Abstract
Purpose
The objectives of this study were to present trends in posaconazole use over time and describe selected outcomes among patients at high risk of invasive fungal infections (IFIs) by use and type of antifungal medicine.
Methods
A retrospective observational study using data from the Premier Healthcare Database between January 2007 and March 2016 was conducted. Inpatient use of posaconazole by formulation and year is described. Separately, four cohorts of patients at high risk of IFI – those with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), hematopoietic stem-cell transplantation (HSCT), and graft-vs-host disease (GVHD) – but without a diagnosis code for IFI during the index encounter were identified as potential candidates for antifungal prophylaxis. Use of antifungal medication(s) in these patients was categorized. Index length of stay (LOS), index hospital costs, and subsequent inpatient and outpatient encounters with IFI at 30, 60, and 90 days post-index encounter are presented by antifungal group for each cohort. The percentage of patients with inpatient and outpatient encounters with IFI at 90 days post-index encounter was determined for each cohort by year.
Results
Use of posaconazole oral suspension increased through 2012, then declined as the tablet formulation became available in 2013. A total of 19,872 AML patients, 12,125 MDS patients, 14,220 HSCT patients, and 5,431 GVHD patients were considered potential candidates for antifungal prophylaxis; however, a large proportion of patients within each cohort (33%–94%) did not receive any antifungal drug during the index hospitalization. Index LOS, hospital costs, and subsequent encounters for IFI varied among cohorts and by antifungal group. Within each cohort, subsequent encounters for IFI at 90 days post-index encounter fluctuated but remained rare across different years.
Conclusion
Over time and as new posaconazole formulations became available, the frequency of use of each formulation changed. In addition, this study suggested a low rate of potential antifungal prophylaxis in high-risk patients. This is one of the first reports attempting to describe antifungal prophylaxis in a contemporary, large, all-payer, geographically representative hospital database.
Introduction
Patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) are at high risk for invasive fungal infection (IFI) when presented with prolonged neutropenia after induction chemotherapy.Citation1 IFI is associated with increased morbidity and mortality and is a serious concern in AML, MDS, and other immunocompromised patients, such as those undergoing hematopoietic stem-cell transplantation (HSCT) or with graft-vs-host disease (GVHD).Citation2,Citation3 Several professional guidelines recommend using antifungal prophylaxis in high-risk patients and consider timely initiation of antifungal use as a critical component in improving patient outcomes.Citation4–Citation6
To date, many antifungal drugs have demonstrated poor efficacy, particularly in the prevention of invasive aspergillosis. The use of fluconazole as prophylaxis is limited by its narrower spectrum of antifungal activity, being effective only against Candida strains.Citation7 Voriconazole does not show any significantly greater benefit than other azoles in antifungal prophylaxis.Citation8–Citation10 When given in capsules, itraconazole is absorbed poorly, and when given as oral suspension it has gastrointestinal side effects.Citation11 Micafungin and caspofungin can only be administered intravenously, are approved only for prophylaxis of Candida infections, and the effectiveness of prophylaxis in hematological patients has not been consistently reported.Citation12–Citation15 Finally, unless there are contraindications to use of azole antifungals, amphotericin is not recommended for use as the primary prophylactic treatment.Citation16
Posaconazole is a new-generation azole that is recommended for use in neutropenic patients, SCT recipients, and patients with severe GVHD for antifungal prophylaxis by National Comprehensive Cancer Network, American Society of Clinical Oncology, and Infectious Diseases Society of America guidelines.Citation5,Citation7,Citation17 Three formulations of posaconazole – oral suspension, delayed-release tablet, and parenteral – are currently US Food and Drug Administration (FDA)-approved for the prophylaxis of invasive Aspergillus and/or Candida infections. In clinical trials, posaconazole has proved to be clinically superior to other triazoles in preventing IFI, especially aspergillosis.Citation17,Citation18 Implementation of clinical guidelines and research findings in current practice, however, has not been well followed. Further, real-world evidence leveraging nationwide, geographically representative data to assess associations between posaconazole use and patient outcomes is lacking.
