Abstract
Background
Ingestion of low-digestible carbohydrates triggers symptoms in patients with irritable bowel syndrome (IBS). These carbohydrates become substrates for microbial fermentation in the colon, yielding short-chain fatty acids (SCFAs) that are readily absorbed. Aiming to compare colonic fermentation in patients with IBS and healthy controls, we analyzed the concentrations of SCFA in serum at fasting and 90 minutes following ingestion of an unabsorbable, but fermentable carbohydrate, lactulose.
Methods
Patients with IBS according to Rome III criteria (n=22) and healthy controls (n=20) ingested 10 g lactulose dissolved in water. Symptoms were graded by questionnaires and SCFA were analyzed using hollow fiber-supported liquid membrane extraction coupled with gas chromatography.
Results
Lactulose induced more symptoms in patients with IBS than in healthy controls (P=0.0001). Fasting serum levels of SCFA did not differ between patients with IBS and controls. However, the postprandial levels of total SCFA (P=0.0002), acetic acid (P=0.005), propionic acid (P=0.0001), and butyric acid (P=0.01) were significantly lower in patients with IBS compared with healthy controls. There was no correlation between the levels of serum SCFA and symptom severity.
Conclusion
Low-serum levels of SCFA after lactulose ingestion may indicate impaired colonic fermentation in patients with IBS. Conceivably, this disturbance is related to symptom generation, but the mechanism is not clear.
Introduction
The pathophysiology of irritable bowel syndrome (IBS) seems to involve disturbances at many levels of the so-called gut-brain axis.Citation1 Recent research has emphasized the role of food in abdominal symptom generation,Citation2 in particular low-digestible carbohydrates.Citation3 Our group has previously shown that the ingestion of the unabsorbable, but fermentable disaccharide lactulose provokes more symptoms in patients with IBS than in healthy individuals.Citation4,Citation5 Intriguingly, the symptoms provoked by lactulose were similar to the patients’ habitual complaints.Citation4 Fermentable carbohydrates that escape digestion and absorption within the small intestine are metabolized by colonic microbiota. HunterCitation6 has previously suggested that the disturbances of colonic fermentation play a significant role in IBS symptom generation, and has proposed the term “enterometabolic disorder” to describe such alterations. Colonic fermentation yields short-chain fatty acids (SCFAs; mainly acetic, propionic, and butyric acids) and gases (mainly CO2, H2, and CH4). Hence, the efficacy of the fermentation may be evaluated by SCFA analysis. Due to regional differences regarding the availability of fermentable substrates and abundance of saccharolytic microbes, fermentation of low-digestible carbohydrates takes place mainly in the cecum and the proximal colon, rather than in the distal parts.Citation7 A correlation has previously been demonstrated between cecal levels of SCFA and portal and aortic blood levels of SCFA in rats,Citation8 and assessment of SCFA in peripheral venous blood may be considered as an indirect measure of colonic fermentation.Citation9
The aim of the present study was to compare serum SCFA levels in patients with IBS and healthy controls before and after the ingestion of lactulose. Since a previous study has shown that the mean orocecal transit time of a 10 g lactulose solution is approximately 90 minutes,Citation10 and that the onset of symptoms after a meal often occurs within 90 minutes,Citation11 we chose this time point for evaluating the lactulose-stimulated response.
Patients and methods
Study participants
Patients with IBS were included consecutively from the outpatient clinic of one of the authors at Lovisenberg Diaconal Hospital (Oslo, Norway). The individuals were diagnosed with IBS according to the Rome III criteria,Citation12 and divided into subgroups according to phenotypes based on the Rome III questionnaire for functional bowel disorders: diarrhea predominant IBS, constipation predominant IBS, and IBS with equally diarrhea and constipation. Patients with organic diseases were excluded. Healthy controls were recruited among the hospital staff at Lovisenberg Diaconal Hospital. They were included if they considered themselves to be healthy, and were not otherwise medically examined. However, they were excluded if they met the Rome III criteria for IBS based on our questionnaires. All participants gave written, informed consent, and the study was carried out according to the Declaration of Helsinki and approved by the Regional Committee for Medical Research Ethics (REK Sør-Øst; reference number 2011/2474).
