Abstract
Background:
Individuals with severe hemophilia A have reduced blood levels of clotting factor VIII (FVIII) leading to recurrent bleeding into joints and muscles. Primary prophylaxis with clotting factor concentrates started early in childhood prevents joint bleeds, thus avoiding joint damage and improving people’s quality of life. There remain significant differences in the implementation of primary prophylaxis worldwide mainly due to the cost of prophylaxis compared with treatment on demand.
Objective:
To evaluate the cost-effectiveness of primary prophylaxis with FVIII concentrates versus secondary prophylaxis, versus treatment on demand, and versus a “hybrid” (primary prophylaxis followed by on-demand treatment in adults) in individuals with severe hemophilia A.
Methods:
A Markov model was developed and run using different sources of clinical, cost, and utility data. The model was populated with a hypothetical cohort of 100 individuals with severe hemophilia A. The perspective of the Italian National Health System was used.
Results:
The baseline results showed that primary and secondary prophylaxis is cost-effective compared both with treatment on demand and with a hybrid strategy. The incremental costs per quality-adjusted life-year gained for individuals with hemophilia A receiving primary and secondary prophylaxis were €40,229 to €40,236 versus an on-demand strategy. However, the sensitivity analyses performed showed that the results were sensitive to the unit cost of clotting FVIII, bleeding frequency, and the discount rate.
Conclusion:
Although primary prophylaxis is a costly treatment, our results show that it is cost-effective compared with treatment on demand.
Introduction
Hemophilia A is a hereditary X-linked disorder caused by deficiency or absence of clotting factor VIII (FVIII) in the blood.Citation1 The disease, which has an incidence of 1 in 5000 to 10,000 male births,Citation2 can be defined as mild, moderate, or severe depending on the degree of FVIII deficiency. Individuals with severe hemophilia A require lifelong treatment with exogenous FVIII to prevent bleeding and associated complications in muscles and joints,Citation3 often leading to hemophilic arthropathy, a chronic painful and disabling condition with a considerable impact on their overall quality of life.Citation4
Treatment with replacement therapy can be either on demand, when infusions of FVIII are given to treat a bleeding episode, or on prophylaxis when regular infusions of clotting concentrates are provided to prevent bleeding episodes. Moreover, prophylaxis is usually defined as primary when started before the age of 2 years and/or prior to the first joint bleed and secondary when started after the age of 2 years and/or after more than 1 joint bleed. Primary prophylaxis is the gold standard treatment for children with severe hemophilia A. Despite this, there remain relevant differences in its implementation worldwide because of different healthcare priorities and available resources within the healthcare systems.Citation1 In addition, the issue of when to stop prophylaxis is still open because few data on orthopedic outcome after a prolonged follow-up period are yet available.Citation5 Recently published evidenceCitation6 has confirmed the long demonstrated benefits of prophylactic over on-demand therapy,Citation1 though primary prophylaxis is more costly to provide.Citation7 Considering the importance of prophylaxis in preventing joint damage and consequently in preserving hemophiliac patient’s quality of life, we have conducted an economic evaluation to assess the cost-effectiveness of prophylaxis with FVIII (recombinant plasma/albumin-free; ReFacto AF™, Pfizer) versus treatment on demand for individuals with severe hemophilia A.
Materials and methods
Our analysis is based on the study of Miners et alCitation7 which evaluated the outcome of treating patients with severe hemophilia A with primary prophylaxis or on demand over a 70-year time frame from the perspective of the UK National Health System.
To adapt Miners’ model to the Italian context, a few clinical parameters were varied and cost data were replaced with the corresponding Italian estimates of resource use.
The analysis conducted is a cost-utility analysis, ie, an economic evaluation that estimates the cost per quality-adjusted life-year (QALY) gained from undertaking one intervention instead of another.Citation8 The QALY is a potential measure of health and is obtained by multiplying the duration of a health state (in years) by a factor representing the quality (“utility”) of that health state. A QALY value of 1 is equivalent to a year of “perfect health” whereas a value of zero corresponds to “death”.
The Markov model
In a Markov model a patient’s possible prognosis is divided into distinct health states. Costs and benefits are assigned to each health state and the movement of an individual between these health states over a given amount of time (cycle) is defined by transition probabilities.Citation9,Citation10 The costs and benefits of comparative treatments are then estimated according to the time spent in each state.
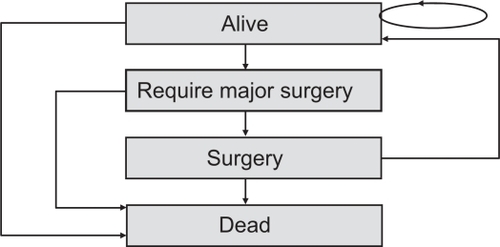
The model () was structured with the following assumptions:
All individuals enter the model at birth in the health state “alive”.
