Abstract
Background:
To compare the cost-effectiveness of treating early responders versus early nonresponders to an atypical antipsychotic (risperidone) and the cost-effectiveness of treating early nonresponders maintained on risperidone versus those switched to olanzapine.
Methods:
This post hoc analysis used data from a randomized, double-blind, 12-week schizophrenia study (Study Code: HGMN, n = 628). Participants were initially assigned to risperidone therapy. Early response was defined as a ≥ 20% improvement on the Positive and Negative Syndrome Scale (PANSS) total score from baseline to two weeks. Early responders continued on risperidone, whereas early nonresponders were randomized in a double-blind manner to continue on risperidone or switch to olanzapine for 10 additional weeks. Early responders and early nonresponders maintained on risperidone were compared for health-state utilities (benefits) and total cost over the 12-week study; early nonresponders maintained on risperidone or switched to olanzapine were compared from randomization (10-week period). Utilities were derived from the PANSS and adverse events. Mixed models were used to assess group differences in utilities. Treatment costs were calculated based on health states. Incremental cost-effectiveness ratios were then utilized to compare treatment groups.
Results:
Early responders to risperidone had significantly greater total utility and lower total treatment costs than early nonresponders to risperidone. Compared with early nonresponders who continued on risperidone, those who were switched to olanzapine had significantly higher total utility scores at endpoint and numerically lower total treatment costs, reflecting significantly lower nonmedication treatment costs, even though medication costs were significantly higher compared with generic risperidone.
Conclusion:
Treatment of early responders was more cost-effective than treatment of early nonresponders to atypical antipsychotic therapy. Switching early nonresponders to olanzapine resulted in improved treatment effectiveness, met the criteria for some dominance, and appeared modestly more cost-effective than maintaining treatment with generic risperidone.
Introduction
Before switching a patient with schizophrenia to a new antipsychotic medication, expert consensus guidelines recommend that clinicians wait 4–8 weeks before changing a patient’s antipsychotic regimen,Citation1,Citation2 in part due to the possibility of a delayed onset of action. However, a comprehensive meta-analysis and several post hoc analyses have tested and rejected the notion of a delayed response to antipsychotics.Citation3–Citation7
A number of studies have demonstrated that early nonresponse to antipsychotics is a strong predictor of subsequent nonresponse to continued treatment with the same antipsychotic. In one study, Correll et al found that every patient whose symptoms had not improved by 20% after one week of treatment were not responders after four weeks.Citation3 In a larger analysis, Kinon et al pooled five randomized, double-blind trials of antipsychotics in the treatment of schizophrenia, and found that a lack of response at two weeks was a robust predictor of poor response at 12 weeks.Citation4 These findings were extended in a naturalistic trial, where early nonresponders were found not only to have worse clinical outcomes, but worse functional and economic outcomes as well.Citation5
All of these studies were retrospective in nature, but a recent 12-week clinical trial (HGMN)Citation8,Citation9 was specifically designed to examine the early response hypothesis in an a priori manner. This study confirmed previous clinical and functional findings demonstrating poor outcomes for individuals with schizophrenia who were not early responders to an atypical antipsychotic (risperidone). HGMN also examined whether early nonresponders who continue on the initial antipsychotic (risperidone) after the first two weeks of therapy differ in treatment outcomes from early nonresponders who were randomized in a double-blind manner to another antipsychotic (olanzapine). Findings indicated that early nonresponders who switched to olanzapine had a small but significantly greater reduction in symptoms at endpoint relative to early nonresponders who remained on risperidone. However, the early responders to risperidone had significantly greater symptom reductions than either group of early nonresponders. The economic implications of these findings have not yet been studied.
The objectives for this analysis were two-fold: to compare the cost-effectiveness of treating early responders versus early nonresponders to an atypical antipsychotic (risperidone) over a 12-week period, and to compare the cost-effectiveness of treating early nonresponders to risperidone who were randomized in a double-blind manner to either continue on risperidone or to switch to olanzapine for an additional 10 weeks of therapy.