This study set out to describe real-world use of different formulations of posaconazole in a hospital setting. Four cohorts of patients at high risk of IFI – those with AML, MDS, HSCT, and GVHD – and those without diagnosis codes for FI during the index hospitalization were identified as likely candidates for antifungal prophylaxis. Within each cohort, the observed antifungal use was identified and categorized as single (posaconazole or other antifungal), multiple (antifungals with and without posaconazole), or none. Selected economic and clinical outcomes are described for each of the cohorts.
Methods
Study design
A retrospective observational study using the Premier Health-care Database (PHD) was conducted to describe real-world use of posaconazole in a hospital setting and among patients at high risk of FIs, as well as to explore use of common antifungal drugs and occurrence of IFIs up to 90 days after discharge.
Data source
The PHD is a large database of geographically diverse US hospitals containing patient- and hospital-level information and representing a variety of payer types. The PHD contains a subset of data from the Premier Quality Advisor platform that offers deidentified, Health Insurance Portability and Accountability Act-compliant data. Use of the PHD data for this study was considered exempt from institutional review-board oversight, as dictated by Title 45 Code of Federal Regulations (CFR), Part 46 of the US, specifically 45 CFR 46.101(b)(4) (http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html). In addition, in accordance with the Health Insurance Portability and Accountability Act privacy rule, data disclosed from the PHD are considered deidentified as per 45 CFR 164.506(d)(2)(ii)(B) through the “expert determination” method.
During the study period, data from standard hospital-discharge files, including patient demographics, disease states, admission and discharge diagnoses, patient disposition, and discharge status were available from more than 654 million stays, representing more than 20% of all US hospital discharges. At the time of analyses, there were more than 151 million patients. The PHD also contains a date-stamped log of billed items, including procedures, devices, medications, laboratory tests, and diagnostic and therapeutic services at the individual patient level. Drug-utilization information is available by day of stay and includes quantity, dose, strength, and hospital cost. Patients can be tracked across inpatient and hospital-based outpatient settings, as well as across visits with a unique identifier within a single hospital. Hospital information included geographic location, population served (urban vs rural), teaching status, and number of beds.
Study population
All patients discharged from an inpatient hospital visit between January 1, 2007 and March 31, 2016 receiving at least one dose of posaconazole, whether used alone or in combination with other antifungals, were identified using medication-billing records in the PHD. The formulation used (oral suspension, oral tablet, parenteral) was also identified from the billing records. Since the tablet formulation of posaconazole was approved by the FDA in November 2013 and the parenteral formulation approved in March 2014, any posaconazole used prior to November 2013 was labeled as the oral suspension. If the formulation after November 2013 could not be determined from the billing record, it was classified as an unknown formulation. Trends in use by formulation or use of combinations of posaconazole formulations were displayed over time.
AML patients that did not achieve remission or were in relapse and patients with MDS, HSCT, or GVHD were identified as high-risk population for IFIs and included in the study. For descriptions of antifungal-medication use in the study cohort, all patients discharged from an inpatient hospital visit between January 1, 2007 and March 31, 2016 with a primary diagnosis code for each condition were identified. A hierarchical approach was used to categorize patients into one of the four groups. All patients with a primary ICD9/10 diagnosis code for AML (205.00, 205.02, C92.00, C92.40, C92.50, C92.02, C92.42, or C92.52) who did not meet the criteria for HSCT were categorized into the AML cohort. All patients with a primary ICD9/10 diagnosis code for MDS (238.72-75, D46.0-2, D46.9 or D46.A-C) who did not meet the criteria for HSCT were categorized into the MDS cohort. Patients with a primary ICD9/10 procedure code for HSCT () who did not meet the criteria for the GVHD cohort were categorized into the HSCT cohort. Patients with a primary or secondary ICD9/10 diagnosis code for GVHD (279.50-3 or D89.810-3) who also had at least one dose of a selected immunosuppressant drug (methylprednisolone, prednisone, beclomethasone, cyclosporine, sirolimus, tacrolimus, mycophenolate mofetil, thalidomide, methotrexate, azathioprine, pentostatin, infliximab, rituximab, etanercept, methoxsalen, denileukin, antithymocyte globulin, daclizumab, basiliximab, or alemtuzumab) on the hospital medication-billing record were categorized into the GVHD cohort.
