Abstract
Objective
This study used a decision-analytic framework to assess the cost-effectiveness of brexpiprazole vs comparator branded therapies for reducing relapses and hospitalizations among adults with schizophrenia from a US payer perspective.
Methods
An economic model was developed to assess patients with stable schizophrenia initiating treatment with brexpiprazole (1–4 mg), cariprazine (1–6 mg), or lurasidone (40–80 mg) over a 1-year period. After 6 months, patients remained on treatment or discontinued due to relapse, adverse events, or other reasons. Patients who discontinued due to relapse or adverse events were assumed to have switched to other therapy, and those who discontinued due to other reasons were assumed to have received no therapy. Primary outcomes were incremental cost per relapse avoided and hospitalization avoided, and the secondary outcome was cost per quality-adjusted life-year (QALY) gained. Sensitivity and scenario analyses were also conducted.
Results
Brexpiprazole was associated with the highest per-patient clinical effectiveness (avoided relapses 0.637, avoided hospitalizations 0.719, QALYs 0.707) among comparators, followed by cariprazine (avoided relapses 0.590, avoided hospitalizations 0.683, QALYs 0.683) and lurasidone (avoided relapses 0.400, avoided hospitalizations 0.536, QALYs 0.623). Annual per-patient health-care costs were lowest for brexpiprazole ($20,510), followed by cariprazine ($22,282) and lurasidone ($25,510). Brexpiprazole was the least costly and most effective treatment strategy for all outcomes. Results were sensitive to relapse rates and daily cost of brexpiprazole. Limitations include data principally obtained from drug-specific randomized withdrawal studies and lack of direct-comparison trials.
Conclusion
This analysis evaluated brexpiprazole treatment for the reduction of schizophrenia relapses and hospitalizations over a 1-year period compared to other recently available branded antipsychotics, and excluded generic antipsychotic treatments. Brexpiprazole treatment may lead to clinical benefits and medical cost savings, and provides a cost-effective treatment option for patients relatively to other branded second-generation antipsychotics.
Introduction
Schizophrenia is a complex and disabling mental disorder characterized by delusions, hallucinations, disorganized speech and behavior, negative symptoms, cognitive impairment, and other symptoms that contribute to social and occupational dysfunction.Citation1 The disorder affects approximately 1.1% of adults in the USA.Citation2 The economic burden of schizophrenia in the US is substantial: estimated at $156 billion in 2013.Citation3 Schizophrenia-relapse rates are high, and further contribute to the economic burden of the disorder. Acher-Svanum et al found that total annual direct mental health-care costs were about three times higher among persons with schizophrenia who experienced relapses in the 6 months prior to the study compared to patients who did not experience relapses in that period.Citation4
The goals of schizophrenia treatment have evolved over the last several decades, and focus on increasing quality of life (QoL) and functioning and striving for remission.Citation5–Citation7 Guidelines recommend psychosocial interventions incorporated into all phases of patient management, with the goal of minimizing stress and maximizing patient functioning.Citation6 The American Psychiatric Association practice guidelines support the use of programs, such as community interventions (eg, Program for Assertive Community Treatment, family interventions, supported employment, cognitive behavioral therapy, social skills training, and programs of early intervention to delay relapse).Citation6 The association recommends antipsychotic agents as the mainstay of treatment for schizophrenia; however, variance in pharmacological profiles create clinically relevant variability in tolerability and efficacy.Citation8 Typical antipsychotics (ie, first-generation antipsychotics) are antagonists at dopamine D2 receptors and effective against psychotic symptoms. However, these agents have a high rate of motoric adverse events (AEs), such as drug-induced parkinsonism and tardive dyskinesia, at therapeutic doses. Atypical antipsychotic agents (ie, second-generation antipsychotics [SGAs]) available in the US include clozapine, risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, lurasidone, paliperidone, iloperidone, cariprazine, and asenapine. These atypical antipsychotics differ from one another in their tolerability profile. Although atypical antipsychotics may have reduced risk of motoric side effects, there is evidence that demonstrates variable risk of weight gain, diabetes, hyperlipidemia, and cardiovascular complications.
