and extent of intra-abdominal adhesions,Citation91 the technical difficulty and subsequent risk for complications of a laparoscopic approach may increase, too. However, the way this influences the surgeons’ choice has never been formally investigated in the context of bariatric surgery. A retrospective study of 139 patients who underwent laparoscopy-assisted gastrectomy for early gastric cancer demonstrated that history of laparotomy was not associated with increased postoperative complications.Citation92 However, 80% of these previous laparotomies were due to appendectomies and gynecological operations. Moreover, operations were performed in a lean population; thus, the results may not be representative for a bariatric population where surgery is mainly limited to the upper gastrointestinal tract.
Our survey showed that a history of previous laparotomies impacts on the choice of bariatric procedure, although it was regarded only as a minor factor. The majority of participating surgeons favored SG, when faced with (several) previous laparotomies, probably intending to avoid complex adhesiolysis of the lower abdomen, which might be necessary for the lower anastomosis in case of RYGB or BPD.
Increased anesthesiological risk (ASA >III)
ASA class ≥III is an independent predictor of postoperative complications after RYGB.Citation93,Citation94 Interestingly, postoperative pulmonary complications differ significantly between RYGB (1.3%), SG (0.84%), and GB (0.3%), which may reflect differences in the duration of surgery.Citation94
The majority of participants of our survey consider a high anesthetic risk when they decide on the type of bariatric surgery. The majority of surgeons tend to prefer SG when the ASA score is >III whereas a small group of surgeons opted for GB in this situation. Differences may be explained by the intention to reduce operation time, which may be shorter for SG than RYGB,Citation94 and also to reduce the risk for postoperative anastomotic complications. Of course, operation time for GB is the shortest.Citation94
Conclusion
Metabolic surgery is currently the most effective treatment for morbid obesity and its concomitant diseases. The armory should include RYGB, SG, BPD, and GB. Traditionally, the effects of bariatric surgery were explained by caloric malabsorption and mechanical restriction. However, animal and human studies challenged these mechanical explanations. Instead, other physiological mechanisms seem to be at play, such as alterations in gut hormone levels, changes in bile acid concentration, an altered composition of gut microbiota, as well as changes in energy expenditure. All these seem to contribute to the beneficial effects of bariatric surgery. The different effects of surgical procedures on body weight and comorbidities such as T2DM may be explained by the specific mechanisms of these operations, but more work is required to fully understand each contribution.
A survey among Swiss surgeons and clinical practitioners revealed that T2DM and BMI are major criteria considered during the choice of bariatric surgeries. In case of T2DM, most of the participants prefer RYGB over BPD as it has fewer side effects, such as hypoproteinemia and diarrhea, although offering preferable improvements in terms of glycemic control. While T2DM remission rate seems slightly higher after RYGB, the smaller glucose excursions after food intake after SG may have benefits. Similarly, in case of a BMI >50 kg/m2, the risk–benefit ratio seems best for SG and RYGB. Technical considerations like a large left liver lobe and the option of a later conversion to RYGB may be considered as reasons to rather perform a SG. Surgeons seem to prioritize perceived safety and avoidance of postsurgical side effects before selecting the procedure.
The presence of GERD should encourage surgeons to perform RYGB because of its beneficial and weight- independent effects on GERD. Most participants of the survey prefer SG in cases of high anesthetic risk (ASA >III) and previous laparotomy in the patients’ history. In summary, choice of surgical procedure can be challenging as several competing factor requires consideration. Although consensus is emerging, no clear decision tree has been established as the underlying evidence is still lacking.
Disclosure
The authors report no conflicts of interests in this work.