For each cohort, the first qualifying visit was identified as the index visit. Patients were then excluded if they had an admission or discharge diagnosis code for an FI during the index visit (ICD9 diagnosis codes 112.XX, 114.X, 115.XX, 116.X, 117.X, 118, 348.89, 484.6, 484.7, 495.4, and 495.6; ICD10 diagnosis codes B36.8, B37.X-B49, H16.069, J67.4, and J67.6). Subsequent inpatient and outpatient encounters in the same hospital system were identified through 90 days after the index visit discharge date.
Antifungal-use groups of interest
Within each high-risk cohort, patients were further categorized into one of antifungal-use groups based upon the use of antifungal medications during the index visit:
single antifungal (posaconazole) – the only antifungal used during the index visit was posaconazole
single antifungal (not posaconazole) – use of only one of fluconazole, itraconazole, voriconazole, micafungin, caspofungin, or amphotericin B during the index visit
multiple antifungals (including posaconazole) – multiple antifungal drugs listed above were used during index encounter, including posaconazole
multiple antifungals (not including posaconazole) – multiple antifungal drugs used during the index visit, but none were posaconazole
no antifungal – no antifungal drugs were used during the index visit
Patient and hospital characteristics
Selected patient and visit characteristics (age, sex, race, ethnicity, admission type, and discharge status) were obtained from the PHD and are presented by high-risk cohort and antifungal-treatment groups. Selected hospital characteristics (teaching status, urban/rural location, US Census geographical regions, and number of beds) were similarly obtained and are presented in the same manner.
Study outcomes
Outcomes of interest during the index hospitalization and following discharge from the index hospitalization were determined. Outcomes during index hospitalization were total hospital length of stay (LOS) and total hospital cost. Outcomes following index hospital discharge were the occurrence of 30-, 60-, and 90-day readmissions and subsequent outpatient visits with the presence of a primary or secondary ICD admission or discharge diagnosis code for IFI ().
Statistical analysis
Descriptive statistics were derived. Continuous data are expressed as means ± SD, minimum and maximum, and medians and IQR. Categorical data are expressed as counts and percentages. Patients with missing values for total hospital cost were excluded from cost analysis. Patients who died during the index encounter were excluded from the denominator for analyses of subsequent encounters.
Results
Posaconazole use in a hospital setting
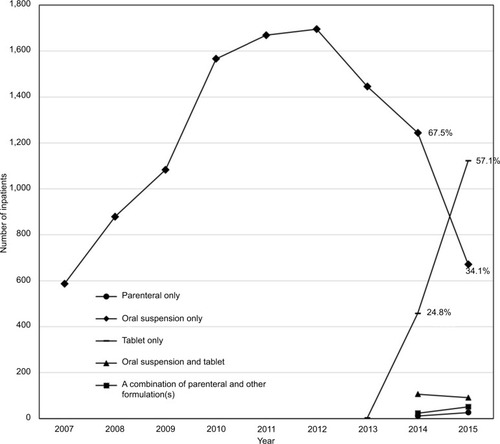
Prior to 2013, the only formulation of posaconazole available in the US was the oral suspension. The number of patients receiving posaconazole oral suspension peaked in 2012 and rapidly declined thereafter (). Much of that decline may have resulted from availability of the oral tablet formulation, which demonstrated rapid growth in use in 2014 and 2015. The proportion of inpatients receiving posaconazole who were administered the tablet formulation increased from 24.8% in 2014 to 62.3% in the first quarter of 2016 (data not shown), while the proportion of inpatients receiving the oral suspension decreased from 67.5% to 32.7% during this same period (data not shown). There was less use of the most recently marketed formulation: parenteral posaconazole. When used, it was frequently prescribed in combination with other formulations during the same hospitalization.
Figure 1 Inpatients receiving posaconazole by formulation and by year.