Although antipsychotic medications can manage the symptoms of schizophrenia effectively and help patients to achieve remission, relapses are common.Citation9 In addition, discontinuation rates for antipsychotic medications are high in both clinical trial and real-world settings. For example, in the US Clinical Antipsychotic Trials of Intervention Effectiveness study,Citation10 the overall discontinuation rate over 18 months for patients with chronic schizophrenia taking antipsychotics was 74%, and in a 3-year European observational study discontinuation rates were 34%–66%.Citation11
Poor tolerability and side effects of antipsychotics are among the primary reasons for premature treatment discontinuation, resulting in inadequate symptom resolution and an increased risk of relapse.Citation12,Citation13 Therefore, additional tolerable treatment options are needed. Brexpiprazole is an SGA approved by the US Food and Drug Administration (FDA) in July 2015 as monotherapy for schizophrenia in adults. The efficacy of brexpiprazole in schizophrenia was demonstrated in two 6-week, randomized, double-blind, placebo-controlled, fixed-dose clinical trials.Citation14,Citation15 Brexpiprazole has demonstrated a low incidence of sedating or activating AEs, a low rate of long-term metabolic effects, and moderate weight gain.Citation14–Citation16 The efficacy of brexpiprazole was also demonstrated in a randomized-withdrawal, double-blind, placebo-controlled, 52-week maintenance study,Citation17 which showed a reduction in risk of relapse of 71% vs placebo over 1 year and an incidence of AEs that was comparable to placebo.
Evidence of efficacy and tolerability remains important in the evaluation and comparison of available therapies; however, it is also important to determine their cost-effectiveness, given limitations on health-care spending. In the absence of head-to-head studies, indirect comparisons to evaluate the cost-effectiveness of treatments for schizophrenia can assist in health-care decision-making. This study used a decision-analytic framework to assess the cost-effectiveness of brexpiprazole in schizophrenia for reducing relapses and hospitalizations among adults with schizophrenia from a US payer perspective.
Evaluating the cost-effectiveness of newly available branded treatments in schizophrenia can shape policies concerning treatment coverage and reimbursement. Decision makers emphasize the need for more timely information.Citation18 In the US, the majority of oral antipsychotics are available as generic products. Given increasing pressures to manage health-care costs, it is expected that generic-drug utilization is generally prioritized over the use of branded treatments. For policy makers evaluating new branded treatments for formulary placement, an appropriate pharmacoeconomic analysis would involve comparisons of newly available and existing branded treatments.
Methods
Model overview
A decision-analytic model was developed to evaluate a hypothetical cohort of adult patients with stable schizophrenia initiating treatment with brexpiprazole, cariprazine, or lurasidone. Lurasidone and cariprazine were selected as comparators because they are the most recently FDA-approved SGAs and long-term prevention studies on them were comparable to those on brexpiprazole in terms of patient population, trial design, and end points. In the long-term maintenance trials, all patients were stabilized before entering a randomized, double-blind phase for at least 12 weeks. Additionally, relapse definitions were comparable across trials. Modeled treatment doses were based on those evaluated in long-term prevention studies from which clinical events were derived: brexpiprazole (1–4 mg), cariprazine (1–6 mg), and lurasidone (40–80 mg). It was assumed that patients remained adherent to treatment during treatment-initiation and -switch periods.
Model outputs are reported over a 1-year model time horizon, which was chosen to be consistent with the clinical trial duration period. It is clinically relevant to model schizophrenia outcomes within 1 year, because clinical effectiveness and treatment discontinuation are typically seen within this period and data beyond 1 year are limited.Citation10,Citation19 Due to the length of the time horizon, discounting (ie, translating future costs and benefits into present-day values) was not applied.
The model incorporated direct costs related to drug acquisition, AE treatment, relapse-related treatment, and patient monitoring. All costs are reported in 2016 US$. Clinical events were estimated from long-term relapse trials (efficacy) and acute trials (AEs) for each model comparator. Cost-effectiveness was evaluated for primary outcomes of incremental cost per relapse avoided and cost per hospitalization avoided and the secondary outcome of cost per quality-adjusted life-year (QALY) gained. The model was programmed using Microsoft Excel 2010.
Model population and structure
The model evaluated a hypothetical cohort of adults with stable- phase schizophrenia consistently with patients enrolled in long-term prevention studies of brexpiprazole,Citation17 cariprazine,Citation20 and lurasidone.Citation21 Initially, patients entered the model and were treated with brexpiprazole, cariprazine, or lurasidone (). Following treatment initiation, patients remained on therapy for the full year or discontinued treatment after 6 months due to relapse/lack of treatment efficacy, AEs, or other reasons (including cost of medication, nonadherence, patient preference, or unknown). Because the median time to discontinuation in the brexpiprazole studyCitation17 was 169 days, the use of 6 months (approximately 183 days) was unlikely to bias model results.
Figure 1 Model structure.
Abbreviation: AEs, adverse events.
