Abstract
Background
According to the World Health Organization, glaucoma is a leading cause of irreversible blindness worldwide. By 2020, 80 million people will be affected by glaucoma in the world, which represents a significant financial burden to society. Glaucoma medications alone make up 38–52% of the total direct cost. The purpose of this research is to conduct a cost-minimization analysis to evaluate brand-name medications versus generic medications for treating glaucoma patients.
Methods
The per-bottle cost (in Canadian dollars) of brand-name drugs for glaucoma was obtained from the wholesaler, McKesson Canada, and, for generic drugs, from the Ontario Drug Benefit (ODB) Formulary. Further, a wastage adjustment fee, a pharmacy mark-up, and an ODB dispensing fee ($CAD) was added to the cost of both brand and generic. Previously published frequencies of medication prescription were utilized to calculate the average annual cost for each class of brand and generic. For each medication class and for mono-, bi-, and tri-drug therapy, the cost differential between brands and generics over a six-year period was computed and analyzed from third-party payer perspective.
Results
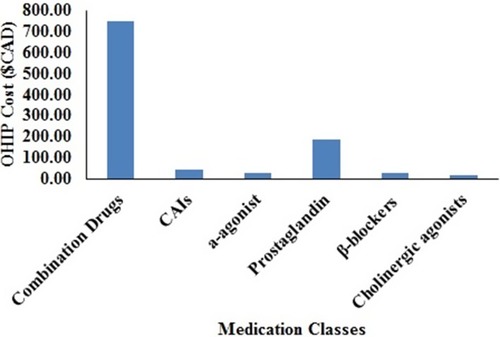
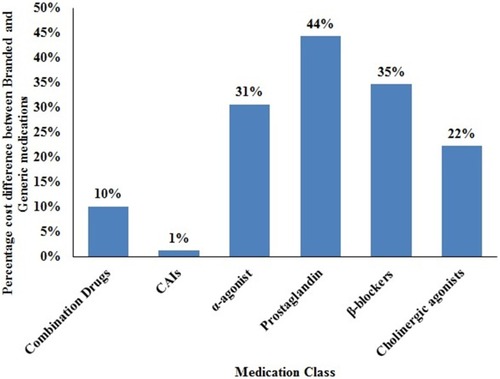
In descending order, the average annual government-funded health care system costs were: combination drugs such as Cosopt® ($748.23) were the most expensive, followed by prostaglandin analogs ($246.36), carbonic anhydrase inhibitors (CAIs) ($45.04), α-agonist ($30.34), β-blockers ($29.29), and cholinergic agonists ($16.51). Brand-name mono-drugs are 34% more expensive compared to generics. Brand-generic percentage cost differential for various medication classes over a six-year period was the highest for prostaglandin analogous (44%), followed by β-blockers (35%), α-agonist (31%), cholinergic agonists (22%), combination drugs (10%), and CAIs (1%).
Conclusion
Brand-name drugs are relatively more expensive than their generic counterparts, with variable cost differentials depending on drug class.
Background
According to the World Health Organization, glaucoma is a leading cause of irreversible blindness worldwide,Citation1,Citation2 with 6.6 million bilaterally blind people. It has been estimated that at least 3 million people in the USCitation3 and 400,000 people in CanadaCitation4 have been affected by glaucoma. By 2020, 80 million people will be affected by glaucoma in the world,Citation4–Citation6 which represents a significant financial burden to society.Citation7 In the US, the total annual treatment cost for glaucoma patients is $2.86 billionCitation3 and in Canada cost is $156 million, which includes direct and indirect costs.Citation6 The glaucoma medications alone make up 38–52% of the total direct cost.Citation8
Brand-name medication refers to drugs that go through the rigor of Phases I–III clinical trials to ensure efficacy and safety, and the company that brings the drug to market is generally responsible for these research and development (R&D) costs. The extensive data generated by the R&D process are essential to gaining subsequent government approval that will allow prescription of the drug. The company generally markets the drug using a proprietary name or “brand name” (Duramed Pharmaceuticals Inc., Diamox®, Cincinnati, OH, USA, goes by the generic name acetazolamide), which has patent protection for a period. After the patent expires or is challenged, other companies (generic manufacturers) can start manufacturing the drug for sale, usually at a lower price. In some provinces in Canada, the government mandates a set percentage cost of generic medication relative to brand.