Patient and hospital characteristics of high-risk patients
presents patient and hospital characteristics for each of the four patient cohorts. presents patient and hospital characteristics by patient cohort for the five antifungal categories. A total of 51,648 patients who met study criteria were categorized: 19,872 (38%) AML patients, 12,125 (23%) MDS patients, 14,220 (28%) HSCT patients, and 5,431 (11%) GVHD patients (). Among these, 58% of AML patients (11,482), 94% of MDS patients (11,382), 33% of HSCT patients (4,736), and 37% of GVHD patients (2,014) did not receive any of the selected antifungal medications during the index hospitalization (). Posaconazole, alone or in combination with another drug, was received in <10% of patients across the four cohorts.
Table 1 Patient, visit, and hospital characteristics of patients with a diagnosis of AML, MDS, HSCT, or GVHD without documented fungal infections
The median patient age was highest in the MDS cohort (median 79 years, IQR 70–85 years) and lowest in the GVHD cohort (median 52 years, IQR 35–61 years). In each cohort, there was a higher proportion of men than women; however, in the subgroup of MDS patients receiving posaconazole plus other antifungals, only 41% were male. Across all four cohorts, approximately 70% of patients were white. Approximately 70%–85% of patients with AML, MDS, or GVHD had an emergent or urgent visit, while only 36% of patients with HSCT had an emergent or urgent visit. Most patients were discharged home, and there were more AML patients transferred to another hospital, hospice, or expired than other cohorts. Consistently across the four cohorts, it appeared that patients receiving posaconazole as the only antifungal during the index encounter had low in-hospital mortality among the five antifungal groups.
More than half of HSCT and GVHD patients were admitted to hospitals with 550+ beds, while fewer patients with AML (37%) or MDS (24%) were treated at such hospitals. With regard to geographical distribution, more patients with HSCT (30%) and GVHD (26%) were treated at hospitals in the Northeast than patients with AML (18%) or MDS (20%). shows that patients treated with antifungals were more frequently seen in hospitals with 550+ beds than hospitals of smaller sizes. Posaconazole, alone or in combination with another antifungal, was found to be more frequently used in AML, MDS, and GVHD patients in the Northeast and in hospitals with 550+ beds, which is consistent with the fact that Northeast hospitals tend to have larger bed capacity than other regions in our study sample (data not shown).
The majority of patients with HSCT (73%) or GVHD (64%) were treated at teaching hospitals, whereas only around half of or fewer patients with AML (52%) or MDS (42%) were treated at teaching hospitals. Across all cohorts and treatment categories (), over 90% of patients were admitted to a hospital in an urban area. Overall, antifungal prophylaxis was more frequently given to all four cohorts by teaching hospitals. In both single and multiple antifungal therapies, posaconazole was more frequently given to AML and MDS patients by teaching hospitals.
Economic and clinical outcomes of high-risk patients
– present economic and clinical outcomes by antifungal categories for AML, MDS, HSCT, and GVHD cohorts, respectively. Across these four cohorts, patients with no antifungal treatment were found to have the shortest LOS, whereas patients with more than one type of antifungal had the longest LOS. There was not a noticeable difference in LOS between patients treated with posaconazole only and patients treated with one other antifungal only or between patients receiving multiple antifungals treated with and without posaconazole. Consistently, in all four cohorts, patients with no antifungal treatment had the lowest total hospital cost, whereas patients with more than one type of antifungal had the highest. Total hospital costs were similar between patients treated with single antifungals whether posaconazole or other agent; and between patients receiving multiple antifungals whether with or without posaconazole.