Materials and methods
Data source
Data for this post hoc analysis came from a 12-week, randomized, double-blind, flexible dose HGMN trial that was explicitly designed to validate whether early responders to risperidone had greater reductions in psychopathology relative to early nonresponders to risperidone, and secondly, to investigate the effects of switching early risperidone nonresponders to olanzapine or continuing them on risperidone.Citation8 HGMN included adult patients aged 18–65 years who met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)Citation10 criteria for schizophrenia, schizoaffective disorder, or schizophreniform disorder. The multicenter trial started in May 2006, ended in December 2007, and was conducted at 64 centers in three countries. Before undergoing any study procedure, the eligible patients were given a complete description of the study and provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee from each participating institution. The clinical trial was registered with ClinicalTrials.gov (identifier NCT00337662).
During the 2–5-day initial screening period (study period 1), study participants were assessed to ensure that they met the inclusion and exclusion criteria. Beyond age and diagnosis, the primary inclusion criteria included a score ≥ 45 on the Brief Psychiatric Rating Scale (BPRS),Citation11 total score extracted from the Positive and Negative Syndrome Scale (PANSS)Citation12 a score of ≥4 (moderate) on at least two BPRS items: conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content, a minimum Clinical Global Impressions-Severity scaleCitation13 rating of 4 (moderately ill), and, within two weeks of the first visit, patients needed to have experienced an exacerbation of their illness that required an increased level of psychiatric care. Patients for whom risperidone or olanzapine was contraindicated, and those who had been hospitalized for more than two weeks preceding the first visit or had another serious or unstable medical condition were excluded.
During study period 2, patients began flexible dose therapy with risperidone 2–6 mg/day. Previous antipsychotic medications were discontinued abruptly, and risperidone was initiated with a dosing schedule of 2 mg/day on day 1, 4 mg/day on days 2–7, and flexible dosing 2–6 mg/day after day 7.
At week 2, patients who met the a priori definition for early nonresponse (<20% improvement on PANSS total score) were randomized to either continue with risperidone treatment 2–6 mg/day or switch to olanzapine 10–20 mg/day for the next 10 weeks. During this third study period, the use of benzodiazepine/hypnotics/anxiolytics was permitted for the treatment of anxiety or insomnia as clinically indicated. In addition, patients who had been receiving a stable dose of an antidepressant or mood stabilizer for ≥30 days prior to the initiation of the study could continue on these medications at a stable dose. Further details of the parent trial are available elsewhere.Citation8
gives a graphical depiction of the study design. The three study cohorts included the early responders (n = 144) and the two groups of early nonresponders (n = 378) who were randomized to either continue risperidone (ENR-RIS, n = 192) or switch to olanzapine (ENR-OLZ, n = 186). Comparisons between early responders and early nonresponders contrasted only the individuals who were treated with risperidone over the 12 weeks of study periods 2 and 3, and the patients randomized to olanzapine were excluded from this comparison. Comparison between the ENR-RIS and ENR-OLZ cohorts were evaluated only over the 10 weeks of study period 3.
Figure 1 HGMN study design.
Abbreviations: mg, milligrams; V, visit; ENR-RIS, early nonresponders to risperidone randomized to continue treatment with risperidone; ENR-OLZ, early nonresponders to risperidone randomized to switch to olanzapine.