A generic is approved by the government for patient use if deemed “bioequivalent.” According to Health Canada, for drugs administered orally, generic manufacturers can either repeat the chemistry, animal, or human studies (Phase I, II, and III clinical trials) already performed by a brand manufacturer or they can prove the value of their drug through comparative bioavailability (CB).Citation9 In CB studies, both brand and generic are given to healthy human volunteers on two different occasions, and serum concentrations are measured for comparison. Generics are required to show the same amount and rate of delivery of the active ingredients as brands,Citation9 and their efficacy is assessed by measuring the bioavailability in the volunteers’ blood after oral use. Most generic manufacturers prefer to show CB data since safety and efficacy of the brand has already been proven and because CB data are less expensive and less time-intensive to gather. However, comparable testing for ophthalmic eye drops is not possible since it is difficult to measure absorption of the active ingredients in the eye.
Generic ophthalmic medications are required to contain the same active ingredients as the brand and with a pre-determined acceptable concentration range, systemic absorption profile, and route of administration. However, the inactive ingredients – such as preservatives, antioxidants, thickening agents, buffers, and substances that adjust tonicity in generic compared to brand – may vary. Minor formulation differences in brand versus generic may affect how well active ingredients are absorbed in the eye. Second, changes in inactive ingredients can also affect the stability of the drug.Citation10 Finally, in case of eye drops, the container in which the solutions and suspensions are placed and the color of the caps and packaging can also vary,Citation11 and patients who rely upon color and/or style of the bottle may find it difficult to recognize the generic. Despite these concerns, generic drugs are widely used because of their cost benefit.Citation12
Due to cost savings, about two-thirds of all drugs dispensed in the US are generic.Citation13 For example, timolol maleate is a beta-blocker that lowers IOP by aqueous inflow suppression. Timoptic XE® (0.5%) (Merck & Co., Whitehouse Station, NJ, USA), an American brand-name drug, is a gel formulation of timolol maleate. Timolol GFS (0.5%) (Falcon Pharmaceuticals [Alcon], Fort Worth, TX, USA) is a generic version of Timoptic XE (0.5%).Citation11 Based on the clinical study by Schenker and Silver,Citation14 Timolol GFS (0.5%) was shown to be equally effective compared to Timoptic XE (0.5%). However, the study by Mammo et alCitation11 demonstrated significant differences between brand and generic in terms of volume, viscosity, and surface tension. In another study, DiamoxR was shown to be equally safe and effective compared to its generic counterpart.Citation15 These data were possible since DiamoxR is an oral medication. In a cost-comparison study, the generic acetazolamide was shown to be 37% less expensive than the branded alternative.Citation15 Additionally, given the cost of brand-name medications may significantly deter adherence.Citation16
Various studies have considered the cost of either brand or generic for glaucoma. Vold et al evaluated the yearly cost of generics in the US, using the Scott and White prescription claims for 1484 patients.Citation17 Fiscella et al conducted a controlled study to calculate the daily patient cost of various brands for glaucoma from the average wholesale price in the US.Citation1 Rylander and Vold conducted a prospective study to calculate the yearly cost of generic.Citation12 The theoretical yearly cost of generics for glaucoma was based on the average wholesale price and common dosing patterns.Citation12 Schlenker et al conducted a cost comparison of US and Canadian glaucoma medications.Citation18 Studies have yet to develop a cost-analysis model for comparing the cost of both generic and brand.