Table 2 Economic and clinical outcomes in AML patients without fungal infection diagnosis
Table 3 Economic and clinical outcomes in MDS patients without fungal infection diagnosis
Table 4 Economic and clinical outcomes in HSCT patients without fungal infection diagnosis
Table 5 Economic and clinical outcomes in GVHD patients without fungal infection diagnosis
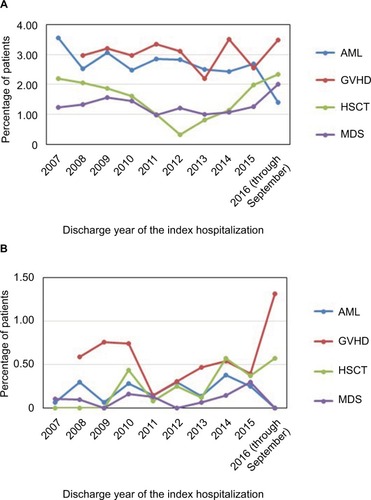
IFI-related readmissions and subsequent outpatient visits were rare events. shows that the percentage of patients with subsequent inpatient and outpatient encounters with IFI at 90 days post-index encounter fluctuated yet remained very low over the study period. For each cohort, there was not a noticeable difference across different years. Patients with no antifungal treatment had the fewest IFI-related readmissions and subsequent outpatient visits, and no remarkable differences were found among the remaining four treatment groups (–).
Figure 2 Inpatients with 90-day subsequent inpatient (A) and outpatient visits (B) with IFI by cohort and by year.
Abbreviations: AML, acute myeloid leukemia; GVHD, graft-vs-host disease; HSCT, hematopoietic stem-cell transplantation; IFI, invasive fungal infection; MDS, myelodysplastic syndrome.
Discussion
Antifungal prophylaxis for high-risk patients has been promoted by various scientific societies due to the rising incidence of life-threatening IFIs and undesired outcomes when initiation of antifungal use is delayed.Citation19 This is one of the first studies to observe and characterize the possible use of antifungal prophylaxis and associated outcomes in a large, representative database. Our findings indicate that across different cohorts of immunocompromised patients, antifungal prophylaxis appears to be underutilized, especially in patients with shorter hospital stays. As patient LOS extended, a greater proportion of the at-risk population started to receive multiple antifungal drugs, even when no FI was present. Although antifungal prophylaxis is currently regarded as the gold standard in situations with a high risk of FI, such as in these immunocompromised patients,Citation4,Citation5,Citation7,Citation9 it is possible that the clinician’s perspective of risk for an individual patient may vary and/or there may be inconsistency in the interpretation and application of guideline recommendations for antifungal prophylaxis.Citation20
The unexpected finding that a substantial number of high-risk patients did not receive antifungal prophylaxis highlights the need for initiatives to promote the adoption of guideline recommendations. The higher proportion of patients receiving antifungal prophylaxis within teaching hospitals may indicate greater knowledge, receptivity, and/or availability of tools for utilization of antifungal prophylaxis or could be due to the fact that teaching hospitals may contain a higher proportion of complex patients considered at extremely high risk of FIs. Regional differences may also indicate varying degrees of knowledge, receptivity, and available resources. Additional investigations may facilitate a better understanding of clinician decision-making and guide development of tools for greater use of antifungal prophylaxis in appropriate patients across all hospitals in the nation.
There are a number of limitations to this study. First, the identification of antifungals was based on text-string searching in hospitals’ charge masters. Since hospitals record their pharmacy costs in a variety of ways, some discharges that used an antifungal of interest may not have been captured. Second, due to the retrospective nature of this study and lack of access to medical records, it was extremely difficult to differentiate antifungal prophylaxis from treatment or empirical treatment. One important assumption of the study was that patients with antifungal use who did not have any diagnosis for an FI were likely receiving the antifungal(s) for prophylaxis. Under this assumption, those who received antifungal prophylaxis in the beginning of their stay but later developed an FI were not a focus of this study, and addition of this subset of patients in future studies could lead to different conclusions. Third, the risk of IFI and corresponding therapeutic strategies to address IFI were not constant during all the phases of treatment of the four cohorts. For example, since induction chemotherapy is the first time that a patient experiences profound immunosuppression, most AML patients are at greater risk of IFI at this stage, but differentiations of the treatment stage of each patient were not included in this study. Fourth, patients discharged from January 1, 2007 to March 30, 2007 may have had a hospitalization during the 90 days prior to their index hospitalization, raising a possibility, albeit small, that some readmissions might be misclassified. It is also important to note that this US study may not be generalizable to other health-care settings. Lastly, the current study design was descriptive. No unadjusted or adjusted comparisons were made.