At the end of the first cycle (1 year) individuals either remain in the state “alive” or move to the health states “require major surgery” or “dead”.
At the following cycle individuals in state “require major surgery” can pass to state “surgery” or “dead”.
At the next cycle, individuals who underwent surgery move directly to state “alive” or “dead”.
Figure 1 Structure of the model. The rectangles represent the possible disease states and the arrows indicate the possible transitions between those states.Citation6
Each cycle lasts 1 year and individuals end their treatment when they reach the age of 70 or are dead. Our model was run for a hypothetical cohort of 100 individuals and was re-run more times to simulate 4 different scenarios (). Of note, scenario 2 was introduced purposely because it represents a “hybrid” program, that is, in this case, patients receive primary prophylaxis until the age of 18 years and subsequently switch to treatment on demand. Miners’ modelCitation7 was run using a combination of data obtained from different sources and was constructed on a few assumptions made by the authors, the majority of which were maintained in our model. However, a few adjustments on transition probabilities and costs were introduced to adapt the model to the Italian context. All input data used in the model are reported in . Modeling was undertaken using Microsoft Excel 2003 (Microsoft Corporation, Redmond, Washington, USA).
Figure 2 The four different scenarios simulated in the model.
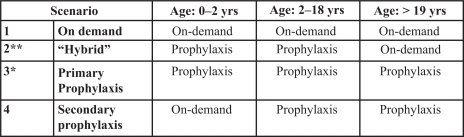
Table 1 Input data used to run the Italian model
Transitions probabilities
Similarly with Miners’ analysis,Citation7 it was assumed that life expectancy for individuals with severe hemophilia was equivalent to that of the general North European male population. Therefore, the probability of death in each year for individuals treated on demand and with primary prophylaxis was based on a single Italian male life-table.Citation11 The probability of death in the year following major surgery was calculated based on the Italian male life-table mentioned above by adding an additional 1%, as explained elsewhere.Citation7 The annual probability of requiring major surgery for individuals on primary prophylaxis was assumed to be equal to that of the Italian general population. The annual rate of major surgery was derived from the patients’ hospital discharge records; the mean annual probability of major surgery in individuals on primary prophylaxis was then calculated by considering data per single age category and found to be equal to 0.08% (mean value).Citation12 For secondary prophylaxis and treatment on demand, the rates were based on Miners’ dataCitation7 but adjustments were made to take into account the increase of major surgery events with age.
Utilities
Because of the lack of suitable Italian data on the effect of the different treatment regimens on health-related quality of life of individuals with severe hemophilia A, the utilities for the health states “alive” and “surgery” imported to the model were derived from Miners et al.Citation13 Similarly, the utility for the health state “require major surgery” was obtained from Laupacis et alCitation14 who assumed that all individuals in that state suffered from a painful condition similar to that of individuals with osteoarthritis.
Resource use
Clotting factor use
For treatment on demand it was considered that individuals with severe hemophilia A received a bolus dose of 40 IU/kg bodyweight once or twice after each bleed. In the case of secondary and primary prophylaxis the dosage was 30 IU/kg, to be administered 2.5 times per week.Citation15
All costs associated with the administration of clotting FVIII were calculated considering the therapeutic regimens and the mean annual bleeding frequency, which were obtained from an Italian study by Tagliaferri et al.Citation16 Data were entered into the model on the mean number of annual bleeds experienced by adolescents and adults treated on demand (33.7 and 36.9, respectively) and by adolescents and adults receiving prophylaxis (2.5 and 5.4 respectively). Additional resources taken into account were the unit costs associated with the hospitalizations for major surgery (€8582) and for bleeding or examinations (€4246).Citation4
Cost analysis
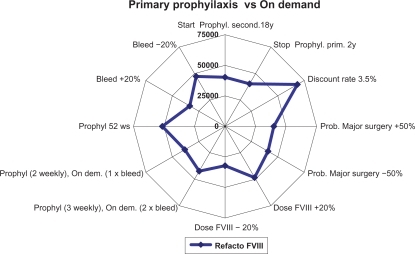
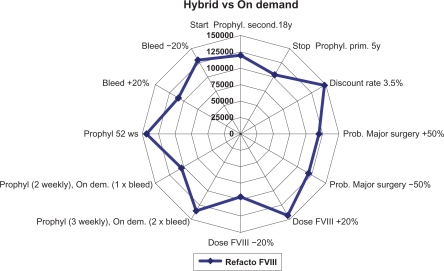
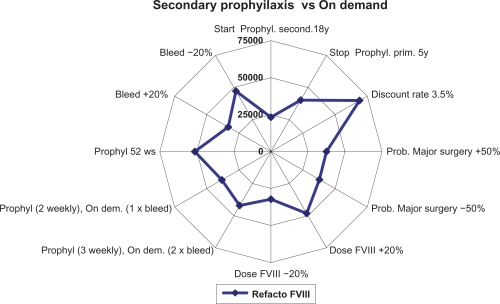
The analysis was conducted from the perspective of the Italian National Health System (Sistema Sanitario Nazionale). A discount rate of 6% was applied to all costs as in Miners’ studyCitation7 and subsequently tested in the sensitivity analysis ( to ).