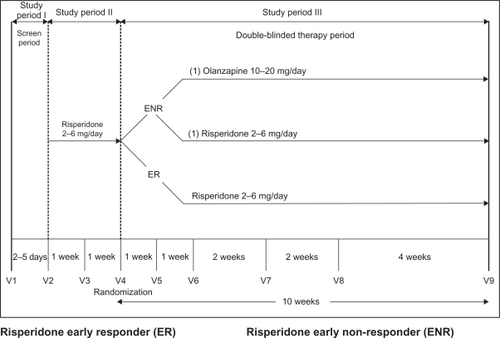
Approximately 17% (106/628) of enrolled patients discontinued risperidone treatment before the end of study period 2. Of the 522 patients who completed the initial two weeks of risperidone treatment (study period 2), 72.4% were classified as early nonresponders, and 27.6% were classified as early responders. A similar portion of patients in each of the three cohorts discontinued study period 3: 30.6% of the early responder group, 30.2% of the ENR–RIS group, and 32.3% of the ENR–OLZ group.Citation8
Cost-effectiveness analysis
The US Public Health Service Task Force on Cost-Effectiveness in Health and Medicine has recommended evaluating different health states based on quality-adjusted life year (QALY) ratings, where a year of life is rated on a scale from 0 (worst possible health) to 1 (perfect health), as evaluated by members of the general public.Citation14 The ratings at specific points in time are known as utilities. QALYs are calculated from utilities by weighting the utilities according to time spent (years) at each health state and summing across the full time period. In this analysis, the timeframe was limited to the 12-week study period.
The methods used for this cost-utility analysis mirrored those from a previously published study.Citation15 In the current analysis, we used disease-specific utility weights for eight health states (based on PANSS scores) identified in previously published research.Citation16,Citation17 Lenert et al identified positive, negative, and cognitive symptoms through factor analysis, and then used cluster analysis to identify eight health states from the symptom factors. Using a standard gamble methodology, utility scores were identified for the eight health states and ranged from 0.42 to 0.88 (see ). In addition, Lenert et al developed disutilities for five common adverse events, ie, weight gain (0.959), orthostatic hypotension (0.912), akathisia (0.898), pseudoparkinsonism (0.888), and tardive dyskinesia (0.857). At each assessment, a patient’s total utility was assessed using the multiplicative calculation of total utility = (PANSS health state utility) × (weight gain disutility) × (orthostatic hypotension disutility) × (akathisia disutility) × (pseudoparkinsonism disutility) × (tardive dyskinesia disutility).Citation16 Instances of adverse events were identified from spontaneously reported adverse events or prespecified definitions from the clinical trial protocol.Citation8 Weight gain was identified by a ≥ 7% increase in baseline body weight, orthostatic hypotension by changes in supine to standing blood pressure and pulse, pseudoparkinsonism by the Simpson–Angus ScaleCitation18 or anticholinergic medication use, akathisia by the Barnes Akathisia Scale,Citation19 and tardive dyskinesia by the Abnormal Involuntary Movement Scale.Citation20 Once a patient reported an adverse event in the post-treatment period, it was assumed to continue for the remainder of the study period.
Table 1 Health state definitions
Building on these PANSS-based health states further, Mohr et alCitation21 estimated monthly treatment costs for the eight different health states in 1997 US dollars using data from a naturalistic cost and outcomes trial.Citation22 We updated these costs to 2008 US dollars using the medical portion of the consumer price index (see ). The medication acquisition cost of olanzapine and risperidone were estimated using the least expensive combination of average wholesale price doses.Citation23 To reflect the discounts and rebates affecting patients whose medication costs would have been paid by Medicaid, average wholesale price was further discounted by 25%.Citation24 Total cost was estimated as the sum of inflation-adjusted monthly cost for the different health states (nonmedication cost) and study drug acquisition costs (medication cost).
The incremental cost-effectiveness ratio identifies the cost in dollars for each unit of increase effectiveness gained. The cost per QALY gained is an important standard in cost-effectiveness research. The incremental cost-effectiveness ratio was to be calculated if one treatment was both more expensive and more effective. Alternatively, if one treatment was both more effective and less costly, it would be considered the dominant treatment, and an incremental cost-effectiveness ratio would not be calculated.