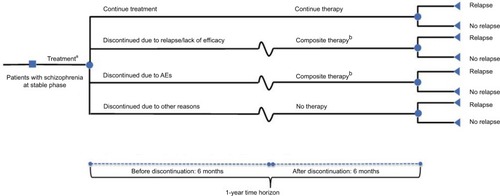
Patients who discontinued due to relapse/lack of treatment efficacy or AEs were assumed to switch to composite therapy, which included generic SGAs (ie, olanzapine, risperidone, quetiapine, ziprasidone, and aripiprazole). Patients who discontinued due to other reasons were assumed to receive no additional therapy. For patients who switched to composite therapy, it was assumed that the likelihood of receiving any one of the therapies was the same. Therefore, rates of relapse and AEs were calculated as averages for the composite therapies. Although patients who switched to composite therapy could experience relapse or AEs, they were assumed to continue treatment throughout the remainder of the year.
Model estimation
Clinical inputs
Key clinical inputs were derived from 52-week maintenance relapse studies for brexpiprazoleCitation17 and cariprazine,Citation20 a 28-week maintenance relapse study for lurasidone,Citation21 and published reports and package inserts (PIs) for SGAs used for composite therapy. Clinical parameters included in the model were rates of treatment discontinuation, relapse/impending relapse, and AEs (–).
Table 1 Probability of treatment discontinuation and relapse at 6 months
Table 3 Estimated cost inputs
In the absence of direct-comparison trials of treatments, an indirect comparison was conducted to determine differences in treatment discontinuation and relapse. Because treatments were all compared to placebo in their long-term maintenance trials, this indirect comparison used placebo as the common comparator to obtain model-efficacy values. Derived rates were calculated for treatment discontinuation, which allowed a more accurate comparison of clinical events across comparator cohorts. In general, derived rates were calculated as the product between the relative clinical rate within a trial (active vs respective placebo) and a pooled placebo clinical rate (see Supplementary material for calculation details). The probabilities of relapse at 6 months for treatments in composite therapy are presented in . Because relapses vary in severity, the model assumed that 77.3% of relapses resulted in an inpatient hospitalization and 22.7% were treated on an outpatient basis.Citation22
Adverse events
The model assumed that patients could experience six types of potential treatment-emergent AEs: akathisia, extrapyramidal symptoms, glucose abnormalities (fasting glucose criteria), lipid abnormalities (fasting total cholesterol criteria), sedation, and weight gain (≥7% weight gain from baseline; ). AE rates were pooled as needed,Citation23 and absolute rates of AEs across comparator trials were used.Citation24,Citation25 AE rates for composite-therapy treatments were obtained from the product labels. Sedation as a unique AE identifier was not reported in the lurasidone PI; therefore, a weighted average was calculated using published data.Citation26
Table 2 Adverse-event rates
Economic inputs
Cost parameters were derived from the literature, and included schizophrenia-care costs related to drug acquisition, relapse, treatment discontinuation/switching, and treatment costs for AEs (). Costs are reported as 2016 US$, and where applicable are inflated to 2016 US$ using the medical care component of the consumer price index.Citation27 The model also included the cost of treatment-related monitoring, considered the office-visit cost of monitoring per outpatient,Citation27 and assumed that a treated patient would require one monitoring visit per month. An additional cost of switching treatments was also applied.Citation19,Citation27 If patients discontinued treatment due to other reasons, the analysis did not assign any composite therapy, and thus no additional treatment-related costs applied. Lastly, the costs of treating relevant AEs () were assumed to occur only within a 6-week period.
Utility inputs
The model used health-state utilities to estimate the impact of treatments on patients’ QoL. Utility weights were obtained from published QoL data among patients with stable schizophrenia.Citation28,Citation29 Utilities associated with relapse with or without hospitalization and AEs were derived from the utility value from stable schizophrenia. Mean (SE) health-state-utility values were 0.88 (4.4%) for stable disease, 0.53 (2.7%) for relapse with hospitalization, and 0.74 (3.7%) for relapse without hospitalization.Citation28,Citation29 Mean (SE) utility decrements associated with AEs were 0.090 (0.005) for akathisia,Citation28 0.099 (0.005) for extrapyramidal symptoms,Citation28 0.067 (0.003) for glucose abnormalities,Citation30 0.099 (0.005) for lipid abnormalities,Citation31 0.084 (0.004) for sedation,Citation32 and 0.036 (0.002) for weight gain ≥7%.Citation32 Because utility-weight decrements were not available for glucose abnormalities, the utility for symptomatic nonsevere hypoglycemia in patients with diabetes was used.