Lee and Hutnik performed a 6-year cost comparison of primary selective laser trabeculoplasty (SLT) with medical therapy in the treatment of open-angle glaucoma,Citation19 the costs of brand were calculated using Lee and Hutnik study. Since cost represents a driving force for utilization, analysis and awareness of cost are becoming increasingly important as health care systems develop “cost-based” models of delivery.
Based on this rationale, a cost-minimization analysis of ophthalmic brand and generic in Ontario is presented from third-party payer perspective over a 6-year time horizon. Data such as these can provide a basis for studies in cost comparison and cost-effectiveness and may also be utilized by decision-makers and policy-makers to calculate the “cost to society” or “burden of the diseases.” Cost-based models of health care delivery will require an expanded awareness by clinicians, whose treatment decisions are likely already being affected by these comparative costs and their management.
Materials and methods
The direct costs (in Canadian dollars) of brand and generic were obtained from a wholesaler, McKesson Canada, and from the 2018 Ontario Drug Benefit (ODB) Formulary,Citation20 respectively. While there are other pharmacy distributors, such as AmerisourceBergen Canada, McKesson Canada was chosen for use in this study since it is one of the largest independent pharmacy distributers. Sixteen McKesson distribution centers provide prescription drugs to more than 800 manufacturers, over 6500 pharmacies, 1300 hospitals, and to other health care institutions in Canada.Citation11 The calculated daily cost for brand is listed in terms of amount per-package size, where package size represents the total volume in milliliters (mL) per bottle. The ODB price for generic reflects the amount calculated per milliliter for single agents and the amount per package size for combination drugs.
Additional cost includes the wastage adjustment fee, Ontario pharmacy mark-up (8%), and ODB dispensing fees. According to the ODB Act, the dispensing fee payable to most pharmacies is $9.93 for each ODB prescription filled. On the other hand, the annual dispensing fee per bottle may vary, depending on the geographic location of the pharmacy. Also, pharmacies can waive or reduce the dispensing fee to attract more patients. For consistency, we calculated the annual dispensing fee per bottle as $9.93. The annual ODB dispensing fee is regulated by the government for Ontario drug benefit payers, such as patients who are 65 years of age or older, residents of long-term care homes, residents of the Homes for Special Care Program, people receiving professional services under the Home Care program, and Trillium drug program registrants. However, if the patient is not an ODB patient and they are privately insured or uninsured, then dispensing fees may vary, depending on the type of private drug plan and the amount of the deductible to the patient. In general, for uninsured patients, pharmacy mark-up and the ODB dispensing fee may be mandated by the pharmacy.
Commonly prescribed anti-glaucoma eye drops in both branded and generic formulations were included in this study. We grouped all the medications into six major classes: carbonic anhydrase inhibitors (CAIs), α-agonists, prostaglandin analogs, β-blockers, cholinergic agonists, and combination drugs.Citation21 lists the details of the medications in each major class.
Table 1 Annual cost data from Mckesson Canada Inc. of glaucoma medications by class of drug
Data on volume per bottle were obtained from Iordanous et al.Citation22 Contemporary medications, as well as older formulations, are sometimes absent from published cost analyses, due primarily to their unavailability at the time of publication.Citation19 In this study, the lack of published data required the following assumptions to be made: Pilopine Hs® gel had a 5 mL package size, which was like Isopto carpine 2%. Thus, volume per bottle for Pilopine Hs gel was assumed to be 5 mL. Similar assumptions were made for Isopto carpine 1% of which had a package size like Isopto carpine 2%. For Alphagan PR and Betoptic S®, the values on volume per bottle were obtained from Iordanous et al.Citation22 ODB formulary was searched to obtain data on volume per bottle for combination and newer medications and Lumigan RC®.
The values of drops per milliliter and dose per day in both eyes (OU) were obtained from the literature.Citation1,Citation19,Citation22 In cases where this value was unknown, values of drops per milliliter and drops per day OU within the class were assumed to be similar. Additionally, no patient data were collected; thus, ethics approval was deemed unnecessary.