Statistical methods
Analysis of baseline characteristics, utilities, costs, and cost-effectiveness were performed for both of the group comparisons of interest, ie, risperidone early responders versus risperidone early nonresponders (excluding patients randomized to olanzapine) and early nonresponders randomized to continue with risperidone (ENR-RIS) versus switch to olanzapine (ENR-OLZ). Baseline characteristics were compared using t-tests for continuous variables and Chi-square tests for categorical variables. The mixed model for repeated measures was used to compare the total utility difference between treatment groups over time. The mixed model for repeated measures included a priori selected covariates for gender, race, diagnosis type, age at illness onset, investigator, as well as baseline PANSS positive, negative, and cognitive scores, and baseline utility category. Reported values represent the predicted mean values (ie, SAS least squares means) after adjusting for these covariates.
Monthly treatment costs were summed across the study period, with missing values imputed using the last observation carried forward method. Only patients with complete baseline covariate information and at least one post-baseline outcome measure were included in the analytical sample. A propensity score was calculated using logistic regression with the a priori selected covariates from the mixed model for repeated measures. An analysis of covariance adjusting for the propensity score was used to compare the total cost, nonmedication cost, and medication cost of the two treatment groups. Due to the skew in the cost data, a sensitivity analysis was conducted assessing mean cost differences using a propensity-score stratified, bootstrap resampling approach.Citation25 The bootstrap resampling provides for a test of mean cost differences without making strong distributional assumptions, while the propensity stratification incorporates adjustment for the a priori set of baseline covariates.
Cost effectiveness was assessed based on the incremental cost-effectiveness ratio, defined as the group difference in mean costs divided by the group difference in utility. The variability of the incremental cost-effectiveness ratio estimate was generated by bootstrap resampling (5000 iterations) of the cost and utility values simultaneously and examining the bootstrap distribution over the quadrants of the cost-effectiveness plane. The degree of dominance criteriaCitation26 was then used to judge the strength of the observed incremental cost-effectiveness ratio. According to these criteria, if 95% of the bootstrap distribution of the incremental cost-effectiveness ratio is in the dominant quadrant (more effective and less costly), then “strict” dominance has been obtained. Alternatively, if only 50% of the bootstrap distribution of the incremental cost-effectiveness ratio is in the dominant quadrant (more effective and less costly), but 90% is in the three more effective or less costly quadrants, then the lesser criteria of “some” dominance has been obtained. All data analyses were completed using SAS version 9.1.3.
Results
The results are reported in two subsections reflecting parallel comparisons underlying the stated objectives: risperidone early responders versus risperidone early nonresponders and early nonresponders randomized to continue with risperidone (ENR-RIS) versus switch to olanzapine (ENR-OLZ). In the tables and figures, the results are juxtaposed to aid in contrasting the results from the two analyses.
Early responders versus early nonresponders to risperidone
This first analysis contrasts those who had an early response to risperidone (n = 139) versus those who did not have an early response to risperidone and were randomized to continue treatment with risperidone (n = 188). In , it can be seen that the population had an average age in their early 40s, average onset of illness in their mid-20s, most were male, the majority were either Caucasian or African-American, and, on average, they had more severe positive than negative or cognitive symptoms of schizophrenia. At the beginning of treatment with risperidone, the early responders had significantly higher levels of positive symptoms and lower total utility scores than early nonresponders.
Table 2 Baseline characteristics of patients
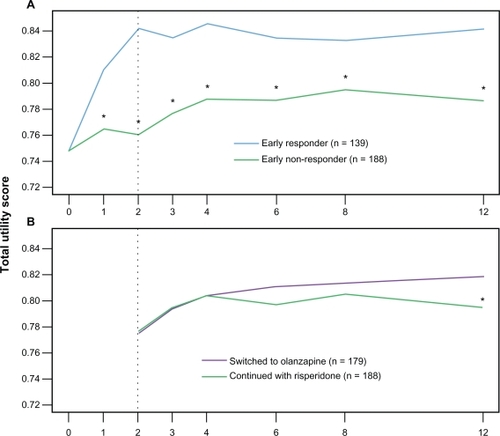
Total utility scores separated significantly between the early nonresponder and early responder groups at week 1 and remained significantly different across the treatment period (see ). On average, the total utility score was 0.835 for the early responder group and 0.780 for the early nonresponder group (P < 0.001). Although only for a period of 12 weeks, this difference represents a gain of 4.6 quality-adjusted life days or 0.013 QALYs.