Analysis
Total direct schizophrenia-related health-care costs, incremental costs, and clinical improvement (ie, number of relapses and hospitalizations avoided) were estimated for each treatment in the model. Incremental cost: effectiveness ratios (ICERs) were expressed as cost per relapse avoided, cost per hospitalization avoided, and cost per QALY gained. These outcomes were calculated at the end of 1 year as the ratio of the difference between the cost of schizophrenia-related care in patients receiving brexpiprazole vs alternative treatment and the difference in the number of patients avoiding relapses or hospitalizations, respectively.
Sensitivity analyses
One-way sensitivity analyses were conducted to quantify the impact of change in individual model parameters on model outcomes. All clinical and economic parameters were varied by 1 SD within a predefined statistical distribution of the base-case values to determine which variables would have the greatest impact on the incremental net monetary benefit (NMB).
To assess uncertainty in the cost-effectiveness analysis, a probabilistic sensitivity analysis (PSA) was also conducted using a second-order Monte Carlo simulation. The PSA was performed by simultaneously drawing from appropriate distribution functions for all model parameters according to their means and SEs (–). All rates were varied using β-distribution and costs varied using γ-distribution. The PSA was repeated 1,000 times, and results reporting the NMB for different willingness-to-pay (WTP) thresholds ($0–$100,000) per selected outcome (avoided relapse, avoided hospitalization, and QALYs) were used to evaluate the robustness of model outcomes.
Scenario analyses were conducted to understand further the impact of model estimate assumptions for AEs and drug costs related to generic options in composite therapy. AE rates were incorporated into the model using absolute estimates from comparator trials. To assess the impact of using derived rates of AEs in the model, a scenario analysis was conducted. Wholesale-acquisition-cost branded pricing was used to estimate drug costs for treatments in the composite-therapy arm. However, given that treatments are generic, a second-scenario analysis using retail pricing from national wholesaler CostcoCitation33 was deemed appropriate to assess the impact of lower-cost drug costs.
Results
In a hypothetical cohort of 1,000 patients, the model estimated that brexpiprazole was the dominant treatment strategy compared to cariprazine and lurasidone over the 1-year time horizon (). In terms of clinical outcomes, treatment with brexpiprazole was associated with higher effectiveness (all outcomes shown per patient; avoided relapses 0.637, avoided hospitalizations 0.719, QALYs 0.707), followed by cariprazine (avoided relapses 0.590, avoided hospitalizations 0.683, QALYs 0.683) and lurasidone (avoided relapses 0.400, avoided hospitalizations 0.536, QALYs 0.623). Brexpiprazole was also associated with lower total schizophrenia-related health-care costs per patient ($20,510), followed by cariprazine ($22,282) and lurasidone ($25,510). In the ICE analyses, brexpiprazole was the dominant (ie, less costly and more effective) treatment strategy compared with lurasidone and cariprazine for all ICERs (). A cost-effectiveness plane displaying results is presented in .
Figure 2 Cost-effectiveness plane per patient.
Abbreviation: QALY, quality-adjusted life-year.
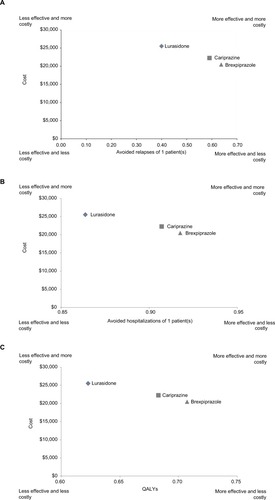
Table 4 Base-case cost-effectiveness analysis
Sensitivity analyses
and show the results of the one-way sensitivity analyses comparing brexpiprazole vs lurasidone and cariprazine, respectively, for the ten most influential variables at a WTP threshold of $30,000 per relapse avoided. As shown in the tornado diagram, when brexpiprazole was compared to lurasidone (), the model parameters with the largest impact on the incremental NMB were the 6-month discontinuation probability due to relapse for brexpiprazole, 6-month discontinuation probability due to relapse for lurasidone, and daily cost of brexpiprazole. Results of one-way sensitivity analyses with the same WTP ($30,000) per relapse-related hospitalization avoided and QALYs showed similar results. When brexpiprazole was compared to cariprazine (), the results were consistent with the comparison of brexpiprazole and lurasidone. Results of the PSA using a WTP range of $0–$100,000 per avoided relapse, per avoided hospitalization, and per QALY are shown in . Based on 1,000 simulations, all results indicated that brexpiprazole was associated with lower cost and better effectiveness, yielding the highest NMB among all comparators.
