Abstract
Introduction
Budget impact analysis (BIA) in health care, sometimes referred to as resource impact, is the financial change in the use of health resources associated with adding a new drug to a formulary or the adoption of a new health technology. Several national and transnational organizations worldwide have updated their BIA guidelines in the past 4 years. The aim of the present review was to provide a comprehensive list of the key recommendations of BIA guidelines from different countries that may be of interest for those who wish to build or to update BIA guidelines.
Methods
National and transnational BIA guidelines were searched in databases including MEDLINE, EMBASE, Cochrane, EconLit, CINAHL, Business Source Premier, HealthSTAR, and the gray literature including regulatory agency websites. Data were reviewed and abstracted based on key elements in a standard BIA model (analytical model structure, input and data sources, and reporting format).
Results
Eight national (Australia, UK, Belgium, Ireland, France, Poland, Brazil, and Canada) and one transnational (International Society for Pharmacoeconomics and Outcomes Research) BIA guidelines were included in this review, and a comprehensive list of BIA recommendations was identified. The review showed that certain recommendations such as patient population assessment, drug-related direct costs, discounting, and disaggregated results were common across the various jurisdictions. BIA guidelines differed from each other in terms of the number and scope of recommendations, the terminology used (eg, the definition of comparators or cost offsets) and the direction of the recommendations (ie, to include or not to include with respect to such items as off-label indications, indirect costs, clinical outcomes, and resource utilization).
Conclusion
While there was a common purpose for all of the BIA guidelines that were identified, substantial differences did occur in the specific recommendations. The pharmaceutical financing system structure might explain why guidelines from the UK, Australia, and Canada have more country-specific recommendations. The desire to be consistent with adopted economic evaluation assumptions might be another reason for some observed differences between countries. Further research is required to assess the source of the heterogeneity between BIA recommendations are identified in different guidelines.
Introduction
The first budget impact analysis (BIA) analytic framework was published by MauskopfCitation1 in 1998. In 2001, Trueman et alCitation2 provided essential suggestions for conducting a BIA, and the Polish BIA guidelines in 2004Citation3 followed the initial framework of BIA proposed by Trueman et al.Citation2 In 2005 in Canada, the Patented Medicine Prices Review Board initiated the development of the Canadian BIA guidelines which were subsequently published in 2007.Citation4 The International Society For Pharmacoeconomics and Outcomes Research (ISPOR) task force published the first transnational guidelines for the execution of a BIA in 2007,Citation5 followed by GermanyCitation6 and FranceCitation7 in 2008.
During the past decade, many jurisdictions around the world have updated their BIA guidelines, including the Ire-land (2018),Citation8 France (2018),Citation9 UK (2017),Citation10 Australia (2016),Citation11 Poland (2016),Citation12 and Belgium (2015).Citation13 ISPOR published their second task force report on good practices for conducting BIA in 2014.Citation14 In Asia (ie, Iran,Citation15–Citation17 ThailandCitation18) and Latin America (ie, Brazil,Citation19 Chile, Colombia, Cuba, and Mexico), there have been initiatives regarding drug reimbursement decision making based on standard economic evaluation and BIA guidelines. Brazil has published their BIA guidelines in 2012, and Chile, Colombia and Mexico require BIA as part of their Health Technology Assessment (HTA) process.Citation20
A number of systematic reviews of BIA empirical studies have recently been published,Citation21–Citation25 and literature reviews of national and transnational BIA guidelines have been conducted as part of national BIA guidelines development (eg, France [2018],Citation9 Belgium [2015],Citation13 and Canada [2008]Citation26). However, the Belgian and the Canadian guidelines did not systematically review the BIA literature. In contrast, the French BIA guidelines provides a comprehensive review of the BIA literature, including 9 national BIA guidelines, 5 recommendations of good practices developed by national and international societies for health economics, and 14 methodological publications on existing BIAs, published between 2000 and 2016.Citation9 Nevertheless, the French review did not provide sufficient details regarding the individual guidelines reviewed and cannot be used as a foundation for constructing a new set of BIA guidelines or updating existing versions. To illustrate, the results were briefly listed in a table in an aggregated form rather than providing a complete detailed list of the BIA recommendations. The present study has been designed to identify and abstract all guideline recommendations relating to three key aspects in designing a standard pharmaceutical BIA (analytical model structure, input data and sources, and reporting format). This paper presents a comparative review of the BIA key element recommendations that are discussed in national and transnational BIA guidelines and, also, provides a list of the relevant components that are needed in order to conduct a comprehensive pharmaceutical BIA.
Methods
Data sources
A systematic search of the literature was undertaken to identify BIA guidelines published from 1998 to June 30, 2018. The following bibliographic databases were searched through the Ovid interface: MEDLINE, EMBASE, Cochrane, Econ-Lit, CINAHL, Business Source Premier, and HealthSTAR. We also searched the gray literature (Supplementary material S1) including International Network of Agencies for Health Technology Assessment (INAHTA) and non-INAHTA members (eg, National Institute for Health and Care Excellence, Pharmaceutical Management Agency as well as EUnetHTA, Health Technology Assessment International, International Health Economics Association, and International Society for Pharmacoeconomics and Outcomes Research). The search strategy included a combination of text words and Medical Subject Headings terms and synonyms of budget/financial analysis, guidelines, and methodology/modeling. The keywords used for the searches are shown in Supplementary material S1.
Inclusion and exclusion criteria
The inclusion criteria were limited to BIA guidelines published since 1998 by different countries or international organizations (eg, ISPOR) that presented recommendations on all three key elements of designing a BIA (ie, analytical model structure, input and data sources, and reporting format).Citation14 The titles and abstracts identified in these searches were screened to find eligible published national and transnational BIA guidelines (peer-reviewed or online multimedia). When a country or transnational BIA guideline was updated, we only included the latest updated version of the BIA guidelines for each organization in order to avoid duplication in data abstraction.