Figure 2 Total utility change over time. A) early responders versus early nonresponders over study periods 2 and 3. B) ENR-RIS versus ENR-OLZ over study period 3. Utility scores vary from 0 (death) to 1 (perfect health). The y-axis is restricted in range from 0.72 to 0.85 to highlight the pattern of change over time.
Abbreviations: ENR-RIS, early nonresponders to risperidone randomized to continue treatment with risperidone; ENR-OLZ, early nonresponders to risperidone randomized to switch to olanzapine.
Over the 12-week study period (study periods 2 and 3), the estimated mean total cost of treating early nonresponders was significantly higher than for treating early responders ($9951 ± $3626 versus $7974 ± $1886; difference = $1977, P < 0.001). The differences were driven primarily by non-medication costs ($9528 ± $3610 versus $7560 ± $1864; difference = $1969, P < 0.001), with study medication costs not differing significantly between the early nonresponder and early responder groups ($422 ± $86 versus $414 ± $100; difference = $8, P = 0.278).
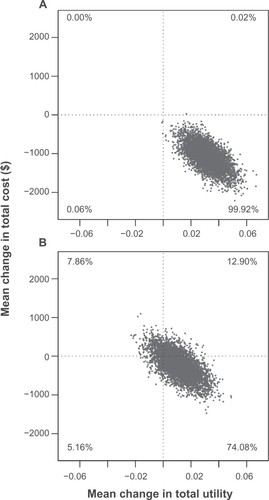
The early responders had significantly greater improvements in total utilities and significantly lower total treatment costs, resulting in early response being considered dominant. gives the bootstrap estimates of both the total utility changes and total cost changes. With nearly every estimate falling within the southeast quadrant, this clearly fits the definition of strict dominance (at least 95% of estimates falling in the more effective and less costly quadrant).Citation26
Figure 3 Distribution of 5000 bootstrap estimates of the incremental cost and effectiveness. A) Early responders versus early nonresponders comparison met criteria for strict dominance (95% of the bootstrap distribution in the more effective and less costly quadrant) and B) ENR-OLZ versus ENR-RIS comparison met criteria for some dominance (50% of the bootstrap distribution in the dominant quadrant with 90% in the three more effective or less costly quadrants).
Early nonresponders randomized to ENR-RIS or ENR-OLZ
This second analysis was a randomized comparison. In it can be seen that, at the time of randomization, the ENR-RIS and ENR-OLZ groups had similar characteristics. At endpoint, total utility scores were higher for the ENR-OLZ group than for the ENR-RIS group (P =0.029, see ). The average total utility score for study period 3 was 0.808 for the ENR-OLZ group and 0.799 for the ENR-RIS group (P = 0.197). This difference represents a gain of 0.6 quality adjusted life days over the 10-week period of study 3.
The total cost was not significantly different for the ENR-RIS and ENR-OLZ treatment groups ($7989 ± $3058 versus $7875 ± $3244; difference = $114, P > 0.05) over study period 3. However, the nonmedication costs ($7621 ± $3044 versus $6634 ± $3233; difference = $987, P < 0.001) were significantly lower for the ENR-OLZ group, but were largely offset by a significantly higher study medication cost ($368 ± $81 versus $1241 ± $253; difference = $873, P ≤ 0.001) for patients treated with olanzapine relative to those treated with generic risperidone.