Lactulose challenge procedure
The participants met (individually) at the outpatient clinic at 08:00 in the morning after an overnight fast. Venous blood samples, from the antecubital vein of the non-dominant arm, were collected and the subjects filled in a questionnaire to quantify their habitual IBS symptoms. The participants then underwent a lactulose provocation test, as described previously.Citation4 Briefly, the subjects ingested 10 g of lactulose dissolved in 200 mL of tap water. Ninety minutes after the intake of the lactulose solution, another blood sample was collected, and the subjects filled in a questionnaire to grade their lactulose-induced symptoms. The participants were not allowed to drink, eat, or smoke during the test, but their activities were otherwise not restricted.
Symptom questionnaires
Habitual symptoms were assessed using the IBS severity scoring system.Citation13 The maximum achievable score of this system is 500 points, and the questionnaire allows grading of symptom severity as follows: mild (75–175 points), moderate (175–300 points), and severe (>300 points). Increase in symptoms following the lactulose ingestion, as compared to baseline, was graded from 0 to 3 for abdominal borborygmi, bloating, and pain, as well as a feeling of sickness and freezing, giving a maximum achievable sum score of 15 points.
Analysis of blood samples
After collection, the samples were centrifuged and the serum was immediately placed in a freezer at −20°C until analysis. Serum was thawed at room temperature and SCFA (acetic, propionic, butyric, iso-butyric, valeric, and iso-valeric acids) were analyzed by using a method developed by Zhao et al.Citation14 This method enables the detection of serum concentrations of SCFA down to as little as 0.04 µM, as described by Jakobsdottir et al.Citation9 All samples were analyzed at least in duplicate. In addition, the ratios of individual SCFA concentrations to the total SCFA concentrations were calculated. Based on the work of Tjellström et alCitation15 on fecal samples, we calculated two fermentation indices, as follows: fermentation index A, reflecting the fermentation of carbohydrates and conceivably the pro-inflammatory properties of SCFA, was calculated as acetic acid concentrations minus propionic and butyric acid concentrations divided by the total concentration of SCFA. Fermentation index B, reflecting the fermentation of proteins and conceivably the anti-inflammatory properties of SCFA was calculated as the sum of iso-butyric and iso-valeric acids.
Statistical analyses
Graphpad Prism version 6 (Graphpad Software Inc., San Diego, CA, USA) was used to analyze the data. Unless otherwise stated, the data are expressed as means and their SD. Paired t-test was used for comparisons within groups and unpaired t-tests for comparing means between groups. Correlations were assessed with Pearson’s correlation coefficient. To compare fractions, the Fishers exact test was conducted. All tests were two-tailed, and P-values less than 0.05 were set as a limit for statistical significance.
Results
Characteristics of study participants
A total of 22 patients with IBS and 20 healthy controls were included in the study. The groups were comparable regarding age, body mass index, and sex ().
Table 1 Characteristics of study participants
Symptoms following lactulose ingestion
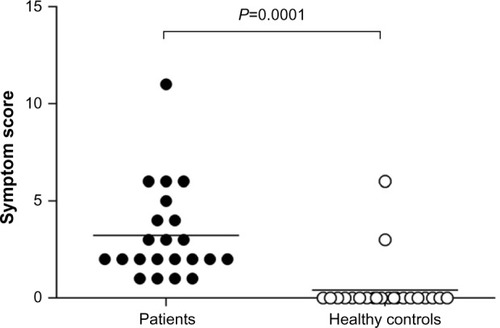
Patients with IBS reported more symptoms in response to lactulose ingestion than healthy controls (). Abdominal symptoms (borborygmi, bloating, and pain) were most pronounced, with a mean sum score of 3.2 points (maximum achievable score 9 points), while systemic symptoms (sickness and freezing) had a sum score of 2.2 points (maximum achievable score 6 points).
Figure 1 Symptom scores 90 minutes after lactulose ingestion in patients with IBS (n=22) and healthy individuals (n=20).
Abbreviation: IBS, irritable bowel syndrome.