Citations that reported BIA for any specific drug or medical device (empirical studies), or review articles of empirical BIAs, abstracts, and conference proceedings and methodological publications other than guidelines for conducting a pharmaceutical BIA were excluded. National guidelines were excluded if they did not explicitly discuss the key elements of a BIA model or if they did not add any additional information beyond the guideline that had been adopted from, and where the latter was already included in the review.
Study selection, data abstraction, and synthesis
Titles and abstracts of all articles were screened (level 1 screening) for inclusion by one reviewer. Following level 1 screening, the full text of the selected articles was retrieved (level 2 screening) and assessed by two independent reviewers for eligibility for final inclusion. The disagreement was resolved through consensus and, if persistent, arbitrated through discussion with a third person.
Using a data abstraction template, all included guidelines were reviewed by two independent reviewers to abstract key elements which were discussed in each BIA guideline. An Excel-based data abstraction form was developed based on the predetermined BIA key elements in accordance with ISPOR BIA guidelines (For sake of simplicity and consistency with other BIA guidelines, in the present review, “ISPOR II Task Force report on BIA Good Practice” was abbreviated to “ISPOR BIA guidelines”).Citation14 All the listed recommendations were for a base-case BIA model. The Excel-based data abstraction form was initially tested using two (Irish and Belgian) BIA guidelines before being used to abstract the data/recommendations from all the included BIA guidelines.
For the purpose of this paper, the BIA key elements were categorized into three groups: analytic model structure, input and data sources, and the reporting format. In each category, we defined primary and secondary elements. The primary elements were the main components within each category (eg, perspective, time horizon, target population, scenarios to compare, costing, modeling, and uncertainty), and secondary elements were more specific and detailed considerations related to the primary elements (eg, off-label use, the degree of implementation, and scenario analysis). The analytic model structure contains a discussion of twelve primary BIA elements (eg, model design, model validation, perspective, time horizon, target population, costing, comparators, discounting and inflation, and handling the uncertainty). The data input category mainly addresses data sources for market-share estimation and epidemiologic analyses. The reporting format section describes details for reporting BIA results based on the payer’s requirements and the standard practices in conducting and reporting BIAs (eg, aggregated and disaggregated results in each year of the time horizon and outcomes are presented in natural and monetary units). All terminologies, categories, and BIA key elements were defined in accordance with ISPOR BIA guidelines.Citation14
Results
Literature search results
A total of 3,804 potential citations were identified through the systematic and the manual searches (having removed duplicates). Fifty-two citations were included after the title and abstract review, of which 43 were excluded for not meeting the eligibility criteria, resulting in a total of 9 national and transnational BIA guidelines published between 1998 and 2018.Citation8–Citation14,Citation19,Citation26 shows the detailed study selection process, and a summary of the included guidelines in the review is shown in .
Figure 1 PRISMA flow diagram of search results.
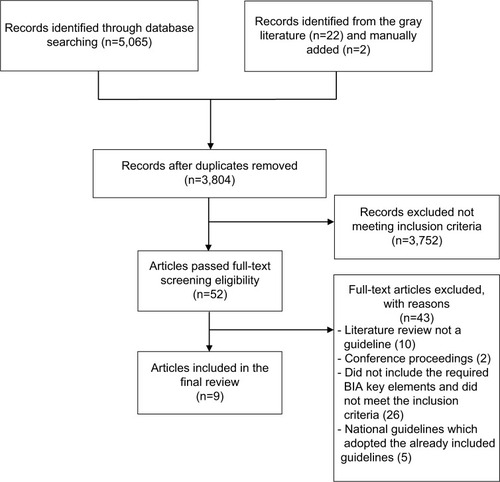
Table 1 Summary of nine included guidelines in the review
Country-specific (national) guidelines from eight countries (Australia, UK, Belgium, Ireland, France, Poland, Brazil, and Canada) were included. The guidelines from five countries were excluded. Germany (2008),Citation6 Thailand (2014),Citation18 and the USACitation27 each adopted the ISPOR BIA guidelines, while the WalesCitation28 and ScotlandCitation29 guidelines were derived from the UK NICE recommendations.Citation10 None of these five countries provided any additional methodological information beyond the source guidelines that they had adopted (which were already included in this review as a primary guideline). A summary of the countries that have developed national BIA guidelines and their associated drug plans is provided in Supplementary material S2.
Guideline recommendations pertaining to the BIA key elements
A comprehensive list of all the BIA guideline recommendations was derived from the nine reviewed guidelines and is presented in . shows the number of guidelines that have made specific recommendations. The following sections provide a synthesis of the key similarities and differences among the nine guidelines.
Figure 2 A schematic list of BIA recommendations in the reviewed guidelines.
Abbreviations: BIA, budget impact analysis; EE, economic evaluation; NICE, National Institute for Health and Care Excellence.
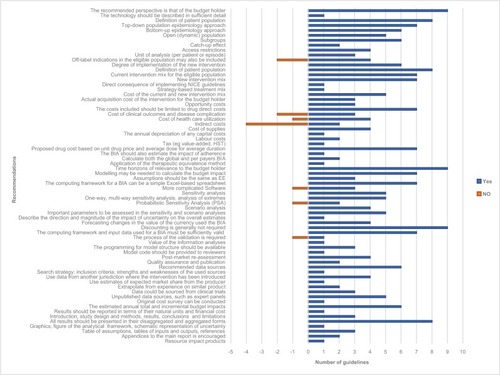
Table 2 BIA categories and recommendations of nine national and transnational BIA guidelines
Analytical model structure
Perspective
In most BIAs, using the perspective of the primary health care budget holder is recommended. However, in the French,Citation9 Polish,Citation12 and CanadianCitation26 BIA guidelines there is a recommendation to use the patient’s perspective as complementary analysis to the base-case analysis. In contrast, AustraliaCitation11 explicitly requires the exclusion of any copayment from any other source beyond the identified budget.
