Abstract
Purpose
Although expert guidelines for the treatment of schizophrenia recommend antipsychotic monotherapy, the use of antipsychotic polypharmacy is common. This study identified characteristics that differentiate patients with schizophrenia who are treated with olanzapine monotherapy versus polypharmacy in usual care in Japan.
Patients and methods
In a large (N = 1850) prospective, observational study, Japanese patients with schizophrenia who initiated treatment with olanzapine were followed for 1 year. Consistent with past research, antipsychotic polypharmacy was defined as the concurrent use of olanzapine and another antipsychotic for at least 60 days. Switching was defined as discontinuing a prior antipsychotic therapy rather than augmenting the medication regimen. Predictors of antipsychotic monotherapy were based on information available at the time of olanzapine initiation. Baseline characteristics were compared using t-tests and χ2 tests. Stepwise logistic regression was used to identify independent predictors of monotherapy.
Results
Patients treated with olanzapine monotherapy (43.2%) differed from those treated with antipsychotic polypharmacy (56.8%) on demographics, treatment history, baseline symptom levels, functional levels, and treatment-emergent adverse events. Stepwise logistic regression identified multiple variables that significantly predicted monotherapy: older age, shorter duration of schizophrenia, outpatient status, comorbid medical conditions, lower body mass index, no prior anticholinergic use, no prior mood stabilizer use, and switching from a previous antipsychotic (typical or atypical).
Conclusion
Consistent with prior research in Japan, antipsychotic polypharmacy appears to be common in the treatment of schizophrenia. Patients treated with monotherapy could be differentiated from those treated with antipsychotic polypharmacy based on a specific set of demographic and baseline clinical characteristics.
Introduction
Treatment guidelines consider antipsychotic medications to be the cornerstone of treatment in schizophrenia.Citation1,Citation2 Antipsychotic monotherapy is recommended over antipsychotic polypharmacy, which is defined as the concurrent use of two or more antipsychotic drugs.Citation2 Despite the consistent preference for monotherapy,Citation1–Citation3 polypharmacy is frequently used in the treatment of schizophrenia,Citation4–Citation7 and its use appears to be increasing over time.Citation5,Citation7
Antipsychotic polypharmacy is initiated for a variety of reasons, but most are related to the need for further control of the positive or negative symptoms of schizophrenia.Citation8,Citation9 Although limited evidence supports the use of polypharmacy in certain situations, the empirical evidence does not support the observed prevalence of antipsychotic polypharmacy.Citation3,Citation10 Although the supporting evidence is limited, the drawbacks are clear: polypharmacy increases the risk of drug–drug interactions,Citation11,Citation12 treatment-emergent adverse events,Citation13 antipsychotic costs,Citation7 and treatment regimen complexity.Citation11 Treatment regimens with multiple antipsychotics are more difficult to evaluate and modify when needed, because the effects of each antipsychotic cannot be easily disentangled.Citation11
Estimates of the prevalence of antipsychotic polypharmacy in schizophrenia have ranged widely from 13% to 70%.Citation5,Citation7,Citation9,Citation14–Citation18 The wide range appears to result from differences in study design characteristics, including patient population, treatment characteristics, and the specific country studied. Patient population factors associated with higher polypharmacy include demographic characteristics such as younger ageCitation18,Citation19 and male gender,Citation5 more frequent concomitant psychotropic medication use,Citation5,Citation6,Citation15,Citation18 and clinical characteristics such as inpatient treatment settingCitation5 and greater symptom severity.Citation6 In addition, polypharmacy appears to vary by antipsychotic treatment, with olanzapine-treated patients being more likely to be treated with monotherapy than patients treated with quetiapine,Citation4,Citation20–Citation22 risperidone,Citation4,Citation22,Citation23 or typical antipsychotics.Citation23 In Japan, the rate of polypharmacy appears to be particularly prevalent, with estimates ranging between 46.7% and 69.3%.Citation24,Citation25
Due to the high prevalence of antipsychotic polypharmacy in schizophrenia and the potential problems associated with its use, research is needed to better understand this phenomenon and help identify the characteristics differentiating monotherapy-treated patients from polypharmacy- treated patients. The objective of this study was to identify demographic and baseline clinical characteristics that differentiate patients with schizophrenia who are treated with olanzapine monotherapy versus olanzapine polypharmacy over a 1-year period in usual care in Japan.
Methods
Data source
The data for this research came from the Olanzapine Post Marketing Surveillance (OPMS) study. The OPMS study is a large multicenter, naturalistic, 1-year study in Japan with 1850 participants meeting the inclusion criteria. The inclusion criteria included having a schizophrenia diagnosis based on the Diagnostic and Statistical Manual of the American Psychiatric Association, Fourth Edition, as well as being initiated with olanzapine treatment. This naturalistic study was designed to be minimally invasive in usual care; all of the treatment decisions were left to the treating physician. Enrollment for the study began in November 2003 and finished in July 2004. The follow-up period continued for 1 year after enrollment or until the patient discontinued treatment with olanzapine. Data were collected at the baseline, 3-month, 6-month, and 12-month visits. The internal review boards at each of the participating institutions approved the study procedures, and informed consent was obtained based on the rules set at these facilities.