The unit costs for recombinant plasma/albumin-free antihemophilic factor (ReFacto AF) of €0.68 were obtained from the Italian Physicians’ Desk Reference (Informatore Farmaceutico 2010).Citation17 Indirect costs were not taken into account in the analysis.
Sensitivity analysis
One-way sensitivity analyses were performed varying the discount rate, the drug dose, and the administration frequency to test the robustness of the analysis.
Results
The results from the baseline analysis confirmed that the mean expected lifetime costs of treating individuals with severe hemophilia A are higher with primary prophylaxis (€166,168,643) than with treatment on demand (€87,426,642) when ReFacto AF was used (). In terms of effectiveness, however, primary and secondary phophylaxis are the most effective strategies as shown by the QALY values obtained: 4137 for treatment on demand and 6094 and 6051 for primary and secondary prophylaxis, respectively, when ReFacto AF is considered (). If we analyze the QALYs in the four different scenarios, prophylaxis is always a cost-effective approach.
Table 2 Total costs and incremental cost-effectiveness ratios (ICERs) for phrophylaxis vs treatment on demand in individuals with severe hemophilia A
The hybrid scenario, in which individuals with severe hemophilia A initially receive prophylaxis and then switch to treatment on demand, is the least cost-effective, with incremental cost-effectiveness ratios (ICERs) of €119,134 versus the on-demand strategy.
Sensitivity analyses
The sensitivity analyses performed ( to ) show that results were sensitive to a number of variables including the cost of the clotting factor, which is a cost driver in the model, the bleeding frequency, and the discount rate.
Discussion
The aim of this analysis was to conduct a cost-utility analysis of prophylaxis versus treatment on demand for individuals with severe hemophilia A in the Italian clinical setting. Though primary prophylaxis is recommended by national and international authorities such as the World Health Organization and the World Federation of Hemophilia,Citation1 many patients in different countries still receive treatment on demand. The importance of primary prophylaxis is due to the fact that it protects patients from the development of hemophilic arthropathy, and today there is strong agreement that to be effective, prophylaxis needs to be started at an early age, before arthropathy has developed.Citation1 Nevertheless, implementation of prophylactic regimen is still hindered and one of the main arguments against its use is the cost of treatment.Citation18
The results of our analysis show that prophylaxis is a cost-effective strategy compared with treatment on demand, as demonstrated by the QALY values obtained. This therapeutic strategy also dominates the hybrid regimen (as described in Methods), which has been considered in this analysis.
Our model has a number of limitations mainly due to its structure, the wide time horizon, the assumptions made, and the data used, some of which were derived from different sources. The fact that the possible indirect costs were not considered is another limitation, though their inclusion may have improved the results of the model. Clinical outcomes were derived from a literature review and were evaluated only for prophylaxis with recombinant plasma/albumin-free (ReFacto AF). Therefore, in a further economic evaluation it could be interesting to compare clinical effectiveness among different alternatives used in Italian setting; but data on costs and outcomes for different clotting factors would need to be collected over a longer period of time. Another important limitation is the assumptions on which the analysis was based, which may be necessary to simplify the model or in case of incomplete data. Specifically, this regarded the transition probabilities, which were lacking in some cases, and the utilities, which were derived from different literature sources and considered to be acceptable for an Italian population. To tackle the possible uncertainties associated with this way of proceeding we performed one-way sensitivity analyses which highlighted that the results obtained were mainly sensitive to the discount rate used and the unit cost of the clotting factor.