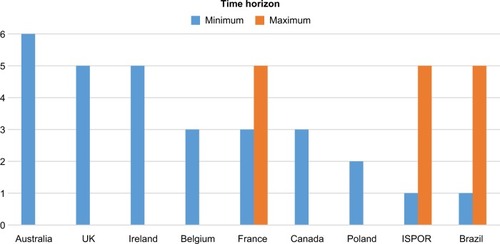
Time horizon
It is recommended in the PolishCitation12 and BelgianCitation13 guidelines to present the budget impact up to the steady state, with a minimum time horizon of 2–3 years. The minimum time horizon in the Canadian BIA guidelinesCitation26 is 3 years, whereas in the updated NICECitation10 and AustralianCitation11 guidelines a longer time duration is recommended (6 and 5 years, respectively). FranceCitation9 and ISPORCitation14 recommend a BIA time horizon varying from 3–5 and 1–5 years in the base-case analysis, respectively. The Brazilian guidelines have also taken a time horizon from 1–5 years.Citation19 The base-case analysis should estimate the annual financial impact over a minimum timeframe of 5 years in the recently updated Irish guidelines.Citation8 A comparison of the time horizon recommended in different guidelines is shown in .
Target population
Some guidelines have defined the target population as the “entire population of patients affected by the assessed indications, targeted by the proposed medicine, over a specified time horizon.Citation8,Citation12,Citation14 French guidelines have introduced two population groups to be included in the analysis, “the target population and the expected treated (forecasted population to be actually treated by the intervention in the real-life practice) population for all indications.”Citation9 Based on the Canadian BIA guidelines, the target population is defined as “all drug plan beneficiaries who are expected to be diagnosed and treated for the conditions of interest and are eligible to use the new drug.”Citation26
Subpopulation analyses can be performed for BIA if there are appropriate justifications: by beneficiary, differences in safety, treatment effect, baseline risks, costs, or market share.Citation8,Citation9,Citation11,Citation13,Citation14,Citation19 For the target population estimation, there are two approaches: top-down or epidemiological and bottom-up or market-share (claim-based analyses). An epidemiological approach is usually preferred if the submission indicates a superior therapeutic conclusion in clinical studies, whereas a market-share approach might be preferred if the submission indicates a noninferior therapeutic conclusion.Citation11 In the epidemiological approach, disease severity shifts, incidence, and prevalence are required, and it is usually inevitable to use data from different sources.Citation26 Apart from the UK,Citation10 Poland,Citation12 and ISPORCitation14 (which only ask for the epidemiologic approach), other guidelines recommend BIA results obtained from both epidemiologic and market-share approaches for all new drug submissions.
The degree of implementation (full replacement or partial substitution of existing technologies or shifts in the target population, market growth, or expansion) is essential in both approaches and recommended by most guidelines. In the Canadian guidelines, it is advised that the treatment displacement assumptions regarding the changes to the market share of each competitor after the introduction of the new drug be tested in the sensitivity analysis.Citation26 The population is dynamic in the Irish, Polish, Belgian, ISPOR and Brazilian guidelines, meaning that patients could be added to or removed from the analysis based on whether they meet the inclusion criteria or not over time.Citation8,Citation12–Citation14,Citation19 In some cases, when the technology applies to a well-defined group of patients, the BIA may require a defined closed population.Citation12
In addition, the French, Belgian, ISPOR (for the current treatment mix) and Brazilian BIA guidelinesCitation9,Citation13,Citation14,Citation19 recommend consideration of off-label usage in all indications for the assessed medicine as complementary to the base- case analysis; this is especially relevant if there is available evidence for cost-effectiveness and, more importantly, it is noted by the payer.Citation9 In the Canadian BIA guidelines, the off-label use is only considered in the sensitivity analysis.Citation26 The catch-up effect which applies to the chronic conditions for patients who switch to the new drug is recommended in the Irish and ISPOR guidelines.Citation8,Citation14 Any planned local regulations and legislations which would limit new drug access in a subpopulation should be considered.Citation12,Citation14,Citation19,Citation26
Scenarios to compare (comparators)
In most of the reviewed guidelines, the current scenario/practice (including “no intervention”) should be “routine care” or the best clinical practice, including the most cost-effective alternatives. The new scenario is the “current scenario” with the new intervention added to or replacing the current interventions entirely or partially.Citation13,Citation14 NICE considers a broader picture of budget impact and defines the current and new scenarios as current and future clinical practice activities (at activity levels) resulting from adopting the NICE guidelines in the NHS.Citation10 In Canada, the comparator definition is more market-oriented. According to the Canadian BIA guidelines, reference scenario is the current market-share distribution of all comparators without new drug, whereas new drug scenario is forecast market share of same comparators with the inclusion of the new drug.Citation26 Multidrug treatment (ie, treatment mix or set,Citation14 treatment set,Citation9 treatment mix,Citation11 and strategy-based treatmentCitation26) rather than individual interventions is recommended in most of the guidelines.Citation8,Citation9,Citation11,Citation12,Citation14,Citation19,Citation26
Cost analysis
Ireland, France, Australia, Poland, ISPOR, Brazil and Canada consider costing based on multi-drug treatment strategy (including adjunct therapies).Citation8,Citation9,Citation11,Citation12,Citation14,Citation19,Citation26 The BIA should, therefore, identify all medicines likely to be affected by the new drug.
