Abstract
Background
Real-world outcomes from staying on an interferon beta (IFNβ) vs switching to another IFNβ could help guide treatment decisions. This study’s objective was to compare outcomes of stable multiple sclerosis (MS) patients on an IFNβ who stayed on therapy vs those who switched to another IFNβ.
Methods
MS patients were identified from the Optum Insights Clinformatics Data Mart Multi-Plan who were 18–64 years old and relapse-free (stable) over 1 year while continuously being treated with an IFNβ. Patients were propensity score matched 3:1 using age, gender, initial IFNβ, adherence, and month and year for patients who stayed on the initial IFNβ (No Switch) to patients who switched to another IFNβ (Switch). Patients had to be continuously enrolled for 1 year prior to and 1 year after the index date (date of the first claim of the switched-to IFNβ or the match date when continuing on initial IFNβ treatment). Patients were enrolled with index dates between January 1, 2005 and September 30, 2014. Relapses were recorded during the 1-year follow-up period after index date.
Results
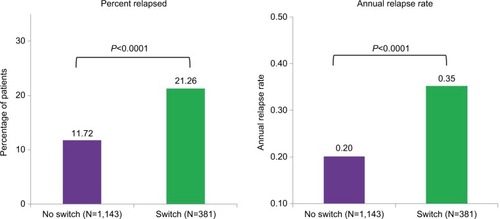
After matching, there were 381 patients in the Switch group and 1,143 in the No Switch group. Baseline characteristics were well matched between groups (average age 46 years, 72% female). The percentage of patients experiencing a relapse during the follow-up was significantly higher in the Switch group than in the No Switch group (21% vs 12%, P<0.0001). Annual relapse rate during the follow-up was significantly higher in the Switch group than in the No Switch group (0.35 vs 0.20, P<0.0001).
Conclusion
MS patients stable on IFNβ therapy who remain on initial therapy had significantly better outcomes (lower annual relapse rate and percentage of patients with relapses) than patients who switched to another IFNβ. This supports the benefits of allowing patients to remain on current IFNβ therapy when stable.
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated demyelinating disease that affects the central nervous system, causing a large range of disabling symptoms. As of 2013, ~2.3 million people worldwide suffer from MS, making it the largest cause of disability in people under the age of 50 years.Citation1 Approximately 85% of MS patients have relapsing-remitting MS (RRMS), characterized by acute relapses lasting from days to months, followed by partial or complete recovery during periods of remission where there is no disease activity. RRMS tends to progress to greater disability over time (secondary progressive MS).Citation1,Citation2 Although no cure exists, RRMS can be treated with disease-modifying therapies (DMTs) which limit the number of relapses a patient experiences and slow the rate of disease progression.Citation3
There are several DMTs that have been approved by both the European Medicines Agency and the US Food and Drug Administration (FDA), including interferon beta (IFNβ) that is recommended as first-line DMTs for RRMS in over 90 countries. IFNβ occurs naturally in the body in response to initiating factors such as viruses. IFNβs block the activity of T cells and reduce the passage of these cells into the central nervous system where they would cause demyelination.Citation4,Citation5 Four IFNβ formulations are available as injections for the treatment of RRMS: subcutaneous (SC) IFNβ-1b (Betaferon®/Betaseron® [Bayer AG, Leverkusen, Germany], Extavia® [Novartis, Basel Switzerland]) every other day, intramuscular (IM) IFNβ-1a (Avonex® [Biogen, Cambridge, MA, USA]) delivered once weekly, SC IFNβ-1a (Rebif® [EMD Serono, Inc., Rockland, MA, USA]) delivered three times per week, and SC peginterferon beta-1a (Plegridy® [Biogen]) delivered once every 2 weeks. All have comparable efficacy with respect to relapse rates and favorable safety profiles and are well tolerated, with few patients experiencing serious treatment-related adverse events.Citation6 Both IFNβ-1a products have also been shown to slow disability progression.Citation6 A continuous use of IFNβs over the course of years can benefit long-term patient outcomes by reducing the number and frequency of relapses and possibly delaying disease progression.Citation7–Citation10
RRMS patients on IFNβ therapy are sometimes switched to an alternative DMT or to a different IFNβ. Reasons for switching may be due to suboptimal response to initial therapy, patient preferences/poor adherence (eg, due to frequency or mode of administration or adverse reactions), or disease progression.Citation11–Citation13 Increasingly, formulary changes by the patient’s insurer also may result in switching without a clinical or patient-related basis for the switch. Formulary restrictions may include differing tiers/higher co-pays for some IFNβs or complete exclusion of some drugs in favor of others.Citation14,Citation15 The outcomes of patients who have been switched when stable on treatment have not been extensively studied, and investigation using real-world data of outcomes following a switch from one IFNβ therapy to another IFNβ therapy vs staying on current IFNβ therapy may help guide treatment decisions and formulary decision-makers. The objective of this study was to compare outcomes of stable MS patients on an IFNβ who stayed on their initial therapy vs those who switched to another IFNβ therapy.