Serum levels of SCFA
Levels of SCFA in serum obtained in the fasted state were not significantly different between patients with IBS and healthy controls, for any of the SCFA. The results (in µM) were as follows: total SCFA 249.6±63.9 vs 279.9±53.4 (P=0.1); acetic acid 172.5±48.9 vs 191.1±40.0 (P=0.2); pro-pionic acid 10.8±1.7 vs 11.5±2.3 (P=0.3); and butyric acid 12.0±3.2 vs 16.0±4.2 (P=0.4; ). Similar results, with no significant differences, were seen for iso-butyric (13.5±4.8 vs 15.3±5.0; P=0.2), iso-valeric (39.0±5.4 vs 47.4±14.0; P=0.07) and valeric acids (1.2±0.5 vs 1.3±0.6; P=0.4).
Figure 2 Serum SCFA levels in patients with IBS (n=22) and healthy controls (n=20).
Abbreviations: IBS, irritable bowel syndrome; SCFA, short-chain fatty acid.
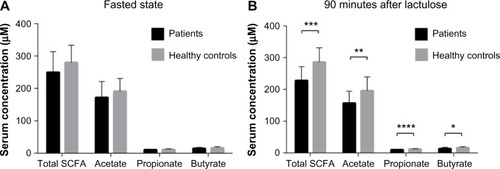
The levels of SCFA in serum 90 minutes after lactulose ingestion were significantly lower in patients with IBS than in healthy controls for all the major SCFA: total SCFA 228.0±43.9 vs 285.9±45.1 (P=0.0002); acetic acid 157.2±37.2 vs 195.4±43.7 (P=0.005); propionic acid 10.2±1.2 vs 12.0±1.5 (P=0.0001); and butyric acid 13.3±4.5 vs 16.7±3.2 (P=0.01; ). Significant differences were also seen for iso-butyric (35.9±12.8 vs 47.8±11.9; P=0.01), iso-valeric (35.9±12.8 vs 47.4±14.0; P=0.004) and valeric acids (1.3±0.4 vs 1.1±0.2; P=0.08).
No correlation was seen between the levels of SCFA in serum obtained 90 minutes after lactulose ingestion and the degree of symptoms, IBS severity scoring system, age, sex, or body mass index. Additionally, there were no differences between the subgroups with IBS.
Fermentation index A was similar in patients with IBS and healthy controls and did not change following lactulose ingestion. Fermentation index B was similar in patients with IBS and healthy controls in the fasting state. However, a significant decline in index B was seen in patients with IBS after the lactulose ingestion (P=0.01).
Discussion
In the present study, we found that patients with IBS had significantly lower serum levels of SCFA following lactulose ingestion than healthy controls. This novel finding suggests an impairment of colonic fermentation in patients with IBS. This might be surprising since the levels of SCFA in serum reflect the levels of SCFA in cecum and the proximal colon, that is, the actual site for fermentation,Citation8 and prior studies have reported increased (not decreased) levels of SCFA in feces of patients with IBS.Citation16 Lack of prior data concerning SCFA in serum in patients with IBS might be due to the shortage of appropriate analytical methods. However, the present method, recently developed by Zhao et al,Citation14 enables accurate quantification of SCFA, including iso-butyric, iso-valeric, and valeric acids, in serum. It may be speculated that it would have been better to collect several blood samples instead of only one single blood sample after 90 minutes,Citation17 especially since the assessment of orocecal transit time was not included in the present study. However, previous studies evaluating the effects of lactulose in patients with IBS have concluded that 90 minutes after lactulose intake is the average time point at which lactulose reaches the cecum.Citation10 Additionally, this is the average time point for the onset of symptoms after a meal.Citation11
The mechanism by which symptoms are evoked is not known, but lactulose provocation likely overloads the fermentation capacity of the proximal colon. An overload of indigestible carbohydrates in the colon may act osmotically to recruit fluid into the gut lumen. SCFA stimulate the uptake of salt and water,Citation18 and decreased fluid absorption in the proximal colon conceivably contributes to rectal overload and diarrhea. However, we found no correlation between the symptom development in patients with IBS and the serum levels of SCFA. Neither did we find any difference in our principal findings between subgroups of IBS. However, we acknowledge that lack of statistically significant differences might also be due to the small patient material.