Scenario analysis
Calculated derived rates of AEs from the first-scenario analysis are presented in . In this scenario, brexpiprazole was also the dominant treatment strategy compared with lurasidone and cariprazine for all ICERs evaluated (). In the second-scenario analysis using retail pricing from CostcoCitation33 for the composite treatments, cost-effectiveness results were similar to the base-case analysis (). For each treatment, the total annual cost per patient was slightly lower compared to results reported in the base-case scenario. Results from these two scenario analyses showed consistent findings with the base-case analyses.
Discussion
Schizophrenia poses substantial human and economic burden, and despite the availability of several SGAs, it remains a difficult disorder to treat effectively. A recent studyCitation34 found that schizophrenia was one of the three most burdensome diseases on an annual per patient basis, estimated at $46,537 in 2014 US$. Relapses have a significant impact on the economic burden of schizophrenia, with relapsed patients incurring three to four times higher health-care costs than nonrelapsed patients, driven primarily by the costs of hospitalization for relapsed patients.Citation4,Citation35
Brexpiprazole is a recently approved SGA treatment option for adults with schizophrenia. To our knowledge, this is the first cost-effectiveness analysis to compare brexpiprazole with other branded SGAs in reducing schizophrenia relapses and hospitalizations. Results of the base-case cost-effectiveness analyses showed that brexpiprazole was the dominant treatment strategy compared with lurasidone and cariprazine for all outcomes assessed. Although this is the only cost-effectiveness analysis of brexpiprazole compared to lurasidone and cariprazine we are aware of, the cost-effectiveness of lurasidone has been explored in previous studies;Citation36–Citation38 however, these models included different populations, comparators, inputs, assumptions, and time horizons, making comparison across studies difficult. Model results should be considered in light of limitations. The analysis was based on data from placebo-controlled trials; as such, results of this analysis may not be generalizable to treatment provided under real-world conditions. In addition, the probability of relapse for the composite therapy risperidone was not derived from a randomized, placebo-controlled withdrawal study, as one was not conducted by the manufacturer. Therefore, probability of relapse came from a maintenance study of risperidone vs haloperidol that did not involve a period of stabilization followed by medication withdrawal.Citation39
The use of observed AE rates from short-term acute-schizophrenia trials due to lack of long-term comparable comparator data could be another study limitation. However, we employed an indirect-comparison method and derived placebo-adjusted rates in scenario analyses. Both scenario and sensitivity analyses suggested that those rates were not identified as major model drivers and had only a minimal impact on the cost-effectiveness results. Furthermore, incorporation of the short-term cost of treating AEs, such as change in glucose, cholesterol, and weight only reflects short-term treatment costs; however, the potential long-term risks of diabetes, obesity, and complications, such as cerebrovascular accident and cardiovascular disease, are not included in the analysis and warrant consideration in a longer-term evaluation.
The model included only the treatment doses that were evaluated in the long-term prevention trials; therefore, efficacy at higher doses for lurasidone was not evaluated and may affect findings. In the long-term, placebo-controlled maintenance trial of lurasidone, patients were randomized to 40–80 mg/day lurasidone or placebo.Citation21 However, the PI for lurasidone recommends a dose of up to 160 mg per day,Citation25 and some patients who have an inadequate response to doses up to 80 mg/day will require higher-dose treatment.Citation40 As noted by Citrome,Citation41 the Tandon et alCitation21 study had some different findings between US and non-US study sites, which may have further limited the effect size observed in this study relative to other similar studies of SGAs. Furthermore, given the objective of the study, this analysis considered only the branded agents that are available in the US for which a generic formulation is not available and where supportive long-term prevention trials have been published.
Finally, this cost-effectiveness analysis takes a US payer perspective, and thus results may not be generalizable to other populations and/or countries in which health-care-resource utilization and clinical practice may be different. This model also assumed that 77.3% of all relapses resulted in an inpatient hospitalization and the remaining relapses (22.7%) were treated on an outpatient basis.Citation22 This assumption was based on a study conducted in England, which may not reflect US treatment patterns. To account for the impact of various parameter estimates on the model results, we conducted both deterministic and probabilistic sensitivity analyses, which showed consistent results that brexpiprazole dominated lurasidone and cariprazine. As noted by Meltzer, attention is essential to important methodological issues in constructing cost-effectiveness analysis of treatments in schizophrenia.Citation42 There are key issues in developing a cost-effectiveness model in schizophrenia that includes perspective, benefits, and future costs. Due to limited available long-term data across comparators, the current model framework was deemed appropriate to evaluate short-term relapse outcomes.