Pharmacy fees in Ontario, including an 8% markup feeCitation23 and a $9.93 annual dispensing fee per bottle,Citation21 were added to the annual cost of the medication class to obtain the average annual cost to Ontario Health Insurance Plan (OHIP). A detailed equation appears below:
The empirical number of bottles used annually per medication was obtained from Iordanous et al,Citation22 which accounted for misadministration of medication by patient and for patient non-compliance. Empirical refill rates for dorzolamide-timolol, brimonidine, travoprost, latanoprost, timolol-XE, dorzolamide, brinzolamide, timolol, levobunolol, and pilocarpine were obtained from Lee and HutnikCitation19 and from Iordanous et al.Citation22
Glaucoma medication utilization patterns were obtained from the literatureCitation22 lists these utility rates by class. The utilization rate or frequency of a medication class prescribed was used to determine the average annual cost of all glaucoma medications in Ontario.Citation22 lists the frequency of mono-, bi-, and tri-drug therapies for glaucoma patients in Ontario. Based on the literature,Citation24 safety and efficacy of the generic drug was assumed to be similar to brand-name drug.
Table 2 Utility rates for various medications class
Table 3 Frequency of mono-, bi-, and tri-drug therapies for treatment of glaucoma in Ontario
Results
The average annual OHIP cost for each medication class of the brand is shown in and of the generic in . For the various medication classes, the average annual OHIP cost per patient is shown in . Combination drugs were the most expensive ($748.23), followed by prostaglandin analogs ($246.36), CAIs ($45.04), α-agonists ($30.34), β-blockers ($29.29), and cholinergic agonists ($16.51).
Table 4 Annual cost data from Ontario Drug Benefit (ODB) Formulary of glaucoma medications by class of drug
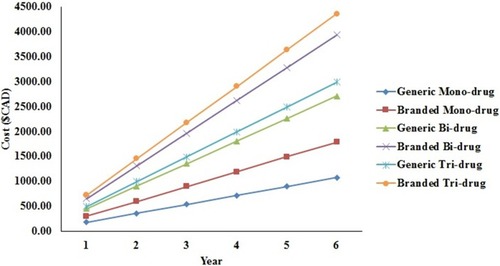
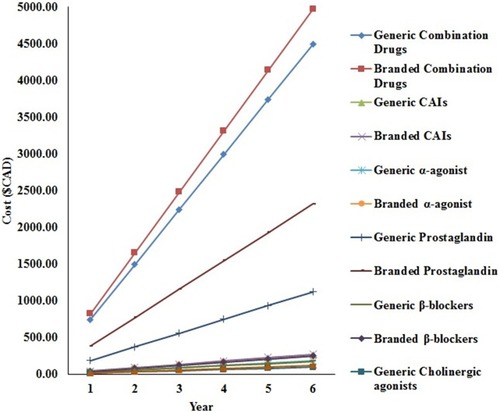
Brand-name drugs were found to be more expensive than their respective generic alternatives. The cost differential between the branded and generic medications for mono-, bi-, and tri-drug therapy for over a 6-year period is shown in . The graph in suggests that, over a 6-year period, branded bi-drug therapy is more expensive compared to generic tri-drug therapy. Also, over a 6-year period, the branded tri-drug cost is approximately $1000 more than a generic tri-drug, while the branded bi-drug cost is approximately $900 more than a generic bi-drug, and the branded mono-drug cost is approximately $500 more than a generic mono-drug.
Figure 2 Difference between branded and generic medications for mono-, bi-, and tri-drug therapy for over 6 years.