Methods
This is a retrospective claims-based analysis using propensity-score matching in cohorts of MS patients on IFNβ therapy. Patients treated with peginterferon beta-1a were excluded from the analysis due to limited data availability since its launch in 2014. Study subjects were selected from the Clinformatics™ Data Mart Multi-Plan claims database from Optum Insight (Eden Prairie, MN, USA). The data were aggregated and de-identified to protect confidentiality. As a result, no review by an institutional review board or ethics committee was required for this study. The database includes linked medical and pharmacy claims in addition to eligibility information. The study period ran from January 1, 2004 to September 30, 2015. Patients were selected who had a diagnosis of MS (ICD-9 code: 340), were 18–64 years old, and had to be continuously enrolled and relapse-free (stable) while being treated with an IFNβ for 1 year prior to the index date, which is defined as the date of the first claim after switching to a new IFNβ or the propensity-scoring match date when continuing on initial IFNβ treatment. Patients were stratified into two groups: those who stayed on the initial IFNβ (No Switch) and those who switched to another IFNβ (Switch) and were followed for 1 year after the index date, during which time relapses (percent of patients relapsed and annual relapse rate) were measured and patients had to be continuously enrolled. Patients were selected with index dates between January 1, 2005 and September 30, 2014 ().
Patient matching
Patients who switched may have had different clinical characteristics compared to those who remained on a single IFNβ therapy. To remove potential confounding between comparison cohorts, patients were propensity score matched 3:1 using the greedy method (also known as nearest neighbor or nearest available matching) without replacement for those who stayed on their baseline therapy (No Switch) vs those who switched to another IFNβ therapy (Switch).Citation16
A logistic regression model was estimated with the dependent variable of switching vs no switching. Independent variables were patient baseline characteristics that may have influenced the decision to switch treatments and/or which may affect disease outcomes, including age, gender, insurance plan type, month and year of index date, prior IFNβ used and prior adherence category (medication possession ratio, <0.6, 0.6–0.8, ≥0.8), number of doctors’ visits in 1 year prior to index date, total medical costs (log-transformed), and presence of MS-related symptoms. For each Switch patient, the three closest No Switch patients were selected based on the propensity scores, which are the estimated probability of treatment switching obtained from the regression model. Propensity score matching improves the comparability of overall probability of treatment switch ing; however, an imbalance in individual measures may still exist. To further minimize the selection bias, identification of matching controls was further stratified by age category, gender, month/year of index date, previous IFNβ used, and prior year adherence category.
Outcome measurement and statistical analysis
Outcomes were assessed for the Switch vs No Switch groups. Relapses were recorded during the 1-year follow-up period after index date. Relapses were operationally defined from claims data as any inpatient hospital stay with primary diagnosis of MS, or any outpatient visit (either emergency room or office visit) with use of corticosteroids (high dose of oral steroid [daily dose of ≥500 mg prednisone] or intravenous), adrenocorticotropic hormone, or total plasma exchange within 30 days after the stay and/or visit.Citation17 Annual relapse rates were defined as the total number of relapses divided by the total number of patient years (total number of days in the study for the group, divided by 365). Descriptive statistics were provided for cohorts before and after propensity score matching. Mean and SD were presented for continuous measures, and count and proportion were presented for categorical measures. Statistical tests were conducted to detect statistically significant differences (ie, P<0.05) between Switch and No Switch cohorts before and after matching. For age, Student’s t-test was used. For count and cost variables, nonparametric Wilcoxon rank-sum test was used. For categorical measures, chi-squared tests or Fisher’s exact tests were used, as appropriate. Statistical analysis was done using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Subgroup analysis
A subgroup analysis was performed comparing percent of patients relapsed and annual relapse rates between the Switch and No Switch groups for the subjects who were using IFNβ-1a IM in the baseline period. Subgroups were too small in the other baseline IFNβ treatment groups (IFNβ-1a SC and IFNβ-1b SC) to include in subanalyses.