Although the point estimate for the incremental cost-effectiveness ratio between the ENR-RIS and ENR-OLZ groups fell in the dominant quadrant (greater effectiveness and lower cost), the variability of this estimate did not meet the definition of strict dominance. In , it can be seen that the criteria of Obenchain et al for some dominance is met, given that >50% of the estimates fall in the dominant quadrant and >90% fall in the three more effective or less costly quadrants.Citation26
Discussion
In this cost-effectiveness analysis, the treatment of early responders was found to be more cost-effective than the treatment of early nonresponders. The treatment of patients who were early responders to risperidone was associated with a cost saving of nearly $2000 per patient over the 12-week period as compared with the treatment of patients who were early nonresponders to risperidone. In addition, differences in total utility scores between early responders and early nonresponders to risperidone were significantly different by week 1, and the difference continued over the full 12-week treatment period. Using only the 12-week study period as the timeframe resulted in 0.013 QALYs gained (out of a possible 0.23 for 12 weeks). In cost-effectiveness vernacular, the early responder is considered a dominant choice over the early nonresponder but, beyond clinical acumen for matching patients with treatments, a clinician cannot choose which individuals will be early responders to a specific antipsychotic medication.
The findings also suggest that switching the antipsychotic for early nonresponders from risperidone to olanzapine after only two weeks of treatment may represent a cost-effective treatment strategy. Early nonresponders to risperidone who were switched to olanzapine had better total utility scores after 10 weeks of treatment (at endpoint). A significant reduction in nonmedication health care costs was offset by the higher medication acquisition cost of olanzapine relative to generic risperidone. The QALYs gained during the 10-week period were quite small, representing only 0.6 of a quality-adjusted life day per patient. In cost-effectiveness research, interpreting the dominance of a treatment when the point estimate is both more effective and less costly, but when only one (in this case the effectiveness) is statistically significant poses a challenge. Obenchain et al recommended considering the statistical significance of both the cost and effectiveness simultaneously and proposed three different levels of dominance.Citation26 Our result, as displayed in , meets their criteria for the lowest level of dominance, ie, some dominance. Although not nearly as compelling as the results for the early responders relative to the early nonresponders to risperidone, switching patients who are early nonresponders to risperidone to treatment with olanzapine may represent a cost-effective strategy.
Our finding that early responders to atypical antipsychotics had better outcomes in terms of QALYs is consistent with the findings of other schizophrenia studies contrasting early responders and early nonresponders to various antipsychotic medications. Previous studies have shown the implications of poor early response, not only in terms of poor response of core symptoms of schizophrenia,Citation3,Citation4,Citation6,Citation7 but also in terms of reduced clinician-assessed quality of life, clinician-assessed functioning, patient-reported well-being, clinician-assessed depression,Citation9 patient-reported functioning, and patients’ perceptions of medication benefits.Citation5 Previous studies have also found that health care costs were just over $2000 higher during an eight-week period for early nonresponders relative to early responders,Citation5 a finding that is very similar to the cost finding in the current analysis. This growing body of research questions the prior recommendation from consensus treatment guidelines to wait 4–8 weeks before changing antipsychotic regimens for individuals who do not show a minimal early response to the initial antipsychotic medication.
Given the broad array of differences in outcomes between early responders and early nonresponders, one of the sobering findings in this study was the large percentage of early nonresponders (72%, 378/522). Although some of these early nonresponders do later respond to treatment, the majority (approximately 80%) do not adequately respond.Citation4,Citation8 In the HGMN primary analysis,Citation8 as well as in this cost-effectiveness analysis, switching medication to olanzapine showed a small but statistically significant improvement in outcomes at endpoint relative to continuing on risperidone. However, the patients who were switched to the alternative therapy did not have outcomes that were nearly as favorable as those for the early responders. These early nonresponders may represent a more difficult-to-treat population, but more research investigating these initial poor responders is needed.