Most of the guidelines agree on the fact that direct health care-related costs for the most relevant perspective should be included in the base-case, similar to the guidelines for economic evaluations.Citation8–Citation10,Citation12–Citation14,Citation19 However, the AustralianCitation11 and CanadianCitation26 BIA guidelines exclude the costs associated with changes in outcomes, costs associated with clinical consequences/complications (eg, adverse drug reactions), and resource utilization (eg, hospitalization, emergency room admission), while other guidelines suggest to review such nondrug related costs. In the latest version of the Irish guidelines, for pharmaceuticals, direct costs include the cost of the drug and any other drug-related costs (concomitant therapies, adverse events, and infusion-related costs such as consumables and staffing).Citation8 The impact on indirect, non-health care-related costs (eg, productivity, transport, capacity, and workforce) are not usually included in a BIA base-case analysis, except for the NICE guidelines ().Citation8,Citation9,Citation13,Citation14
Other differences between BIA guidelines were related to the scope of costs (eg, costs related to personnel training, budget transfers between different governments and patients).Citation8,Citation9,Citation13,Citation14 According to the Irish, Polish, and ISPOR guidelines, it is important to consider additional resources that must be taken from the existing services when implementing a new technology, which are called “opportunity costs.” Opportunity costs are the costs that arise when implementing the technology or clinical guidelines that might not being reflected in the “actual costs” at the time of doing BIA analysis.Citation8,Citation12,Citation14 In the case of including condition-related costs (ie, health outcomes and resource use), the actual opportunity costs are relevant in the ISPOR guidelines. In such cases analysts may use cost accounting approaches if actual opportunity costs are not available for a particular jurisdiction.Citation14 According to the Irish guidelines “actual costs” are cash payments which occur from implementing the technology or clinical practice guidelines.Citation8 The BIA should clearly state which unit of analysis is adopted in measuring the outcomes. There are two possible units of analysis: per patient or episode of care. Specified interventions may range from once-daily, repeated, periodic, or continuous interventions; it needs to be clear the number of times or the length of time people might experience the intervention or how many treatment events might arise.Citation8,Citation11,Citation19
Cost of the treatment should be adjusted to consider markups, discounts, inventory allowance,Citation8,Citation14,Citation26 business-related costs to the pharmacy covered by the drug plans, and dispensing fees and patient copayments, as requested by drug plans in Canada.Citation26 In the Canadian BIA guidelines, drug prices can be obtained from provincial formulary websites, public drug plan databases, and manufacturers’ market access department for preparing BIA reports.Citation26 There are also recommendations on how to deal with New Chemical Entities and generic drug prices for BIAs in the Canadian BIA guidelines.Citation26 In Australia, Pharmaceutical Benefits Advisory Committee (PBAC) also recommends “dispensed price for maximum amount” for BIA.Citation11 It is recommended that uncertainties regarding the drug reimbursement price should be targeted through a sensitivity analysis.Citation26
In the Irish guidelines, the value-added tax could be considered if applicable,Citation8 and in the Belgian and Canadian BIA guidelines, protocol-driven costs should be excluded (eg, costs related to the patient enrollment process and additional laboratory tests specific to the clinical trial design).Citation13,Citation26 None of the guidelines recommends inflation and discount rates; however, in the Canadian, Brazilian, Irish, and ISPOR BIA guidelines, they are permitted in the certain circumstances and if there is justification for being included (eg, confirmed information on pricing policy, implementation of an approved new policy rule in the near future, or price changes after patent expiration).
Modeling
Transparency, validity, simple, and user-friendly design along with explicit definitions and assumptions are the most favorable features of a BIA model. It is recommended that the model be designed based on the projected disease condition and be flexible enough to capture long-term outcomes/costs in the chronic diseases.Citation9 Similar to cost-effectiveness analyses, in the Belgian and Brazilian BIA guidelines, decision trees or Markov models can be helpful to be consistent with the economic evaluations.Citation13,Citation19 Most guidelines recommend using an Excel-based model (rather than more complicated software) to calculate the budget impact.Citation9–Citation11,Citation14,Citation19,Citation26 This allows for extending the analysis to the appropriate time horizon and using different data sources. Face, internal, and external validities have to be checked and documented. The model validity and transparency could be assessed using recommendations provided by ISPOR and the Society for Medical Decision Making task force report.Citation30
Handling the uncertainty
Decreasing the uncertainty is an essential consideration in BIA. Although probabilistic sensitivity analysis is not recommended in the Canadian BIA guidelines, one-way, univariate deterministic sensitivity analysis or multivariate scenario analysis are acceptable for the most important variables such as prices, population and market shares.Citation26 Sensitivity analysis of data obtained from clinical trials,Citation11 drug dosage,Citation26 price,Citation26 and market data from other jurisdictionsCitation14 are also recommended.Citation8,Citation9,Citation11,Citation12,Citation14,Citation19,Citation26
Scenario analysis is recommended by Ireland, France, Australia, Belgium, and ISPOR.Citation8,Citation9,Citation11,Citation13,Citation14 PBACCitation11 has provided a very detailed list of recommended scenarios to be considered in reporting the budget impact results, eg, the effects of promotional efforts on prescriber and consumer behavior. Risk sharing agreements with the manufacturers and a more extended introduction phase for the proposed drug have also been recommended by the UK and Australia for managing uncertainty in early BIA results.Citation10,Citation11
Input and data sources
National statistics and registries are recommended sources for epidemiologic data (eg, disease prevalence and incidence).Citation8,Citation9,Citation12,Citation14,Citation19,Citation26 The best sources for the claim-based and market research information are the payer databaseCitation14 and the manufacturer’s marketing department.Citation14,Citation26 In the Irish, ISPOR, Brazilian and Canadian guidelines, data from foreign markets are acceptable if local information are not available ().Citation8,Citation14,Citation19,Citation26 The BIA reports from manufacturers with clear supporting data could also be helpful.Citation14,Citation26 Consensus expert opinion is an option when market intelligence for forecasting the new drug market share is not available.Citation8,Citation12,Citation14,Citation19,Citation26
Reporting format
There are specific requirements for reporting the results in the reviewed guidelines. Newly updated guidelines have put more attention to the details and the manner BIA results are reported, mainly based on the policymakers’ interest and requirements.