To our knowledge, this is the first attempt to undertake a cost-utility analysis on hemophilia A considering prophylaxis versus treatment on demand in an Italian clinical setting. Though no officially established threshold on cost per QALY is available for Italy, our results show that the incremental costs per QALY gained for patients receiving primary and secondary prophylaxis were €40,236 to €40,229 versus the on-demand strategy. However, both ICERs were within the two commonly accepted thresholds of €36,500 per QALYCitation19 and 60,000 per QALYCitation20 calculated by two different authors for the Italian setting. It is worth noting that recent guidelines by the Italian Health Economics Association recommend that a threshold of €25.000 to €40.000 be adopted.Citation21
In spite of all the limitations of pharmacoeconomic models, these instruments have a key role when priorities in resource allocation have to be established. In fact, they provide decision-makers in the health care systems with useful tools to make more rational and effective decisions.
A more accurate estimate of the cost-effectiveness of prophylaxis may be obtained when appropriate data are collected. The randomized study ESPRIT (Evaluation Study on Prophylaxis: a Randomized Italian Trial),Citation22 comparing the efficacy and safety of prophylaxis and on-demand regimens in preventing joint deterioration and reducing the number of bleeds, may provide meaningful evidence to be used in future prospective pharmacoeconomic evaluations.
Disclosure
The study was financially supported by Pfizer Italia Srl, Rome, Italy.
References
- LjungRProphylactic therapy in hemophiliaBlood Rev20092326727419775786
- PipeSAntihemophilic factor (recombinant) plasma/albumin-free method for the management and prevention of bleeding episodes in patients with hemophilia ABiologics2009311712519707401
- MinersARevisiting the cost-effectiveness of primary prophylaxis with clotting factor for the treatment of severe haemophilia AHaemophilia200915488188719473422
- GringeriAMantovaniLGScaloneLMannucciPMFor the COCIS Study GroupCost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group2003102723582363
- Donadel-ClaeyssensSEuropean Paediatric Network for Haemophilia Management; Current co-ordinated activities of the PEDNET (European Paediatric Network for Haemophilia Management)Haemophilia200612212412716476085
- Manco-JohnsonMJAbshireTCShapiroADProphylaxis versus episodic treatment to prevent joint disease in boys with severe hemophiliaN Engl J Med2007357653554417687129
- MinersAHSabinCATolleyKHLeeCACost-utility analysis of primary prophylaxis versus treatment on-demand for individuals with severe haemophiliaPharmacoeconomics2002201175977412201795
- DrummondMFSchulpherMJTorranceGWO’BrienBJStoddartGLMethods for the Economic Evaluation of Health Care Programmes3rd edOxfordOxford University Press2005
- SonnenbergFABeckJRMarkov models in medical decision making: a practical guideMed Decis Making19931343223388246705
- BriggsASculpherMAn introduction to Markov modelling for economic evaluationPharmacoeconomics199813439740910178664
- Demo.istat.it [homepage on the internet]. Italian National Institute of StatisticsAge specific death rates by gender [updated 2007] http://demo.istat.it/unitav/index.html?lingua=ita. Accessed January 11, 2010.
- Salute.gov.it. [homepage on the internet]. Italian Ministry of Health http://www.salute.gov.it/ricoveriOspedalieri/ric_informazioni/default.jsp. Accessed February 12, 2010.
- MinersAHSabinCATolleyKHJenkinsonCKindPLeeCAAssessing health-related quality-of-life in individuals with haemophiliaHaemophilia19995637838510583523
- LaupacisABourneRRorabeckCThe effect of elective total hip replacement on health-related quality of lifeJ Bone Joint Surg Am19937511161916268245054
- SantagostinoEMannucciPMBianchi BonomiAGuidelines on replacement therapy for haemophilia and inherited coagulation disorders in ItalyHaemophilia20006111010632734
- TagliaferriAFranchiniMCoppolaAEffects of secondary prophylaxis started in adolescent and adult haemophiliacsHaemophilia200814594595118540895
- Informatore FarmaceuticoElsevier2010
- GeraghtySDunkleyTHarringtonCLindvallKMaahsJSekJPractice patterns in haemophilia A therapy – global progress towards optimal careHaemophilia2006121758116409179
- LucioniCRavasioRCome valutare i risultati di uno studio farmacoeconomico?PharmacoEconomics – Italian Research Articles20043121130
- MessoriASantarlasciBTrippoliSVaianiMControvalore economico del farmaco e beneficio clinico: Stato dell’arte della metodologia e applicazione di un algoritmo farmacoeconomicoPharmacoEconomics – Italian Research Articles200355367
- Associazione Italiana di Economia Sanitaria (AIES)Proposta di linee guida per la valutazione economica degli interventi sanitariPharmacoEconomics – Italian Research Articles2009118393
- GringeriAProspective controlled studies on prophylaxis: an Italian approachHaemophilia20039Suppl 1S38S42