Brand-generic percentage cost differential is shown in for mono-, bi-, and tri-drug therapy for over 6 years. Brand-name mono-drugs are 34% high-priced compared to their generic counterparts over 6 years. Brand-name bi- and tri-drugs are 27% costly compared to generics. The difference between brands and generics cost for the various medication classes is shown in . The greatest difference exists between generic and branded prostaglandin analogous ($841.55), followed by combination drugs ($478.90), β-blockers ($73.60), α-agonist ($65.84), cholinergic agonists ($24.78), and CAIs ($3.34). Generic prostaglandin costs $841.55 less than branded prostaglandin. These lower costs create a strong incentive for switching patients to a generic prostaglandin, also in addition to creating a more competitive generic market for this class of anti-glaucoma medication. Moreover, efficacy of the generic drug, stability in the bottle, bottle design/composition, and side effects do not factor into this switch.
Figure 3 Percentage difference between branded and generic medications for mono-, bi-, and tri-drug therapy for over 6 years.
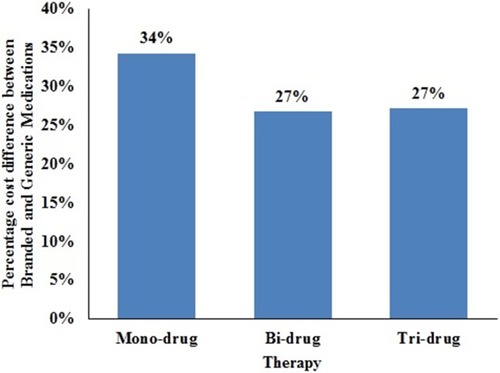
Figure 4 Difference between branded and generic medications for various medication classes over 6 years.
shows the percentage difference between brand and generic for various medication classes over 6 years. Brand-name prostaglandins are 44% pricey compared to generic equivalents followed by β-blockers (35%), α-agonist (31%), cholinergic agonists (22%), combination drugs (10%), and CAIs (1%). Prostaglandins are first line of treatment and β-blockers are the second line of treatment for primary open-angle glaucoma patients. Low-income families are enticed to switch to generic equivalents of prostaglandins and β-blockers given the cost.
Discussion
In this cost analysis study, brand-name drugs were shown to be high-priced than generic drugs, but the cost savings were inconsistent among the classes. Since first-line agents have the lowest generic costs, the profits may be linked to volume of sales. Despite government reform that mandated a set generic price on a percentage basis, cost differences were highly variable, depending on the drug class. The cost difference between brands and generics may be less significant if therapy is short term and of low prevalence. However, for chronic diseases that are prevalent, such as glaucoma, the cost differences become significant over time.
It was not the intent of this study to include indirect costs, such as the quality-of-life costs associated with eye-drop toxicity, allergies, and/or noncompliance. Unlike a cost-effective analysis, this study did not address the costs of treatment success or failure. Rather, the intent here was to produce a study that could be used as a foundation for more extensive economic analyses that include indirect costs and the influences of treatment outcomes. Importantly, this research represents a contemporary cost-minimization analysis study as glaucoma medications transition into the generic market, and it creates awareness of the actual cost differential between anti-glaucoma brands and generics.
A product’s life cycle involving five stages: development, introduction, growth, maturity, and decline may apply to brand-name and generic drugs.Citation25 In the introduction stage, sales are low, prices are high for the manufacturer to recoup development costs. In the growth stage, sales increase and prices are generally maintained at a high level. Maturity stage is the most profitable stage when sales increase. During the decline stage, prices may fall to liquidate inventory. However, competition may decrease the market share and/or prices. Patent expiry is an important milestone in the life cycle of brands.Citation26 Generics are introduced when brand patent expires. Brand manufactures may reduce production, drop price, or may phase out due to low profits. Thus, timewise, generics could be analyzed separately then brands. However, data show the demand elasticity of generics is relatively independent of time.Citation26 Further, customers may choose brands over generics to ensure safety and efficacy irrespective of price. This study, based on the literatureCitation24 assumed similarity in efficacy or safety of GM compared to BM. A review of the literature revealed that very few studies have been conducted in this regard. Thus, computing Quality Adjusted Life Years (QALYs) due to generic compared to brand may represent a natural extension of this study.