Total and incremental impact on the primary payer’s budget should be presented in the Polish, Irish, French, and Australian guidelines.Citation8,Citation9,Citation11,Citation12 The Canadian guidelines only require the incremental impact on the annual budget.Citation26 Results should be both aggregated and disaggregated in each year of the time horizon in the Irish, French and Australian guidelines.Citation8,Citation9,Citation11
The budget impact can be presented in natural (eg, number of unpaid working days) and monetary units separately for the different health care payers.Citation8 A table of assumptions, inputs, and outputs, a schematic representation of any uncertainty analyses (eg, Tornado diagram), appendices, and references should be included.Citation9,Citation14,Citation19 Estimated financial implications for the health budget (other health sectors), the impact of uncertainty (quantify how precise are the results), activities to support the quality use of medicines, and postmarketing surveillance amendments are recommended by PBAC.Citation11 In their new resource impact assessment (RIA) manual, NICE classifies results as “substantial” if the implementation of a single recommendation in the UK costs higher than a specific threshold.Citation10
NICE recommends publishing the resource planner, a word file of resource impact reports, resource impact statements, quality assurance and publication, as well as making postpublication amendments. RIA results should be published at the same time as NICE evidence-based guidelines and performed in parallel with economic evaluations.Citation10
Discussion
In the present review, we identified BIA guidelines from Ire-land, France, UK, Australia, Poland, Belgium, ISPOR, Brazil and Canada reviewed and all their recommendations related to the analytical model structure, input and data sources, and reporting format of BIAs.Citation8–Citation14,Citation19,Citation26 It is the first peer-reviewed evidence in the health literature in which a systematic review of national and transnational BIA guidelines was published as robust and comprehensive basis for the future research.
There are some similarities in guidelines recommendations (eg, using drug-related direct costs from the primary payer’s perspective, top-down or bottom-up approaches for population assessment, simple [not complicated] modeling techniques, and deterministic sensitivity analysis as the minimum requirements for a BIA base-case analysis). Differences between guidelines were related to number, scope, and direction (yes/no) of recommendations (eg, inclusion of off-label indications, indirect costs, clinical outcomes, and health care resource utilization; duration of time horizon; dealing with uncertainty [eg, deterministic analysis vs PSA], and reporting format). Moreover, there are differences in the terminologies which are used in different guidelines/countries for defining specific concepts in designing a BIA (eg, multidrug treatment in assessing the comparators, target population definition such as “open population”, or cost offsets).
Some guidelines were closely aligned in their recommendations (eg, French, Australian, Belgian, and ISPOR BIA guidelines), while others had included more country-specific recommendations (eg, Canada, Australia, and the UK). In some guidelines/countries such as ISPOR, UK, Belgium, Ireland, and Australia, if an economic evaluation was performed, the BIA model should be consistent with the clinical and economic assumptions in economic evaluation. In the UK, BIA is called RIA and the estimation of costs and savings is based on direct consequence of implementing NICE guidelines (not just drug comparators).Citation10
The results of our review are similar to the French literature reviewCitation9 of BIA guidelines in terms of key aspects in designing BIA. However, our review used BIA categories more aligned with the ISPOR BIA guidelines.Citation14 The literature review that was conducted as part of the Belgian guidelines was not published with sufficient detail,Citation13 and the literature review results in the French guidelines were summarized in an aggregated format.Citation9 Thus, there were insufficient details to provide a complete taxonomy of BIA guideline recommendations. A previous Canadian BIA literature reviewCitation26 included the older versions of the Polish (2004), Australian (2002), and ISPOR (2007) BIA guidelines. Our literature review was different in terms of 1) the review design (systematic), 2) the scope (focused on only BIA guidelines recommendations), 3) inclusion criteria (all BIA guidelines published since 1998, excluding any versions that were replaced by newer updates), and 4) reporting format (applicable details for future research).
The present review is the most recent systematic review of published national and transnational BIA guidelines that have been created or updated since 1998. A potential limitation of this study includes having only one reviewer for the level 1 (title and abstract) screening which we believe that did not contribute to considerable bias. We did not include results from countries that simply adopt BIA guidelines from other jurisdictions (Germany, Thailand, USA, Scotland, and Wales) which might be considered a limitation in that it would underestimate the frequency of use for some recommendations. We also did not include published BIA methodologic papers as we were only interested in reviewing BIA guideline recommendations.
Conclusion
To maintain sustainability in financing the health care systems, it is increasingly important to improve informed pricing and reimbursement decision making at national and transnational levels. Our literature review showed that over last 20 years, countries have become actively interested in comprehensive financial and economic evaluations and have tried to keep their BIA guidelines updated. Through a systematic review of national and transnational BIA guidelines published or updated since 1998 following Mauskopf’sCitation1 publication, we provided a full list (not a summary) of the details for conducting a standard pharmaceutical BIA in accordance with the most up-to-date national and transnational BIA guidelines recommendations. The remaining challenge is how to embrace the heterogeneity of recommendations and terminologies that is evident across different guidelines. Further research is required to analysis each countries’ pharmaceutical financing system in more detail to assess any true relationship between country-specific health care parameters and BIA recommendations. The results of this review can be a starting point for countries who are initiating the development of national standard BIA guidelines based on their pharmaceutical reimbursement requirements. The present review can provide useful practical methodological information for BIA users and producers and provide a contribution to the future research in the field of pharmaceutical BIA.