Conclusion
These findings suggest that treatment with brexpiprazole may lead to clinical benefits and medical cost savings. Brexpiprazole treatment resulted in fewer relapses and hospitalizations, lower total cost of treatment, and higher QoL compared to cariprazine and lurasidone. Given the heterogeneity of treatment response in schizophrenia, health plans may consider making multiple treatment options available, and brexpiprazole offers a cost-effective treatment option.
Acknowledgments
Editorial support for the preparation of this manuscript was provided by Ann Cameron, PhD of Xcenda LLC, and funded by Otsuka America Pharmaceutical Inc. Princeton, NJ, USA, and Lundbeck LLC, Deerfield, IL, USA. She has no conflicts of interest to declare. This work was presented as a poster at the 28th Annual US Psychiatric and Mental Health Congress, San Diego, CA, September 10–13, 2015.
Supplementary materials
Figure S1 One-way sensitivity analysis for avoided relapses using $30,000 as the WTP threshold (brexpiprazole vs lurasidone)
Abbreviations: WAC, whole acquisition cost; WTP, willingness to pay.
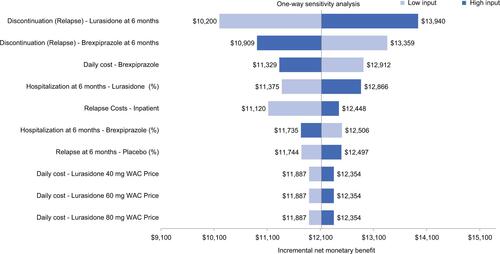
Figure S2 One-way Sensitivity analysis for avoided relapses using $30,000 as the WTP threshold (brexpiprazole vs cariprazine)
Abbreviations: WAC, whole acquisition cost; WTP, willingness to pay.
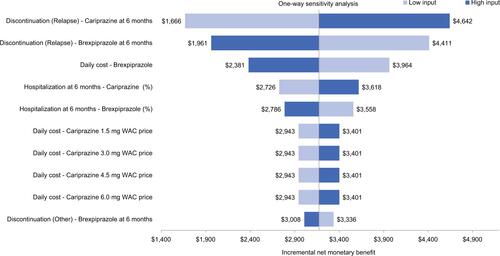
Table S1 Mean probabilistic sensitivity analysis results
Table S2 Scenario analysis: derived rates of adverse events
Table S3 Scenario analysis: results of cost-effectiveness analysis using derived AE rates
Table S4 Scenario analysis: results of cost-effectiveness analysis using generic drug costs for treatments in composite therapy arm
References
- Rexulti [package insert]TokyoOtsuka Pharmaceutical2016
- Latuda [package insert]Marlborough (MA)Sunovion Pharmaceuticals2013
- CitromeLBrexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic – what is the number needed to treat, number needed to harm and likelihood to be helped or harmed?Int J Clin Pract201569997899726250067
- Vraylar (cariprazine) [summary review]2015 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/204370Orig1Orig2s000SumR.pdfAccessed June 19, 2018
- DurgamSStaraceALiDAn evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trialSchizophr Res20141522–345045724412468
- Zyprexa [package insert]IndianapolisLilly USA2014
- Risperdal [package insert]Titusville (NJ)Janssen Pharmaceutical2009
- Seroquel [package insert]Wilmington (DE)AstraZeneca Pharmaceuticals2009
- Geodon [package insert]New YorkPfizer2009
- Abilify [package insert]TokyoOtsuka Pharmaceutical2012
- FleischhackerWWHobartMOuyangJEfficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomised, double-blind, placebo-controlled studyInt J Neuropsychopharmacol2017201112127566723
Disclosure
MSA is an employee at Otsuka Pharmaceutical Development and Commercialization, Inc. SAK was a full time employee of Otsuka Pharmaceutical Development and Commercialization, Inc at the time of this research. In the past 36 months, LC has engaged in collaborative research with or received consulting or speaking fees from Acadia, Alexza, Alkermes, Allergan, AstraZeneca, Avanir, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Forum, Genentech, Janssen, Jazz, Lundbeck, Merck, Medivation, Mylan, Neurocrine, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, Valeant, and Vanda. AMD and SL are employees of Xcenda, a consulting company that received funds from Otsuka America Pharmaceutical and Lundbeck LLC to conduct this study. The authors report no other conflicts of interest in this work.