Conclusion
To date, there has been limited retrospective research using a large, all-payer, geographically representative hospital database to describe high-risk patients who require antifungal prophylaxis. This current study, despite its limitations, adds real-world knowledge to this field. The results revealed that contrary to guideline recommendations, some high-risk patients did not receive any antifungal prophylaxis. Additional research is necessary to confirm this finding and determine reasons for potential underutilization of antifungal prophylaxis in high-risk patients, in order to develop interventions and tools to improve guideline adherence and clinical outcomes.
Acknowledgments
The authors wish to acknowledge Carol Cohen, who provided editorial support for the manuscript, and also Hetty Waskin and Michael Westmoreland for their invaluable clinical insights that significantly enhanced the manuscript.
Supplementary material
Table S1 Primary ICD9/10 procedure code for hematopoietic stem-cell transplantation
Table S2 Primary or secondary ICD admission or discharge diagnosis code for invasive fungal infection
Table S3 Patient, visit, and hospital characteristics by antifungal use
Disclosure
RF and JG are employees of Premier, which received funding from Merck for conducting this study. AHS was formerly with Merck & Co., Inc. The authors declare no other conflicts of interest in this work.
References
- RüpingMJVehreschildJJCornelyOAPatients at high risk of invasive fungal infections: when and how to treatDrugs200868141941196218778118
- FlemingSYannakouCKHaeuslerGMConsensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014Intern Med J20144412B1283129725482741
- MarrKABowEChillerTFungal infection prevention after hematopoietic cell transplantationBone Marrow Transplant200944848348719861982
- FlowersCRSeidenfeldJBowEJAntimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guidelineJ Clin Oncol201331679481023319691
- FreifeldAGBowEJSepkowitzKAClinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of AmericaClin Infect Dis2011524e56e9321258094
- BadenLRBensingerWAngaroneMPrevention and treatment of cancer-related infectionsJ Natl Compr Canc Netw201210111412144523138169
- PappasPGKauffmanCAAndesDRClinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of AmericaClin Infect Dis2016624e1e5026679628
- CornelyOAMaertensJWinstonDJPosaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropeniaN Engl J Med2007356434835917251531
- TackeDBuchheidtDKarthausMPrimary prophylaxis of invasive fungal infections in patients with haematologic malignancies: 2014 update of the recommendations of the Infectious Diseases Working Party of the German Society for Haematology and OncologyAnn Hematol20149391449145624951122
- VehreschildJJBöhmeABuchheidtDA double-blind trial on prophylactic voriconazole (VRC) or placebo during induction chemotherapy for acute myelogenous leukaemia (AML)J Infect200755544544917822770
- NucciMBiasoliIAkitiTA double-blind, randomized, placebo-controlled trial of itraconazole capsules as antifungal prophylaxis for neutropenic patientsClin Infect Dis200030230030510671332
- ScottLJMicafungin: a review of its use in the prophylaxis and treatment of invasive Candida infectionsDrugs201272162141216523083111
- HiramatsuYMaedaYFujiiNUse of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantationInt J Hematol200888558859519039629
- HirataYYokoteTKobayashiKAntifungal prophylaxis with micafungin in neutropenic patients with hematological malignanciesLeuk Lymphoma201051585385920214445
- NeofytosDHuangYTChengKSafety and efficacy of intermittent intravenous administration of high-dose micafunginClin Infect Dis201561Suppl 6S652S66126567284
- UzunOAnaissieEJAntifungal prophylaxis in patients with hematologic malignancies: a reappraisalBlood1995866206320727662953
- SoysalAPrevention of invasive fungal infections in immunocompromised patients: the role of delayed-release posaconazoleInfect Drug Resist201583213126392781
- UllmannAJLiptonJHVesoleDHPosaconazole or fluconazole for prophylaxis in severe graft-versus-host diseaseN Engl J Med2007356433534717251530
- BoutatiEIAnaissieEJFusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for managementBlood199790399910089242529
- SungAHRhodesTArduinoJInconsistency in defining profound and prolonged neutropenia for antifungal prophylaxis decisionsPoster presented at: ICAAC–ICC2015September 17–21, 2015San Diego, CA