We have previously shown that lactulose causes abnormal accumulation of fluid in the small intestine in patients with IBS,Citation19 a phenomenon that might indicate the presence of an underlying motility disorder. When the buildup of fluid in the small intestine enters the cecum, symptoms may be evoked due to poor cecal accommodation,Citation20 particularly if the influx occurs in unregulated flushes, thereby overloading the colonic fermentation and salvaging capacity.Citation21 Logically, microbial fermentation will continue distally throughout the colon as long as substrates remain. Indeed, a recent study may indicate that fermentation, measured as pH, persists throughout the whole colon in patients with IBS.Citation22 Increased levels of SCFA in feces in patients with IBSCitation16 may thus indicate a distal displacement of colonic fermentation. Hence, the levels of SCFA in patients with IBS may be different from healthy subjects throughout the colon, from abnormally low in the proximal part to abnormally high in the distal part.
Distal displacement of colonic fermentation may have several clinical consequences.Citation23 For example, SCFA stimulate the enterochromaffin cells in the colorectal mucosa to increase the production of serotonin,Citation24 a neurotransmitter exerting important physiological effects on both sensory and motoric neural transmission. In addition, SCFA from the distal colon bypasses the portal circulation, enabling systemic access and activation of specific SCFA receptors present in both the autonomic and somatic nervous system.Citation25 However, delayed fermentation could be positive since many of the diseases that occur in the gastrointestinal tract are located in the lower part, like colorectal cancer and inflammation, and regarding these conditions, high concentrations of SCFA could be protective.Citation26
A widespread treatment method for IBS is dietary advice, guiding patients to avoid foods high in low-digestible and fermentable short-chain carbohydrates (FODMAP; fermentable oligo-, di- and mono-saccharides, and polyols). The rationale behind this treatment concept is probably to reduce the fermentation burden on the colon.Citation27 However, a low FODMAP diet is probably not a measure to improve the colonic fermenting capacity. Indeed, recent studies suggest that FODMAP restriction may induce unfortunate changes of gut microbiota.Citation28
In the present study, we calculated two fermentation indices according to Tjellström et al:Citation15 Index A, reflecting the fermentation of carbohydrates (high index suggesting a trend toward a pro-inflammatory condition) and index B, indicating the fermentation of proteins (high index suggesting a trend toward an anti-inflammatory condition). According to these indicators, we found that the fermentation of proteins decreases in the postprandial state in patients with IBS compared with healthy controls. This might suggest that the colonic content in patients with IBS has pro-inflammatory properties, conceivably related to subclinical inflammation, as reported in patients with IBS by other groups.Citation29 However, we acknowledge that the two proposed fermentation indices have not yet been formally validated.
Conclusion
We found that the fermentation of lactulose leads to lower serum levels of SCFA in patients with IBS than in healthy controls, suggesting impaired colonic fermentation. When developing new treatments, it might be wise to target the fermentation process itself. Efforts to improve colonic fermentation capacity may be essential for patients with IBS.
Acknowledgments
Our submitted manuscript is a follow-up on our previous article entitled “Abnormal accumulation of intestinal fluid following ingestion of an unabsorbable carbohydrate in patients with irritable bowel syndrome: an MRI study”.Citation19 The abstract of this paper was presented at the 22nd United European Gastroenterology Week (UEGW) in Vienna in 2014 as a poster presentation with interim findings.Citation30 The actual paper, however, has never been published before.
Disclosure
The authors report no conflicts of interest in this work.