Results
After matching, there were 1,143 patients in the No Switch group and 381 patients in the Switch group. Baseline characteristics before and after matching are shown in , and were well matched between the groups. After matching, only one match characteristic (percent with fatigue/malaise as an MS-related symptom) showed a difference between the groups.
Table 1 Baseline characteristics
Both the proportion of patients experiencing a relapse and the annual relapse rate during the follow-up year were significantly higher (P<0.0001) in the Switch group than in the No Switch group (). Specifically, the percentage of patients relapsed was 82% higher in the Switch group (21.3% vs 11.7%) and the annual relapse rate was 75% higher in the Switch group (0.35 vs 0.20 relapses per patient year).
Subgroup analysis
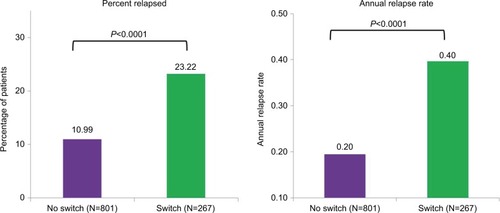
For the subgroup analysis of patients treated with IFNβ-1a IM in the baseline period, results were similar to the findings in the overall analysis. After matching, there were 801 patients in the No Switch group and 267 patients in the Switch group. Baseline characteristics before and after matching are shown in , and were again well matched between groups, with statistically significant differences (P<0.05) again found only between the Switch and No Switch groups after matching on percent with fatigue/malaise as an MS-related symptom.
Table 2 Baseline characteristics of IFN β-1a IM users
Study end point results were more striking in the patients treated with IFNβ-1a IM in the baseline period than in the overall study results (). In the subgroup of patients who stayed on IFNβ-1a IM, the percent relapsed was 113% higher in patients who were switched from IFNβ-1a IM to another IFNβ (23.22% vs 10.99%). Similarly, the annual relapse rate was twice as high in the Switch group (0.40 vs 0.20 relapses per patient year) in the subgroup analysis.
Figure 3 Percentage of patients relapsed and annual relapse rate – IFN β-1a IM subgroup.
presents data on what IFNβ patients were switched to for switched patients in the post-matched cohort. The majority of switched patients (70%) were initially treated with IFNβ-1a IM. Of these patients, 83% were switched to IFNβ-1a SC, and 17% were switched to IFNβ-1b SC. Patients initially treated with IFNβ-1a SC were mostly switched to IFNβ-1a IM (68%), while patients initially treated with IFNβ-1b were more often switched to IFNβ-1a SC (62%).
Table 3 The switching pattern among patients who switched IFNβ treatments
Discussion
In this propensity score-matched study in clinically stable (relapse-free) MS patients on IFNβ therapy, patients who remained on the same therapy had significantly better outcomes than those who switched to another IFNβ therapy. Both the percentage of patients experiencing a relapse and the annual relapse rates during the 1-year follow-up period were significantly higher in the Switch group than in the No Switch group. Patients who remained on their initial IFNβ therapy had 45% lower odds of experiencing a relapse and a 43% lower annual relapse rate than those patients who switched to a different IFNβ therapy. The subset of patients stable on IFNβ-1a IM who remained on therapy had similar results to the overall selected population, showing significantly better outcomes than those who switched to another IFNβ.
Although it is difficult to compare relapse rates in this study to those of previous research, due to differences in how relapses are defined and/or differences in important MS-related characteristics such as MS subtype, disability progression, and even IFNβ therapy type,Citation8 it is reasonable to conservatively suggest that relapse rates in the switch group in this study may approach rates observed in patients discontinuing IFNβ therapy altogether,Citation18 and the differences between relapse rates in the Switch vs No Switch groups approximate the differences observed in clinical trials of IFNβ therapy vs placebo.Citation19,Citation20
Legitimate clinical reasons for switching patients from one IFNβ therapy to another do exist. For example, switching between IFNβs may have beneficial effects when first-line IFNβ therapy had suboptimal response (patients did not stabilize), but disease activity is not suitable for escalation to a second-line DMT.Citation11,Citation12 Further, subjects with intolerable side effects from their current medication should be switched to another DMT within the same line of treatment. Although these clinical reasons for switching between IFNβ therapies in stable patients are valid, switching patients for economic reasons may be both clinically harmful as well as cost-ineffective. The costs of moderate to severe relapses (such as those reported in this study) are high. Goldberg et al reported an average cost of relapses in the US of $2,381 for moderately severe and $16,589 for severe relapses (weighted average $6,834 in 2008 dollars).Citation21,Citation22 Given these costs, and the higher risks of relapse in patients who are switched, formulary decision-makers need to evaluate the trade-offs of any potential cost savings of switching stable patients.