References
- le RouxCWAylwinSJBatterhamRLGut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parametersAnn Surg2006243110811416371744
- le RouxCWWelbournRWerlingMGut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypassAnn Surg2007246578078517968169
- BueterMLowensteinCOlbersTGastric bypass increases energy expenditure in ratsGastroenterology201013851845185319931268
- SaeidiNMeoliLNestoridiEReprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypassScience2013341614440641023888041
- StylopoulosNHoppinAGKaplanLMRoux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese ratsObesity (Silver Spring)200917101839184719556976
- WerlingMFandriksLOlbersTRoux-en-Y gastric bypass surgery increases respiratory quotient and energy expenditure during food intakePLoS One2015106e012978426098889
- WerlingMOlbersTFandriksLIncreased postprandial energy expenditure may explain superior long term weight loss after Roux-en-Y gastric bypass compared to vertical banded gastroplastyPLoS One201384e6028023573244
- GlicksmanCPournarasDJWrightMPostprandial plasma bile acid responses in normal weight and obese subjectsAnn Clin Biochem201047Pt 548248420595403
- PournarasDJGlicksmanCVincentRPThe role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic controlEndocrinology201215383613361922673227
- PournarasDJle RouxCWAre bile acids the new gut hormones? Lessons from weight loss surgery modelsEndocrinology201315472255225623794408
- RyanKKTremaroliVClemmensenCFXR is a molecular target for the effects of vertical sleeve gastrectomyNature2014509749918318824670636
- LiJVAshrafianHBueterMMetabolic surgery profoundly influences gut microbial-host metabolic cross-talkGut20116091214122321572120
- LiouAPPaziukMLuevanoJMJrMachineniSTurnbaughPJKaplanLMConserved shifts in the gut microbiota due to gastric bypass reduce host weight and adipositySci Transl Med20135178178ra141
- TremaroliVKarlssonFWerlingMRoux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulationCell Metab201522222823826244932
- AngrisaniLSantonicolaAIovinoPFormisanoGBuchwaldHScopinaroNBariatric surgery worldwide 2013Obes Surg201525101822183225835983
- WeberMMullerMKBucherTLaparoscopic gastric bypass is superior to laparoscopic gastric banding for treatment of morbid obesityAnn Surg20042406975982 discussion 982–97315570203
- GumbsAAGagnerMDakinGPompASleeve gastrectomy for morbid obesityObes Surg200717796296917894158
- BueterMle RouxCWGastrointestinal hormones, energy balance and bariatric surgeryInt J Obes (Lond)201135Suppl 3S35S3921912386
- KornerJBesslerMCiriloLJEffects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulinJ Clin Endocrinol Metab200590135936515483088
- LiFJiangCKrauszKWMicrobiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesityNat Commun20134238424064762
- OstoMAbeggKBueterMle RouxCWCaniPDLutzTARoux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestinePhysiol Behav2013119929623770330
- LaferrereBHeshkaSWangKIncretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetesDiabetes Care20073071709171617416796
- LutzTABueterMThe physiology underlying Roux-en-Y gastric bypass: a status reportAm J Physiol Regul Integr Comp Physiol201430711R1275R129125253084
- AsarianLBachlerTNeuroendocrine control of satiationHorm Mol Biol Clin Investig2014193163192
- AtkinsonRLBrentELAppetite suppressant activity in plasma of rats after intestinal bypass surgeryAm J Physiol19822431R60R647091396
- PournarasDJOsborneAHawkinsSCThe gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective studyObes Surg2010201566019826888
- BeharyPCeglaJTanTMBloomSRObesity: lifestyle management, bariatric surgery, drugs, and the therapeutic exploitation of gut hormonesPostgrad Med2015127549450226040552
- le RouxCWBatterhamRLAylwinSJAttenuated peptide YY release in obese subjects is associated with reduced satietyEndocrinology200614713816166213
- ShinACZhengHTownsendRLSigaletDLBerthoudHRMeal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgeryEndocrinology201015141588159720179262
- DimitriadisEDaskalakisMKampaMPeppeAPapadakisJAMelissasJAlterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational studyAnn Surg2013257464765423108120
- HansenCFBueterMTheisNHypertrophy dependent doubling of L-cells in Roux-en-Y gastric bypass operated ratsPLoS One201386e6569623776529
- MumphreyMBPattersonLMZhengHBerthoudHRRoux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in ratsNeurogastroenterol Motil2013251e70e7923095091
- DirksenCJorgensenNBBojsen-MollerKNGut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypassInt J Obes (Lond)201337111452145923419600
- FenskeWKBueterMMirasADGhateiMABloomSRle RouxCWExogenous peptide YY3-36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypassInt J Obes (Lond)2011