Acknowledgments
The authors appreciate the contributions made by Arash Foroutan (second reviewer for level 2 screening) and Fergal Mills (Innomar Strategies) to this project.
Supplementary materials
Supplementary material S1
Systematic literature review process
MEDLINE, EMBASE, Cochrane, EconLit, CINAHL, Business Source, Ovid HealthSTAR, and the gray literature including International Network for Agencies for Health Technology Assessment (INAHTA) and non-INAHTA members (eg, NICE, PHARMAC) as well as European network for health technology assessment (EUnetHTA), Health Technology Assessment International (HTAi), iHEA, and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) were searched using a combination of text words and Medical Subject Headings terms and synonyms of budget/financial analysis, guidelines, and methodology/modeling. The keywords used for the searches are as following:
Search strategy
MEDLINE
The gray literature list
Websites of health technology assessment or regulatory agencies
Supplementary material S2
Countries with developed budget impact analysis (BIA) guidelines and the types of drug programs where they are applied.
In Australia, there is a government-run Pharmaceutical Benefits Scheme that subsidizes prescription medication, and there is a copayment for patients at the point of dispensing.Citation1 The BIA guidelines as a part of the Australian guidelines on the preparation of new drug submissions to Pharmaceutical Benefits Advisory Committee (PBAC) (2016) is the first full revision of PBAC guidelines since 2006. After 2010, any recommendation by PBAC that has a financial impact on the Federal government’s budget is reviewed by the cabinet.Citation2 There is a close relationship between the estimated financial impact of a drug on the Australian drug budget and the rate of PBAC positive recommendations for reimbursement.Citation3
Belgium has a Bismarck-type social insurance system (multipayer) in which the insurers, called Sickness Funds, are financed by both employers and employees.Citation4 In Belgium, since 2002, Health Care Knowledge Centre (KCE) under the supervision of the Minister of Public Health and Social Affairs is in charge of conducting studies that support the political decision making on health care and health insurance.Citation5 The Belgian guidelines for economic evaluations now include guidance for a BIA in an updated version (2015). The Belgian official Health Technology Assessment institute, KCE, and Belgian stakeholders from both government and industry contributed to improving their recent national economic evaluations and BIA guideline.Citation5
In Brazil, the Unified Health System provides free universal care for all Brazilians as well as vaccinations and pre-natal care. A highly decentralized system has led to complex patterns of funding and service provision with the Federal, State, and Municipal governments involved. Brazil’s system remains highly privatized with the private sector receiving substantial funds from all levels of government.Citation6 Brazil (Ministry of Health [CONITEC]) has been developing the necessary analytical instruments for the evaluation of new technologies for health. In this context, the development of national recommendations for budget impact studies in the health area became more important. The methodology for the development of budgetary impact studies in the health area was adapted to the Brazilian needs, through several presentation and discussion sessions among the professionals of the institutions involved.Citation7
Canada is an example of a “National Health Insurance” model. Canada’s publicly funded health care system is called “Medicare” in which ten provincial and three territorial health care insurance plans share roles and responsibilities for health care services with the Federal government.Citation8 Drug benefit funding is primarily a composite of provincial/territorial governments and private insurance programs. Federally, the Patented Medicine Prices Review Board sets ex-factory price ceilings for patented medications. Although a BIA had been required to be submitted to most provincial public drug plans in the 1990s, before 2007, there was no standardized method of conducting a BIA in Canada. In 2005, Patented Medicine Prices Review Board initiated the development of the Canadian BIA Guidelines on behalf of the National Prescription Drug Utilization Information System, and this was published in 2007.Citation9
In France, the pharmaceutical reimbursement decision-making process consists of two steps: 1) the technical assessment by French National Authority for Health La Haute Autorité de Santé (HAS) and 2) enlisting the drug with price-fixing by the “health care products pricing committee” of the Ministry of Health (Comité Economique des Produits de Santé [CEPS]).Citation10 Since January 2016, cost-effectiveness analysis and BIAs are required to be submitted by manufacturers to HAS and CEPS for highly specialized medicines with an expected 2-year sales revenue more than €50 million.Citation11 In France, BIA for new drug submissions should be prepared for the French statutory social insurance scheme. HAS updated the French BIA guidelines for new drug submissions in December 2017, however, it is not still clear that how BIA results would be applied in the reimbursement price negotiation process.