References
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders5th edArlington (VA)APA2013
- National Institute of Mental HealthSchizophrenia2016 Available from: http://www.nimh.nih.gov/health/statistics/prevalence/schizophrenia.shtmlAccessed June 19, 2018
- CloutierMAigbogunMSGuerinAThe economic burden of schizophrenia in the United States in 2013J Clin Psychiatry201677676477127135986
- Ascher-SvanumHZhuBFariesDEThe cost of relapse and the predictors of relapse in the treatment of schizophreniaBMC Psychiatry201010220059765
- VolavkaJCitromeLOral antipsychotics for the treatment of schizophrenia: heterogeneity in efficacy and tolerability should drive decision-makingExpert Opin Pharmacother200910121917192819558339
- LehmanAFLiebermanJADixonLBPractice guideline for the treatment of patients with schizophrenia, second editionAm J Psychiatry20041612 Suppl156
- BrissosSMolodynskiADiasVVFigueiraMLThe importance of measuring psychosocial functioning in schizophreniaAnn Gen Psychiatry2011101821702932
- LehmanAFLiebermanJADixonLBPractice guideline for the treatment of patients with schizophrenia2nd ed2010 Available from: https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdfAccessed June 19, 2018
- EmsleyRChilizaBAsmalLHarveyBHThe nature of relapse in schizophreniaBMC Psychiatry2013135023394123
- LiebermanJAStroupTSMcevoyJPEffectiveness of antipsychotic drugs in patients with chronic schizophreniaN Engl J Med2005353121209122316172203
- HaroJMSuarezDNovickDBrownJUsallJNaberDThree-year antipsychotic effectiveness in the outpatient care of schizophrenia: observational versus randomized studies resultsEur Neuropsychopharmacol200717423524417137759
- Ascher-SvanumHNyhuisAWStaufferVReasons for discontinuation and continuation of antipsychotics in the treatment of schizophrenia from patient and clinician perspectivesCurr Med Res Opin201026102403241020812791
- Liu-SeifertHAdamsDHKinonBJDiscontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugsBMC Med200532116375765
- CorrellCUSkubanAOuyangJEfficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trialAm J Psychiatry2015172987088025882325
- KaneJMSkubanAOuyangJA multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophreniaSchizophr Res20151641–312713525682550
- KaneJMSkubanAHobartMOverview of short- and long-term tolerability and safety of brexpiprazole in patients with schizophreniaSchizophr Res20161741–3939827188270
- FleischhackerWWHobartMOuyangJEfficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomised, double-blind, placebo-controlled studyInt J Neuropsychopharmacol2017201112127566723
- NeumannPJMethods of cost-effectiveness analysis in the evaluation of new antipsychotics: implications for schizophrenia treatmentJ Clin Psychiatry199960Suppl 3915
- CitromeLKamatSASapinCCost-effectiveness of aripiprazole once-monthly compared with paliperidone palmitate once-monthly injectable for the treatment of schizophrenia in the United StatesJ Med Econ201417856757624758296
- DurgamSEarleyWLiRLong-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled trialSchizophr Res20161762–326427127427558
- TandonRCucchiaroJPhillipsDA double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophreniaJ Psychopharmacol2016301697726645209
- National Institute for Health and Care ExcellencePsychosis and schizophrenia overview2018 Available from: https://pathways.nice.org.uk/pathways/psychosis-and-schizophreniaAccessed June 19, 2018
- CitromeLBrexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic – what is the number needed to treat, number needed to harm and likelihood to be helped or harmed?Int J Clin Pract201569997899726250067
- Rexulti [package insert]TokyoOtsuka Pharmaceutical2016
- Latuda [package insert]Marlborough (MA)Sunovion Pharmaceuticals2013
- CitromeLLurasidone for the acute treatment of adults with schizophrenia: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed?Clin Schizophr Relat Psychoses201262768522776634
- US Bureau of Labor StatisticsConsumer price index: medical care Available from: https://data.bls.gov/cgi-bin/surveymost?cuAccessed June 19, 2018
- LenertLASturleyAPRapaportMHPublic preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scoresSchizophr Res200471115516515374583
- FuriakNMAscher-SvanumHKleinRWCost-effectiveness of olanzapine long-acting injection in the treatment of patients with schizophrenia in the United States: a micro-simulation economic decision modelCurr Med Res Opin201127471373021265593
- LevyARChristensenTLJohnsonJAUtility values for symptomatic non-severe hypoglycaemia elicited from persons with and without diabetes in Canada and the United KingdomHealth Qual Life Outcomes200867318823555