- ElliottJAJacksonSKingSGut hormone suppression increases food intake after esophagectomy with gastric conduit reconstructionAnn Surg2015262582483026583672
- LaureniusALarssonIMelansonKJDecreased energy density and changes in food selection following Roux-en-Y gastric bypassEur J Clin Nutr201367216817323299713
- AdrianTEGariballaSParekhKARectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteersDiabetologia20125592343234722696033
- HirasawaATsumayaKAwajiTFree fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120Nat Med2005111909415619630
- ThomasCGioielloANoriegaLTGR5-mediated bile acid sensing controls glucose homeostasisCell Metab200910316717719723493
- TadrossJAle RouxCWThe mechanisms of weight loss after bariatric surgeryInt J Obes200933Suppl 1S28S32
- YeJHaoZMumphreyMBGLP-1 receptor signaling is not required for reduced body weight after RYGB in rodentsAm J Physiol Regul Integr Comp Physiol20143065R352R36224430883
- AhmadNNPfalzerAKaplanLMRoux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesityInt J Obes2013371215531559
- KohliRMyronovychATanBKBile acid signaling: mechanism for bariatric surgery, cure for NASH?Dig Dis201533344044626045281
- DubocHTacheYHofmannAFThe bile acid TGR5 membrane receptor: from basic research to clinical applicationDig Liver Dis201446430231224411485
- ParkerHEWallisKle RouxCWWongKYReimannFGribbleFMMolecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretionBr J Pharmacol2012165241442321718300
- WatanabeMHoutenSMMatakiCBile acids induce energy expenditure by promoting intracellular thyroid hormone activationNature2006439707548448916400329
- OckengaJValentiniLSchuetzTPlasma bile acids are associated with energy expenditure and thyroid function in humansJ Clin Endocrinol Metab201297253554222162464
- BackhedFDingHWangTThe gut microbiota as an environmental factor that regulates fat storageProc Natl Acad Sci U S A200410144157181572315505215
- LiJVReshatRWuQExperimental bariatric surgery in rats generates a cytotoxic chemical environment in the gut contentsFront Microbiol2011218321949514
- TurnbaughPJLeyREMahowaldMAMagriniVMardisERGordonJIAn obesity-associated gut microbiome with increased capacity for energy harvestNature200644471221027103117183312
- FuretJPKongLCTapJDifferential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markersDiabetes201059123049305720876719
- KongLCTapJAron-WisnewskyJGut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genesAm J Clin Nutr2013981162423719559
- CamilleriMGoresGJTherapeutic targeting of bile acidsAm J Physiol Gastrointest Liver Physiol20153094G20921526138466
- CarrascoFPapapietroKCsendesAChanges in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypassObes Surg200717560861617658019
- DasSKRobertsSBMcCroryMALong-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgeryAm J Clin Nutr2003781223012816767
- ThivelDBrakoniekiKDuchePMorioBBoirieYLaferrereBSurgical weight loss: impact on energy expenditureObes Surg201323225526623224568
- PeterliRBorbelyYKernBEarly results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypassAnn Surg20132585690694 discussion 69523989054
- PeterliRSteinertREWoelnerhanssenBMetabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trialObes Surg201222574074822354457
- SaeidiNNestoridiEKucharczykJUygunMKYarmushMLStylopoulosNSleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in obese ratsInt J Obes (Lond)201236111396140223044855
- StefaterMAPerez-TilveDChambersAPSleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivityGastroenterology20101387242624362436 e2421242320226189
- AlvarezVCarrascoFCuevasAMechanisms of long-term weight regain in patients undergoing sleeve gastrectomyNutrition201632330330826611808
- HankirMBueterMGsellWIncreased energy expenditure in gastric bypass rats is not caused by activated brown adipose tissueObes Facts20125334935822722324
- le RouxCWBorgCMWallisKGut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferationAnn Surg20102521505620562614
- MingroneGPanunziSDe GaetanoABariatric surgery versus conventional medical therapy for type 2 diabetesN Engl J Med2012366171577158522449317
- MingroneGPanunziSDe GaetanoABariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trialLancet2015386999796497326369473
- SchauerPRBhattDLKirwanJPBariatric surgery versus intensive medical therapy for diabetes – 3-year outcomesN Engl J Med20143702002201324679060
- SchauerPRKashyapSRWolskiKBariatric surgery versus intensive medical therapy in obese patients with diabetesN Engl J Med2012366171567157622449319
- ChangSHStollCRSongJVarelaJEEagonCJColditzGAThe effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012JAMA Surg2014149327528724352617