Additionally, the prices presented of brands and generics may get influenced by the current stage of their product life cycles. However, there are limitations to the product life cycle concept. Life cycle curves vary substantially by brands and generics.Citation25 Second, manufactures differ so not all brands and generics go through every stage of product life cycle. Even if they go through every stage of product life cycle, length of time spent in each stage may vary with drugs.Citation27 Third, it is difficult to see transitions in product life cycle phases. Fourth, market conditions or consumer tastes may change dynamically adding uncertainty to the product life cycle. Fifth, a product life cycle may be an unreliable indicator of the product’s true-life span.
The information presented in the study is highly relevant during an era when cost represents a predominant factor in health care decision making. The cost-minimization analysis performed herein may be helpful to clinicians, hospital administrators, payers and policy-makers in their decision-making efforts since cost will have to be balanced with therapeutic efficacy and safety. Further, switching to generics may help improve patients’ adherence.Citation16
Conclusion
If the brand-generic cost differential is large and the generics prove to be comparable in safety and efficacy, then all levels of the health-care system may embrace the movement to generics. However, if the generic is less tolerable and/or less efficacious, clinicians may need to challenge the cost-based approach to ensure maximum patient outcomes. Similarly, if the brand-generic cost differential is relatively small, clinicians may choose not to gamble on potential problems with the switch to generic. The small differential may not be worth the risk is highlighted by our study’s finding that a significant cost differential does exist between generic and brand for prostaglandin and combination glaucoma eye drops. Clinicians must be vigilant in monitoring safety and efficacy, especially for these classes, which are frequently used in the management of glaucoma. Further, clinicians must acknowledge that the historical comfort level associated with these brands may not be assumed, especially when cost represents the driving factor for utilization.
Abbreviations
ODB, Ontario Drug benefit formulary; R&D, research and development; CB, comparative bioavailability; IOP, intraocular pressure; OHIP, Ontario Health Insurance Plan; CAI, carbonic anhydrase inhibitors; SLT, selective laser trabeculoplasty; OU, oculus uterque (both eyes); QALYs, Quality Adjusted Life Years; NGOs, non-governmental organizations.
Ethics approval and consent to participate
No human subjects were recruited for the study; hence ethics approval was deemed unnecessary.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Availability of data and materials
All data is presented within the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- Fiscella RG, Green A, Patuszynski DH, Wilensky J. Medical therapy cost considerations for glaucoma. Am J Ophthalmol. 2003;136(1):18–25. doi:10.1016/s0002-9394(03)00102-812834665
- Kingman S. Glaucoma Is Second Leading Cause of Blindness Globally. World Health Organization; 2004.
- Foundation BF. Glaucoma: facts & figures. Bright Focus Foundation; 2017 Available from: http://www.brightfocus.org/glaucoma/article/glaucoma-facts-figures. Accessed 324, 2016.
- Glaucoma Research Society of Canada. Quick facts; 2018 Available from: https://www.glaucomaresearch.ca/about/about-glaucoma/. Accessed 531, 2018.
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.08122416488940
- MEDEC’s Ophthalmic Sector Committee. Economic benefits of treatment. MEDEC; 2018 Available from: http://www.medec.org/webfm_send/434. Accessed 321, 2018.
- Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011;152(4):515–522. doi:10.1016/j.ajo.2011.06.00421961848
- Lee PP, Walt JG, Doyle JJ, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006;124(1):12. doi:10.1001/archopht.124.1.1216401779
- Health Canada. The safety and effectiveness of generic drugs; 2012 Available from: https://www.canada.ca/en/health-canada/services/healthy-living/your-health/medical-information/safety-effectiveness-generic-drugs.html. Accessed 321, 2018.