Limitations
The current analysis was restricted to patients treated with risperidone (early responders and early nonresponders), with early nonresponders randomized in a double-blind manner to continue on risperidone or switch to olanzapine. Therefore, our results may not generalize to other combinations of antipsychotic medication switches.
The total utility scores used in this analysis were calculated from symptom scores and adjusted for adverse event disutilities. The total utility score represents a weighted measure of both the efficacy and adverse events of the different medications as rated by a representative sample of individuals in the US.Citation16 QALYs have been recommended for use in cost-effectiveness research with antipsychotics because they allow direct comparison of therapies across a number of domains and can be understood in terms of willingness to buy a unit for this outcome.Citation27 An additional strength of the PANSS-based QALY ratingsCitation16,Citation17 is that they were based on health domains that are specifically affected by psychotic illnesses and, therefore, may be more sensitive to important changes in symptoms of schizophrenia than generic measures of quality of life.Citation27
Our simplifying assumption that once an adverse event occurred it did not resolve during the study may have served to underestimate the total utility slightly. However, given the short duration of the trial and the size of adverse event disutilities relative to the disease state utilities, we anticipate this assumption had a negligible effect on the final results.
A challenge in conducting cost-effectiveness research using multinational clinical trials is that the patterns of resource use and the cost of those resources vary greatly by geography. In some countries, psychiatric hospitalization is inexpensive relative to the cost of medications, whereas in the US, psychiatric hospitalization represents one of the most expensive aspects of care. However, the mean duration of psychiatric hospitalization in the US is relatively short compared with some countries (eg, Brazil and Japan). To circumvent this issue in our analysis, cost was assigned based on previously identified monthly costs in the US for the different health states,Citation21 rather than direct collection of and costing of resource use, which was not included in the HGMN trial. Costing based on health states has been used previouslyCitation15 and involves some additional assumptions, including that health states are the main driver of costs and that the previously identified costs for the health states generalize to this patient population.
Finally, although the statistical analysis was adjusted for several important baseline variables, the results of the comparison between early responders and early nonresponders to risperidone are still open to biases from other confounders not accounted for in the statistical model. The patients who were early responders to risperidone may have differed in other significant ways from those who were early nonresponders.
Conclusion
Treatment of early responders was significantly more cost-effective than the treatment of early nonresponders to atypical antipsychotic therapy (risperidone). Early nonresponders to antipsychotic therapy, following only two weeks of treatment, appear to represent a group of patients who are more challenging to treat and tend to incur greater health care costs. In this study, switching early nonresponders from risperidone to olanzapine at two weeks provided significantly lower nonmedication health care costs, significantly higher medication costs, and modest improvements in utility scores. One potential approach to the cost-effective treatment of early nonresponders may be to switch their medications after two weeks. Further research into the effectiveness of switching paradigms and alternative treatments is needed.
Acknowledgements
The authors would like to thank Michael D Stensland, PhD of Agile Outcomes Research, In. and Susan L Dennett, PhD of Strategic Health Outcomes, Inc for medical writing support on behalf of Eli Lilly and Company.
Disclosure
This study was funded by Eli Lilly and Company. HA, XP, DF, VS, SKW, and BK are all full-time employees and minor stockholders of Lilly. JK serves as a consultant and is on the speaker’s bureau for Astra-Zeneca (speaker), Bristol-Myers Squibb (speaker), Cephalon, Dainippon Sumitomo, GlaxoSmithKline, Intracellullar Therapeutics, Janssen (speaker), Johnson & Johnson, Eli Lilly and Company (speaker), Otsuka America Pharmaceutical Inc (speaker), Pfizer, PGxHealth, Proteus, Takeda, Vanda, and Wyeth.