Disruption of care from the patient’s perspective should also be of concern. Rood et al found that insurance-driven medication changes had negative effects on patients and physician practices, including adverse medical outcomes, decreased satisfaction with the health care system, and problems that burdened the physician practice. Formulary changes involving neurologic medications were among those that caused the most problems.Citation15 Other study has shown that stable MS patients with long-term experience on the same DMTs tend to be more adherent to their prescribed medications, regardless of mode or timing of administration.Citation23 Finally, Ganther-Urmie et al reported that most patients did not believe that formulary drugs were safer or more effective than nonformulary drugs – a finding with implications for adherence when patients are switched to a new therapy due to formulary decisions.Citation24
Of the three IFNβ formulations included in this study, slightly over half of the pre-matching study cohort and 70% of switched patients were on IFNβ-1a IM (). Consequently, the other baseline IFNβ treatment groups (IFNβ-1a SC and IFNβ-1b SC) were too small to include in subanalyses. Given the study criterion that patients had to be relapse-free for 1 full year prior to the index date, twice as many IFNβ-1a IM patients met this criteria as IFNβ-1a SC or IFNβ-1b patients. Patients also had to be adherent to therapy for at least 1 year. This could also be why there were more IM patients meeting the selection criteria, since IM patients experience fewer side effects and almost no injection site reactions.Citation6,Citation25
This research used administrative claims data, which have inherent limitations. While the study data included MS symptoms, they did not include MS subtype, baseline disease severity, or disability status, any of which may have been unobserved confounders of the reported relapse rate differences and were not available to use as a matching criterion in the propensity scoring. Other aspects of the clinical characteristics of these patients, such as MRI scans and/or disability measurement, were also unavailable from claims data. Relapse rates as measured from claims data also do not capture milder episodes, so true differences in relapse rates in this study population are not known, and are reported only for more severe (but also more costly) cases. Claims data also do not provide reasons for switching IFNβ therapies and there were no patient or physician-reported effectiveness measures to confirm relapse occurrence. Finally, data derived from a commercial managed care health insurance population may not be generalizable to other patient populations.
Conclusion
MS patients stable on IFNβ therapy who remained on initial therapy had significantly better outcomes than those who switched to another IFNβ. The subset of patients stable on IFNβ-1a IM who remained on therapy was less than half as likely to suffer relapses as those who switched to another IFNβ. These findings support the benefits of allowing patients to remain on current IFNβ therapy when stable. Further studies are needed to better understand when to switch therapies vs staying on initial therapy for MS patients.
Author contributions
The authors had full editorial control of the manuscript and provided their final approval of all content. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was sponsored by Biogen (Cambridge, MA, USA). Writing and editorial support for the preparation of this manuscript was provided by Mark Stephens of Prima Health Analytics (Weymouth, MA, USA); funding was provided by Biogen. Biogen reviewed and provided feedback on the manuscript. The abstract of this paper was presented at the American Academy of Neurology 69th Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in the Supplement to Neurology, Vol 88, Issue16 (April 2017): http://n.neurology.org/content/88/16_Supplement/P2.396.abstract.
Disclosure
NW is currently employed as a consultant at Neopath Healthcare Analytics, Newton, MA, USA. CW is currently employed at Atara Biotherapeutics, South San Francisco, CA, USA. NW and CW were employees of Biogen at the time the study was carried out. CW is a stockholder of Biogen. CC reports no conflicts of interest in this work.