- HelmioMVictorzonMOvaskaJComparison of short-term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: A prospective randomized controlled multicenter SLEEVEPASS study with 6-month follow-upScand J Surg2014103317518124522349
- KaramanakosSNVagenasKKalfarentzosFAlexandridesTKWeight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind studyAnn Surg2008247340140718376181
- KehagiasIKaramanakosSNArgentouMKalfarentzosFRandomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI <50 kg/m2Obes Surg201121111650165621818647
- KeidarAHershkopKJMarkoLRoux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trialDiabetologia20135691914191823765186
- PaluszkiewiczRKalinowskiPWroblewskiTProspective randomized clinical trial of laparoscopic sleeve gastrectomy versus open Roux-en-Y gastric bypass for the management of patients with morbid obesityWideochir Inne Tech Maloinwazyjne20127422523223362420
- JimenezACerielloACasamitjanaRFloresLViaplana-MasclansJVidalJRemission of type 2 diabetes after Roux-en-Y gastric bypass or sleeve gastrectomy is associated with a distinct glycemic profileAnn Surg2015261231632225569030
- SovikTTTahaOAasheimETRandomized clinical trial of laparoscopic gastric bypass versus laparoscopic duodenal switch for superobesityBr J Surg201097216016620035530
- DixonJBO’BrienPEPlayfairJAdjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trialJAMA2008299331632318212316
- FischerLWekerleALBrucknerTBariSurg trial: Sleeve gastrectomy versus Roux-en-Y gastric bypass in obese patients with BMI 35–60 kg/m(2) – a multi-centre randomized patient and observer blind non-inferiority trialBMC Surg2015158726187377
- BenoitSCHunterTDFrancisDMDe La Cruz-MunozNUse of bariatric outcomes longitudinal database (BOLD) to study variability in patient success after bariatric surgeryObes Surg201424693694324570089
- DaviesSWEfirdJTGuidryCATwenty-first century weight loss: banding versus bypassSurg Endosc201529494795425106724
- GiordanoSTolonenPVictorzonMLaparoscopic Roux-en-Y gastric bypass versus laparoscopic adjustable gastric banding in the super-obese: peri-operative and early outcomesScand J Surg201510415925623917
- FrancoJVRuizPAPalermoMGagnerMA review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric bandingObes Surg20112191458146821455833
- O’BrienPEMcPhailTChastonTBDixonJBSystematic review of medium-term weight loss after bariatric operationsObes Surg20061681032104016901357
- NguyenNTLongoriaMGelfandDVSabioAWilsonSEStaged laparoscopic Roux-en-Y: a novel two-stage bariatric operation as an alternative in the super-obese with massively enlarged liverObes Surg20051571077108116105411
- SchwartzMLDrewRLChazin-CaldieMFactors determining conversion from laparoscopic to open Roux-en-Y gastric bypassObes Surg20041491193119715527633
- NguyenNTRiversRWolfeBMFactors associated with operative outcomes in laparoscopic gastric bypassJ Am Coll Surg20031974548555 discussion 555–54714522321
- OgdenCLYanovskiSZCarrollMDFlegalKMThe epidemiology of obesityGastroenterology200713262087210217498505
- CheFNguyenBCohenANguyenNTPrevalence of hiatal hernia in the morbidly obeseSurg Obes Relat Dis20139692092423810611
- HeacockLParikhMJainRBalthazarEHindmanNImproving the diagnostic accuracy of hiatal hernia in patients undergoing bariatric surgeryObes Surg201222111730173323011460
- PallatiPKShaligramAShostromVKOleynikovDMcBrideCLGoedeMRImprovement in gastroesophageal reflux disease symptoms after various bariatric procedures: review of the Bariatric Outcomes Longitudinal DatabaseSurg Obes Relat Dis201410350250724238733
- PattersonEJDavisDGKhajancheeYSwanstromLLComparison of objective outcomes following laparoscopic Nissen fundoplication versus laparoscopic gastric bypass in the morbidly obese with heartburnSurg Endosc200317101561156512874685
- StrikCStommelMWTen BroekRPvan GoorHAdhesiolysis in patients undergoing a repeat median laparotomyDis Colon Rectum201558879279826163959
- NunobeSHikiNFukunagaTPrevious laparotomy is not a contraindication to laparoscopy-assisted gastrectomy for early gastric cancerWorld J Surg20083271466147218340481
- HutterMMRandallSKhuriSFHendersonWGAbbottWMWarshawALLaparoscopic versus open gastric bypass for morbid obesity: a multicenter, prospective, risk-adjusted analysis from the National Surgical Quality Improvement ProgramAnn Surg20062435657662 discussion 662–65616633001
- SchumannRShikoraSASiglJCKelleySDAssociation of metabolic syndrome and surgical factors with pulmonary adverse events, and longitudinal mortality in bariatric surgeryBr J Anaesth20151141839025311316
- LutzTBueterMWohin geht die Anti-Obesitas Therapie?Prakt Tierarzt2012931210871095