References
- LehmanAFLiebermanJADixonLBPractice guideline for the treatment of patients with schizophrenia, second editionAm J Psychiatry2004161915615000267
- Canadian Psychiatric AssociationClinical practice guidelines. Treatment of schizophreniaCan J Psychiatry20055013 Suppl 1S7S57
- CorrellCUMalhotraAKKaushikSMcMenimanMKaneJMEarly prediction of antipsychotic response in schizophreniaAm J Psychiatry2003160112063206514594760
- KinonBJChenLAscher-SvanumHPredicting response to atypical antipsychotics based on early response in the treatment of schizophreniaSchizophr Res20081021–323024018423985
- Ascher-SvanumHNyhuisAWFariesDEClinical, functional, and economic ramifications of early nonresponse to antipsychotics in the naturalistic treatment of schizophreniaSchizophr Bull20083461163117118156640
- AgidOKapurSArenovichTZipurskyRBDelayed-onset hypothesis of antipsychotic action: A hypothesis tested and rejectedArch Gen Psychiatry200360121228123514662555
- LeuchtSBuschRHamannJKisslingWKaneJMEarly-onset hypothesis of antipsychotic drug action: A hypothesis tested, confirmed and extendedBiol Psychiatry200557121543154915953491
- KinonBJChenLAscher-SvanumHEarly response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophreniaNeuropsychopharmacology201035258159019890258
- KinonBJChenLAscher-SvanumHChallenging the assumption that improvement in functional outcomes is delayed relative to improvement in symptoms in the treatment of schizophreniaSchizophr Res20101181–317618220080036
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth EditionNew York, NYAmerican Psychiatric Publishing Inc2000
- OverallJEGorhamDRThe Brief Psychiatric Rating ScalePsychol Rep196210799812
- KaySROplerLAFiszbeinAPositive and Negative Syndrome Scale (PANSS) User’s ManualNorth Tonawanda, NYMulti-Health Systems Inc2000
- GuyWECDEU Assessment Manual for PsychopharmacologyPublication ADM 76-338 Revised.Rockville, MDUS Department of Health, Education, and Welfare1976
- GoldMRSiegelJERussellLBWeinsteinMCCost-Effectiveness in Health and Medicine1st edNew York, NYOxford University Press1996
- KaneJMKimEKanHJComparative utility of aripiprazole and haloperidol in schizophrenia: Post hoc analysis of two 52-week, randomized, controlled trialsAppl Health Econ Health Policy20097210911919731968
- LenertLASturleyAPRapaportMHPublic preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scoresSchizophr Res200471115516515374583
- LenertLASturleyAPRapaportMHCorrigendum to “Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores”Schizophr Res2005801135136
- SimpsonGMAngusJWA rating scale for extrapyramidal side effectsActa Psychiatr Scand Suppl197021211194917967
- BarnesTRA rating scale for drug-induced akathisiaBr J Psychiatry19891546726762574607
- National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS)ECDEU Assessment Manual for Psychopharmacology, RevisedRockville, MDUS National Institute of Health1976
- MohrPEChengCMClaxtonKThe heterogeneity of schizophrenia in disease statesSchizophr Res2004711839515374576
- MahmoudRAEngelhartLMJanagapCCOsterGOllendorfDRisperidone versus conventional antipsychotics for schizophrenia and schizoaffective disorder: Symptoms, quality of life and resource use under customary clinical careClin Drug Investig2004245275286
- Analysource Data [Website on the Internet]AWP Cost of Antipsychotics Available from: http://www.analysource.com. Accessed March 31, 2008.
- RosenheckRALeslieDLSindelarJCost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophreniaAm J Psychiatry2006163122080208917151158
- FariesDLeonAHaroJObenchainRAnalysis of Observational Health Care Data Using SASCary, NCSAS Press2010
- ObenchainRLRobinsonRLSwindleRWCost-effectiveness inferences from bootstrap quadrant confidence levels: Three degrees of dominanceJ Biopharm Stat200515341943615920889
- PolskyDDoshiJABauerMSGlickHAClinical trial-based cost-effectiveness analyses of antipsychotic useAm J Psychiatry2006163122047205617151153