References
- CamilleriMPeripheral mechanisms in irritable bowel syndromeN Engl J Med2012367171626163523094724
- CheyWDThe role of food in the functional gastrointestinal disorders: introduction to a manuscript seriesAm J Gastroenterol2013108569469723545712
- StaudacherHMIrvingPMLomerMCWhelanKMechanisms and efficacy of dietary FODMAP restriction in IBSNat Rev Gastroenterol Hepatol201411425626624445613
- ValeurJMorkenMHNorinEMidtvedtTBerstadACarbohydrate intolerance in patients with self-reported food hypersensitivity: comparison of lactulose and glucoseScand J Gastroenterol200944121416142319883270
- MorkenMHNysaeterGStrandEAHauskenTBerstadALactulose breath test results in patients with persistent abdominal symptoms following Giardia lamblia infectionScand J Gastroenterol200843214114517943632
- HunterJOFood allergy–or enterometabolic disorder?Lancet199133887654954961678455
- ToppingDLCliftonPMShort-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharidesPhysiol Rev20018131031106411427691
- JakobsdottirGJadertCHolmLNymanMEPropionic and butyric acids, formed in the caecum of rats fed highly fermentable dietary fibre, are reflected in portal and aortic serumBr J Nutr201311091565157223531375
- JakobsdottirGBjerregaardJHSkovbjergHNymanMFasting serum concentration of short-chain fatty acids in subjects with microscopic colitis and celiac disease: no difference compared with controls, but between gendersScand J Gastroenterol201348669670123600961
- YuDCheesemanFVannerSCombined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBSGut201160333434021112950
- ArsieEColettaMCesanaBMBasiliscoGSymptom-association probability between meal ingestion and abdominal pain in patients with irritable bowel syndrome. Does somatization play a role?Neurogastroenterol Motil20152741642225581334
- LongstrethGFThompsonWGCheyWDHoughtonLAMearinFSpillerRCFunctional bowel disordersGastroenterology200613051480149116678561
- FrancisCYMorrisJWhorwellPJThe irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progressAliment Pharmacol Ther19971123954029146781
- ZhaoGLiuJFNymanMJonssonJADetermination of short-chain fatty acids in serum by hollow fiber supported liquid membrane extraction coupled with gas chromatographyJ Chromatogr B Analyt Technol Biomed Life Sci20078461–2202208
- TjellströmBHogbergLStenhammarLEffect of exclusive enteral nutrition on gut microflora function in children with Crohn’s diseaseScand J Gastroenterol201247121454145923016828
- TanaCUmesakiYImaokaAHandaTKanazawaMFukudoSAltered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndromeNeurogastroenterol Motil201022551251519903265
- FlorentCFlourieBLeblondARautureauMBernierJJRambaudJCInfluence of chronic lactulose ingestion on the colonic metabolism of lactulose in man (an in vivo study)J Clin Invest19857526086133973020
- BinderHJRole of colonic short-chain fatty acid transport in diarrheaAnnu Rev Physiol20107229731320148677
- UndsethRBerstadAKlowNEArnljotKMoiKSValeurJAbnormal accumulation of intestinal fluid following ingestion of an unabsorbable carbohydrate in patients with irritable bowel syndrome: an MRI studyNeurogastroenterol Motil201426121686169325271767
- PritchardSEMarcianiLGarsedKCFasting and postprandial volumes of the undisturbed colon: normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRINeurogastroenterol Motil201426112413024131490
- ReadNWDiarrhoea: the failure of colonic salvageLancet1982282964814836125648
- FarmerADMohammedSDDukesGEScottSMHobsonARCaecal pH is a biomarker of excessive colonic fermentationWorld J Gastroenterol201420175000500724803812
- BourduSDapoignyMChapuyERectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in ratsGastroenterology200512871996200815940632
- ReigstadCSSalmonsonCERaineyJFIIIGut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cellsFASEB J2015291395140325550456
- NohrMKEgerodKLChristiansenSHExpression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory gangliaNeuroscience2015229012613725637492
- ValeurJMorkenMHNorinEMidtvedtTBerstadAIntestinal fermentation in patients with self-reported food hypersensitivity: painful, but protective?Clin Exp Gastroenterol20103657021694848
- OngDKMitchellSBBarrettJSManipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndromeJ Gastroenterol Hepatol20102581366137320659225
- HalmosEPChristophersenCTBirdARShepherdSJGibsonPRMuirJGDiets that differ in their FODMAP content alter the colonic luminal microenvironmentGut20156419310025016597
- OhmanLSimrenMPathogenesis of IBS: role of inflammation, immunity and neuroimmune interactionsNat Rev Gastroenterol Hepatol20107316317320101257
- UndsethRJakobsdottirGNymanMBerstadAValeurJLow serum levels of short-chain fatty acids after lactulose ingestion may indicate impaired microbial fermentation in patients with irritable bowel syndromeUnited Eur Gastroenterol J20142Suppl 1A556