- Ascher-SvanumHFuriakNMLawsonAHCost-effectiveness of several atypical antipsychotics in orally disintegrating tablets compared with standard oral tablets in the treatment of schizophrenia in the United StatesJ Med Econ201215353154722304338
- TreurMBacaEBobesJThe cost-effectiveness of paliperidone extended release in SpainJ Med Econ201215Suppl 1263423016569
- Costco [homepage] Available from: https://www.costco.comAccessed June 19, 2018
- MacewanJPSeaburySAigbogunMSPharmaceutical innovation in the treatment of schizophrenia and mental disorders compared with other diseasesInnov Clin Neurosci2016137–8172527672484
- AlmondSKnappMFrancoisCToumiMBrughaTRelapse in schizophrenia: costs, clinical outcomes and quality of lifeBr J Psychiatry200418434635115056580
- Ng-MakDChuangCCBakerTLiJRajagopalanKLoebelALurasidone versus brexpiprazole in adult schizophrenia: cost-effectiveness analysisPoster presented at: 29th Annual US Psychiatric and Mental Health CongressOctober 21–24San Antonio, TX
- O’DayKRajagopalanKMeyerKPikalovALoebelALong-term cost-effectiveness of atypical antipsychotics in the treatment of adults with schizophrenia in the USClinicoecon Outcomes Res2013545947024049452
- RajagopalanKHassanMO’DayKMeyerKGrossmanFCost-effectiveness of lurasidone vs aripiprazole among patients with schizophrenia who have previously failed on an atypical antipsychotic: an indirect comparison of outcomes from clinical trial dataJ Med Econ201316795196123701260
- CsernanskyJGMahmoudRBrennerRA comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophreniaN Engl J Med20023461162211777998
- LoebelASilvaRGoldmanRLurasidone dose escalation in early nonresponding patients with schizophrenia: a randomized, placebo-controlled studyJ Clin Psychiatry201677121672168027454547
- CitromeLSchizophrenia relapse, patient considerations, and potential role of lurasidonePatient Prefer Adherence2016101529153727563237
- MeltzerDPerspective and the measurement of costs and benefits for cost-effectiveness analysis in schizophreniaJ Clin Psychiatry199960Suppl 333237
- BeasleyCMSuttonVKHamiltonSHA double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapseJ Clin Psychopharmacol200323658259414624189
- PeuskensJTrivediJMalyarovSPrevention of schizophrenia relapse with extended release quetiapine fumarate dosed once daily: a randomized, placebo-controlled trial in clinically stable patientsPsychiatry (Edgmont)20074113450
- AratoMO’ConnorRMeltzerHYA 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) studyInt Clin Psychopharmacol200217520721512177583
- PigottTACarsonWHSahaARAripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week studyJ Clin Psychiatry20036491048105614628980
- Vraylar (cariprazine) [summary review]2015 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/204370Orig1Orig2s000SumR.pdfAccessed June 19, 2018
- DurgamSStaraceALiDAn evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trialSchizophr Res20141522–345045724412468
- Zyprexa [package insert]IndianapolisLilly USA2014
- Risperdal [package insert]Titusville (NJ)Janssen Pharmaceutical2009
- Seroquel [package insert]Wilmington (DE)AstraZeneca Pharmaceuticals2009
- Geodon [package insert]New YorkPfizer2009
- Abilify [package insert]TokyoOtsuka Pharmaceutical2012
- Agency for Healthcare Research and QualityHCUPnet: Healthcare Cost and Utilization Project Nationwide Inpatient Sample2013 Available from: http://hcupnet.ahrq.govAccessed June 19, 2018
- ParkTKuntzKMCost-effectiveness of second-generation antipsychotics for the treatment of schizophreniaValue Health201417431031924968989
- Truven Health AnalyticsMicromedex 2.0: introduction to Red Book onlineAccessed June 19, 2018
Appendix 1
Calculation of treatment discontinuation relative risks
Treatment discontinuation relative risks were calculated for active treatment vs placebo using a 3-step method. First, patients who were terminated by the sponsor were removed from the efficacy sample, and the discontinuation rate due to relapse (or due to AE or other reasons) was recalculated for both the treatment and placebo groups. Of note, in the maintenance trial of cariprazine, no patients were terminated by the sponsor; therefore, this step was skipped for the cariprazine calculation.Citation11 Next, to ensure that all probabilities were calculated within the same time frame, any transition probabilities other than 6 months were converted by using the following formula where EXP refers to the exponential function and LN refers to the natural logarithm function: 1-EXP(LN(1-Probability)/(Number of weeks in the original trial/26)). Finally, the 6-month probability from step 2 was adjusted by applying the relative risk method where the product between the relative clinical rate within trial (active vs respective placebo) and a pooled placebo clinical rate was calculated.