The Republic of Ireland has a new NHS which was launched in 2005 and is controlled by the Health Service Executive.Citation12 The Irish “Health Information and Quality Authority” (The Authority) has the responsibility to evaluate the clinical and cost-effectiveness of health technologies, and provides evidence-based reports to the Minister of Health and Health Service Executive and develops guidelines for doing HTA in Ireland. The latest updated version of the Irish BIA guidelines on health technologies was published by The Authority in 2018.Citation13
Health care in Poland is primarily financed by the National Health Fund (Narodowy Fundusz Zdrowia) and state budget or local government budgets. The state budget plays a complementary role to National Health Fund in the system. The primary role of the local governments is to ensure access to the services, mostly by performing ownership functions toward health care institutions. In Poland, the BIA guidelines are a part of the latest updated Health Technology Assessment guidelines which initially issued by the Agency for Health Technology Assessment and Tariff System in 2007 and were updated in 2009 and 2016.Citation14
National Health Service (NHS) in the United Kingdom is an example of a single-payer health care system for a country. In the UK, the NHS institution in England and Wales pays for medicines if NICE provides a favorable recommendation. NICE published their updated guidelines on the resource impact (budget impact) assessment process on May 2017. It is proposed that a cap called “budget impact test”Citation15 of £20 million, in any of the first 3 years, be considered to signal the need for negotiation with manufacturers for special arrangements to better manage the introduction of new technologies recommended by NICE.Citation16 Moreover, NICE has recently proposed a Fast Track technology Appraisal process for the new technologies which fall below an incremental cost-effectiveness ratio of £10,000 per quality adjusted life years. The budget impact test would be removed as a criterion for entry into the Fast Track Technology Appraisal process.Citation16,Citation17
References
- The GuardianFour healthcare systems divided by the English language: Australia, Canada and Ireland have universal healthcare systems, although run on different lines to Britain’s NHS. And then there is the USA [press release]LondonThe Guardian201167
- GhabriSMauskopfJThe use of budget impact analysis in the economic evaluation of new medicines in Australia, England, France and the United States: relationship to cost-effectiveness analysis and methodological challengesThe European Journal of Health Economics2017
- MauskopfJChirilaCMasaquelCRelationship between financial impact and coverage of drugs in AustraliaInternational journal of technology assessment in health care20132919210023217275
- The Belgian Health Care Knowledge CentreDrug Reimbursement Systems: International Comparison and Policy Recommendations, KCE reports 147CBrussels, BelgiumThe Belgian Health Care Knowledge Centre2010 Available from: https://kce.fgov.be/sites/default/files/atoms/files/KCE_147C_Drug_reimbursement_systems_4.pdfAccessed Mar 23, 2018
- NeytMCleemputIVan De SandeSThiryNBelgian guidelines for budget impact analysesActa Clinica Belgica: International Journal of Clinical and Laboratory Medicine2015703175180
- The World BankBrazil – Health Financing Profile (English)Washington, DCThe World Bank2014
- Ferreira-Da-SilvaALRibeiroRASantosVCEliasFTd’OliveiraALPolanczykCAGuidelines for budget impact analysis of health technologies in BrazilCadernos de saude publica201228712231238 Portuguese22729254
- MossialosEWenzlMOsbornRSarnakD2015 international profiles of health care systemsCanadian Agency for Drugs and Technologies in Health2016
- Patented Medicine Prices Review BoardGuidelines for Conducting Pharmaceutical Budget Impact Analyses for Submission to Public Drug Plans in CanadaCanadaPatented Medicine Prices Review Board2007 Available from: http://www.pmprb-cepmb.gc.ca/cmfiles/bia-may0738lvv-5282007-5906.pdfAccessed February 1, 2018
- France – PharmaceuticalsGlobal Health Care Systems Road Map2009 Available from: https://www.ispor.org/HTARoadMaps/France.asp#4Accessed March 22, 2018
- GhabriSAutinEPoullieAIJosselinJMThe French National Authority for Health (HAS) Guidelines for Conducting Budget Impact Analyses (BIA)Pharmacoeconomics201836440741729247437
- The GuardianFour healthcare systems divided by the English language: Second part of Guardian Healthcare’s guide to healthcare systems in English speaking countries: Republic of Ireland and United States of America [press release]LondonThe Guardian201167
- Health Information and Quality AuthorityGuidelines for the Budget Impact Analysis of Health Technologies in IrelandCork, IrelandHealth Information and Quality Authority2018 Available from: https://www.hiqa.ie/reports-and-publications/health-technology-assessment/guidelines-budget-impact-analysis-healthAccessed January 27, 2018
- The Agency for HealthTechnology Assessment and Tariff SystemHealth Technology Assessment Guidelines (Poland)Warsaw, PolandThe Agency for HealthTechnology Assessment and Tariff System2016 Available from: http://www.aotm.gov.pl/www/wp-content/uploads/wytyczne_hta/2016/20161104_HTA_Guidelines_AOTMiT.pdfAccessed January 27, 2018
- National Health Service“Budget Impact Test” rather than “net budget impact” or threshold/cap to clarify to stakeholders that it is not the maximum funding by NHSLondonNational Health Service
- National Institute for Health and Care ExcellenceProposals for changes to the arrangements for evaluating and funding drugs and other health technologies appraised through NICE ’s technology appraisal and highly specialised technologies programmesLondonNational Institute for Health and Care Excellence2016
- National Institute for Health and Care ExcellenceNICE and NHS England consultation on changes to the arrangements for evaluating and funding drugs and other health technologies assessed through NICE’s technology appraisal and highly specialised technologies programmesLondonNational Institute for Health and Care Excellence2017 Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/TA-HST-consultation-response-paper-March-Board.pdfAccessed March 26, 2018
Disclosure
The authors report no conflicts of interest in this work.