References
- Multiple Sclerosis International Federation (MSIF)Atlas of MS. MS International Federation Available from: https://www.msif.org/about-us/who-we-are-and-what-we-do/advocacy/atlas/ Published 2013Accessed July 7, 2017
- HurwitzBJThe diagnosis of multiple sclerosis and the clinical subtypesAnn Indian Acad Neurol200912422620182569
- GoodinDSFrohmanEMGarmanyGPDisease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice GuidelinesNeurology200258216917811805241
- FilippiniGMunariLIncorvaiaBInterferons in relapsing remitting multiple sclerosis: a systematic reviewThe Lancet20033619357545552
- National Institute for Health and Care Excellence (NICE)Beta Interferon and Glatiramer Acetate for the Treatment of Multiple SclerosisLondon, UKNational Institute for Health and Care Excellence (NICE)2002 Available from: https://www.nice.org.uk/guidance/TA32Accessed July 7, 2017
- LimmrothVPutzkiNKachuckNJThe interferon beta therapies for treatment of relapsing-remitting multiple sclerosis: are they equally efficacious? A comparative review of open-label studies evaluating the efficacy, safety, or dosing of different interferon beta formulations alone or in combinationTher Adv Neurol Disord20114528129622010041
- KinkelRPDontchevMKollmanCAssociation between immediate initiation of intramuscular interferon beta-1a at the time of a clinically isolated syndrome and long-term outcomes: a 10-year follow-up of the Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological SurveillanceArch Neurol201269218319021987393
- NikfarSRahimiRAbdollahiMA meta-analysis of the efficacy and tolerability of interferon-β in multiple sclerosis, overall and by drug and disease typeClin Ther201032111871188821095482
- CohanSChenCBarabanEStuchinerTGroteLRodriguezMResults of sustained long-term use of interferon beta-1a in a community-based cohort of patients with relapsing multiple sclerosisJ Drug Assess2015411627536455
- HerndonRMRudickRAMunschauerFEEight-year immunogenicity and safety of interferon beta-1a-Avonex treatment in patients with multiple sclerosisMult Scler200511440941916042223
- GajofattoABacchettiPGrimesBHighAWaubantESwitching first-line disease-modifying therapy after failure: impact on the course of relapsing-remitting multiple sclerosisMult Scler2009151505818922831
- GajofattoABenedettiMDTreatment strategies for multiple sclerosis: When to start, when to change, when to stop?World J Clin Cases20153754555526244148
- FrancisGSRiceGPAlsopJCPRISMS Study GroupInterferon beta-1a in MS: results following development of neutralizing antibodies in PRISMSNeurology2005651485516009884
- HuskampHADeverkaPAEpsteinAMEpsteinRSMcguiganKAFrankRGThe effect of incentive-based formularies on prescription-drug utilization and spendingN Engl J Med2003349232224223214657430
- RoodMNCruz-KnightWCunaginJThe effect of insurance-driven medication changes on patient careJ Fam Pract2012617E17
- ParsonsLSReducing bias in a propensity score matched-pair sample using greedy matching techniquesProceedings of the Twenty-Sixth Annual SAS Users Group International ConferenceCary, NCSAS institute, Inc2001214226 Available from: http://www2.sas.com/proceedings/sugi26/p214-26.pdfAccessed February 20, 2017
- ChastekBJOleen-BurkeyMLopez-BresnahanMVMedical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claimsJ Med Econ201013461862520883151
- SigerMDurkoANicpanAKonarskaMGrudzieckaMSelmajKDiscontinuation of interferon beta therapy in multiple sclerosis patients with high pre-treatment disease activity leads to prompt return to previous disease activityJ Neurol Sci20113031–2505221333308
- CrossAHNaismithRTEstablished and novel disease-modifying treatments in multiple sclerosisJ Intern Med2014275435036324444048
- European Medicines AgencyGuideline on similar biological medicinal products containing interferon beta22013 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/03/WC500139622.pdfAccessed February 9, 2017
- GoldbergLDEdwardsNCFincherCDoanQVAl-SabbaghAMeleticheDMComparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosisJ Manag Care Pharm200915754355519739877
- O’BrienJAWardAJPatrickARCaroJCost of managing an episode of relapse in multiple sclerosis in the United StatesBMC Health Serv Res2003311712952552
- Garcia-DominguezJMMuñozDComellasMGonzalboILizánLPolanco SánchezCPatient preferences for treatment of multiple sclerosis with disease-modifying therapies: a discrete choice experimentPatient Prefer Adherence2016101945195627713622
- Ganther-UrmieJMNairKVValuckRMccollumMLewisSJTurpinRSConsumer attitudes and factors related to prescription switching decisions in multitier copayment drug benefit plansAm J Manag Care200410320120815032257
- MinagaraAMurrayTJPROOF Study InvestigatorsEfficacy and tolerability of intramuscular interferon beta-1a compared with subcutaneous interferon beta-1a in relapsing MS: results from PROOFCurr Med Res Opin20082441049105518315940