- Kahook MY, Fechtner RD, Katz LJ, Noecker RJ, Ammar DA. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:1–8.22029631
- Mammo ZN, Flanagan JG, James DF, Trope GE. Generic versus brand-name North American topical glaucoma drops. Can J Ophthalmol. 2012;47(1):55–61. doi:10.1016/j.jcjo.2011.12.00422333853
- Rylander NR, Vold SD. Cost analysis of glaucoma medications. Am J Ophthalmol. 2008;145(1):106–113. doi:10.1016/j.ajo.2007.08.04118154755
- Haas JS, Phillips KA, Gerstenberger EP, Seger AC. Potential savings from substituting generic drugs for brand-name drugs: medical expenditure panel survey, 1997–2000. Ann Intern Med. 2005;142(11):891–897. doi:10.7326/0003-4819-142-11-200506070-0000615941695
- Schenker HI, Silver LH. Long-term intraocular pressure lowering efficacy and safety of timolol maleate gel-forming solution 0.5% compared with timoptic XE 0.5% in a 12-month study. Am J Ophthalmol. 2000;130(2):145–150. doi:10.1016/s0002-9394(00)00458-x11004287
- Gallardo MJ, Fiscella RG, Whitson JT. Generic Glaucoma Medications. Is It Safe to Switch? 2006.
- Stein JD, Shekhawat N, Talwar N, Balkrishnan R. Impact of the introduction of generic latanoprost on glaucoma medication adherence. Ophthalmology. 2015;122(4):738–747. doi:10.1016/j.ophtha.2014.11.02225680226
- Vold SD, Riggs WL, Jackimiec J. Cost analysis of glaucoma medications: a 3-year review. J Glaucoma. 2002;11(4):354–358.12169974
- Schlenker MB, Trope GE, Buys YM. Comparison of United States and Canadian glaucoma medication costs and price change from 2006 to 2013. J Ophthalmol. 2015;2015.
- Lee R, Hutnik CML. Projected cost comparison of selective laser trabeculoplasty versus glaucoma medication in the Ontario Health Insurance Plan. Can J Ophthalmol. 20;41(4):449–456. doi:10.1016/S0008-4182(06)80006-2
- Ontario Drug Benefit (ODB) Formulary. Drugs and Devices Division; 2018 Available from: http://www.health.gov.on.ca/en/pro/programs/drugs/odbf_mn.aspx. Accessed 119, 2018.
- Ministry of Health and Long-term Care O, Canada. Ontario drug benefit program: dispensing fees. Ministry of Health and Long-term Care; 2019 Available from: http://www.health.gov.on.ca/en/public/programs/drugs/programs/odb/opdp_dispensing_fees.aspx. Accessed 531, 2019.
- Iordanous Y, Kent JS, Hutnik CM, Malvankar-Mehta MS. Projected cost comparison of trabectome, iStent, and endoscopic cyclophotocoagulation versus glaucoma medication in the Ontario Health Insurance Plan. J Glaucoma. 2014;23(2):e112–8. doi:10.1097/IJG.0b013e31829d9bc7.
- Service Ontario. Ontario drug benefit act; 2018 Available from: http://www.e-laws.gov.on.ca/html/regs/english/elaws_regs_960201_e.htm. Accessed 318, 2018.
- Kim YI, Kim JH, Lee TY, Lee KW. Efficacy and safety of glaucoma patients’ switch from a 2% dorzolamide/0.5% timolol fixed-combination brand-name drug to its generic counterpart. J Ocul Pharmacol Ther. 2015;31(6):335–339. doi:10.1089/jop.2014.017026133057
- NetMBA. The product life cycle. NetMBA; 2010 Available from: http://www.netmba.com/marketing/product/lifecycle/. Accessed 830, 2016.
- Zhang J. AS. Market Analysis of Currently Available Prescription Drugs. Association for Business and Economics Research (ABER); 2009.
- H. B. Benefits and limitations of product life cycle; 2016 Available from: http://www.marketing91.com/benefits-and-limitations-of-product-life-cycle/. Accessed 531, 2018.