References
- MauskopfJPrevalence-Based Economic EvaluationValue in Health19981425125916674550
- TruemanPDrummondMFHuttonJDeveloping Guidance for Budget Impact AnalysisPharmacoeconomics200119660962111456210
- OrlewskaEMierzejewskiPProposal of Polish Guidelines for Conducting Financial Analysis and Their Comparison to Existing Guidance on Budget Impact in Other CountriesValue in Health200471
- Patented Medicine Prices Review BoardGuidelines for Conducting Pharmaceutical Budget Impact Analyses for Submission to Public Drug Plans in Canada2007 http://www.pmprb-cepmb.gc.ca/cmfiles/bia-may0738lvv-5282007-5906.pdfAccessed Feb 1st, 2018
- MauskopfJSullivanSAnnemansLPrinciples of Good Practice for Budget Impact Analysis: Report of the ISPOR Task Force on Good Research Practices—Budget Impact AnalysisValue in health2007105
- Institute for Quality and Efficiency in Health Care (IQWiG)Methods for assessment of the relation of benefits to costs in the German statutory health care system2008 http://www.rees-france.com/en/article.php3?id_article=523Accessed Jan 27, 2018
- Collège des Économistes de la Santé (CES)Guide méthodologique pour la mise en place d’une analyse d’impact budgetaire 2008 http://www.ces-asso.org/docs/Rapport_AIB.pdfAccessed Feb 20, 2018
- Health Information and Quality AuthorityGuidelines for the Budget Impact Analysis of Health Technologies in Ireland2018 https://www.hiqa.ie/reports-and-publications/health-technology-assessment/guidelines-budget-impact-analysis-healthAccessed Jan 27, 2018
- GhabriSAutinEPoullieAIJosselinJMThe French National Authority for Health (HAS) Guidelines for Conducting Budget Impact Analyses (BIA)Pharmacoeconomics2017
- National Institute for Health and Care ExcellenceAssessing resource impact process2017 https://www.nice.org.uk/about/what-we-do/into-practice/resource-impact-assessmentAccessed Jan 27th, 2018
- Department of health, Australian governmentGuidelines for preparing a submission to the Pharmaceutical Benefits Advisory ComitteePharmaceutical Benefits Advisory Comittee guidelines2016 https://pbac.pbs.gov.au/Accessed Jan 27th, 2018
- The Agency for Health Technology Assessment and Tariff System (Polish)Health Technology Assessment Guidelines2016 http://www.aotm.gov.pl/www/wp-content/uploads/wytyczne_hta/2016/20161104_HTA_Guidelines_AOTMiT.pdfAccessed Jan 27th, 2018
- NeytMCleemputIVan De SandeSThiryNBelgian guidelines for budget impact analysesActa Clinica Belgica: International Journal of Clinical and Laboratory Medicine2015703175180
- SullivanSDMauskopfJAAugustovskiFBudget impact analysis - Principles of good practice: Report of the ISPOR 2012 budget impact analysis good practice II task forceValue in Health201417151424438712
- ForoutanNJamshidiHRSalamzadehJForoutanARasekhHPRM18 Conducting Pharmaceutical Budget Impact Analyses in Iran: In Accordance With ISPOR Task Force Report on Good Practice for Budget Impact AnalysisValue in Health2012157A463
- ForoutanNRasekhHRSalamzadehJJamshidiHRNafarMBudget impact analysis of conversion from cyclosporine to sirolimus as immunosuppressive medication in renal transplantation therapyClinicoEconomics and outcomes research: CEOR2013554555324159260
- JamshidiHRForoutanNSalamzadehJ“Budget Impact Analyses”: A Practical Policy Making Tool for Drug Reimbursement DecisionsIranian Journal of Pharmaceutical Research: IJPR20141331105110925276214
- LeelahavarongPBudget impact analysisJournal of the Medical Association of Thailand = Chotmaihet thangphaet201497Suppl 5S6571
- Ferreira-Da-SilvaALRibeiroRASantosVCEliasFTd’OliveiraALPolanczykCAGuidelines for budget impact analysis of health technologies in BrazilCadernos de saude publica20122871223123822729254
- NanavatyMGalaSNyandegeARameshVMwamburiMUnderstanding Health Technology Assessment (HTA) Bodies in Major Latin-American (LATAM) Markets: Systematic Evaluation in 5 Latam CountriesValue in Health2016197A491
- FaleirosDRAlvaresJAlmeidaAMBudget impact analysis of medicines: updated systematic review and implicationsExpert review of pharmacoeconomics & outcomes research201616225726626923561
- GarattiniLvan de VoorenKBudget impact analysis in economic evaluation: a proposal for a clearer definitionThe European journal of health economics: HEPAC: health economics in prevention and care201112649950221874376
- MauskopfJEarnshawSA Methodological Review of US Budget-Impact Models for New DrugsPharmacoEconomics201634111111113127334107
- OrlewskaEGulacsiLBudget-impact analyses: a critical review of published studiesPharmacoeconomics2009271080782719803537
- van de VoorenKDurantiSCurtoAGarattiniLA critical systematic review of budget impact analyses on drugs in the EU countriesAppl Health Econ Health Policy2014121334024158922
- MarshallDADouglasPRDrummondMFGuidelines for conducting pharmaceutical budget impact analyses for submission to public drug plans in CanadaPharmacoeconomics200826647749518489199
- Academy of Managed Care PharmacyA Format for Submission of Clinical and Economic Evidence of Pharmaceuticals in Support of Formulary Consideration Version 42016 http://www.amcp.org/practice-resources/amcp-format-formulary-submisions.pdfAccessed Jan 27, 2018
- All Wales Medicines Strategy GroupForm B Guidance notes2016 http://www.awmsg.org/docs/awmsg/appraisaldocs/inforandforms/Form%20B%20guidance%20notes.pdfAccessed Feb 1st, 2018
- Scottish Medicine ConsortiumGuidance to manufacturers for completion of new product assessment form (NPAF)2013 https://www.scottishmedicines.org.uk/Submission_Process/Submission_guidance_and_forms/Templates-Guidance-for-Submission/Templates-Guidance-for-SubmissionAccessed Feb 1st, 2018
- EddyDMHollingworthWCaroJJTsevatJMcDonaldKMWongJBModel transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7Med Decis Making2012325733743 doi: 710.1177/0272989X1245457922990088