Measures and definitions
A number of clinical variables were assessed at baseline and used to help differentiate patients who were treated with olanzapine monotherapy versus polypharmacy. The Clinical Global Impression-Schizophrenia (CGI-SCH) scale was used to measure the symptom severity level. The ratings are made on an anchored scale ranging from no symptoms (0) to severe symptoms (6).Citation26 Assessment of the concurrent validity of the CGI-SCH scale and the more rigorous Positive and Negative Syndrome ScaleCitation27 found the validity coefficients to range from 0.61 for depressive symptoms to 0.86 for positive symptoms with the remaining coefficients ranging from 0.75 to 0.80. Moderately high inter-rater reliability has also been reported (interclass correlation coefficients ranging from 0.73 to 0.82) for all but the depressive subscale (0.64).Citation26
Health-related quality of life was measured using the European Quality of Life 5-Dimensions visual analog scale (EQ-5D VAS). The EQ-5D VAS, on this generic measure of health-related quality of life, ranges from 0 to 100.
In addition to these published scales and demographic information, several other baseline characteristics were used to predict later antipsychotic monotherapy use. Outpatient (versus inpatient) status was defined based on the patients’ treatment setting at the baseline visit. Prior medication use was assessed using indicator variables for prior use of antipsychotics, anticholinergics, anxiolytics/hypnotics, mood stabilizers, antidepressants, or other medications. An indicator variable for existing medical comorbidities was coded if any of the following medical conditions were present at baseline: hypertension, hyperlipidemia, hepatic dysfunction, renal dysfunction, or other. Social activity was coded if patients reported one or more social activities in the 4 weeks prior to baseline. Work status and living status were defined based on the patients’ status during the 4 weeks prior to baseline. Switching from a typical antipsychotic was coded if the patient discontinued a typical antipsychotic prior to initiating olanzapine. Similarly, switching from an atypical antipsychotic was coded if the patient discontinued an atypical antipsychotic prior to the initiation of olanzapine. The patients who did not switch antipsychotics to olanzapine either initiated antipsychotic therapy with olanzapine or augmented their previous antipsychotic treatment regimen with olanzapine.
Consistent with past research, antipsychotic polypharmacy was defined as using one or more antipsychotics in conjunction with olanzapine for a period of at least 60 consecutive days.Citation4 Conversely, antipsychotic monotherapy was defined as the use of olanzapine as the primary antipsychotic during the 1-year study.
Statistical methods
The differences between the baseline characteristics of patients treated with either monotherapy or polypharmacy were examined using χ2 tests for categorical variables and t-tests for continuous variables. Stepwise logistic regression was used to identify independent predictors of later monotherapy use. provides a list of the baseline predictors used in the stepwise logistic regression. A t-test was used to compare early (3-month) change in CGI-SCH global severity between monotherapy- and polypharmacy-treated patients, with missing values imputed using the last observation carried forward approach. The level of significance was set at α = 0.05, and all analyses were computed using SAS (v 9.1.3; SAS Institute Inc, Cary, NC).
Table 1 Baseline characteristics of patients on monotherapy and polypharmacy
Results
Sample description
The OPMS study registered and enrolled 1949 patients, of whom 1850 (94.9%) met all of the entry criteria for the study. Participants who were excluded from the study included 27 who were in violation of the contract or registration, 20 who had no case report form, 49 who did not return after the initial visit, and three who did not initiate treatment with olanzapine. For the entire sample, the average age was 44.8 ± 15.5 years, 984 were male (53.2%), 43.2% were outpatients, and the mean duration of illness was 18.3 ± 14.7 years.
displays the baseline characteristics for patients who were treated with olanzapine monotherapy (43.2%) or antipsychotic polypharmacy (56.8%). There were significant differences between these two groups on various demographics (age and gender), baseline clinical status (outpatient treatment, duration of illness, body mass index [BMI], medical comorbidities), baseline clinical and functional measures (CGI-SCH global severity, EQ-5D VAS, working for pay, living independently), and prior medication use (switch from a typical or an atypical antipsychotic, anticholinergic use, antidepressant use, anxiolytic use, mood stabilizer use, and other medication use). Only the rates of tardive dyskinesia, level of social activities, and starting dose of olanzapine did not significantly differ between the two groups. displays the commonly used concomitant antipsychotics and the average doses among the 1050 antipsychotic polypharmacy patients.
Table 2 Concomitant antipsychotic medications used in polypharmacyTable Footnotea
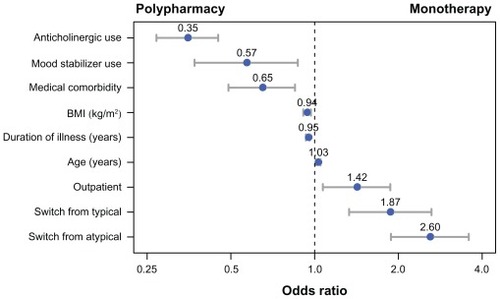
In the stepwise logistic regression analysis, fewer variables independently differentiated patients who were treated with antipsychotic monotherapy or polypharmacy. presents the odds ratios and confidence intervals for the final stepwise logistic regression model. The model was reasonably accurate in identifying patients who were treated with olanzapine monotherapy. The c-statistic of 0.765 indicated that the model could accurately classify a randomly selected individual who was administered monotherapy and a randomly selected individual who was administered polypharmacy 76.5% of the time. For the continuous predictor variables in the model (age, duration of illness, and BMI), the odds ratios represent the change in odds of monotherapy for every one-unit increase in the predictor. For example, the significant odds ratio of 1.03 for age indicates that for every year older a patient was, the odds the patient would be treated with monotherapy were 1.03 times higher.
Figure 1 Significant predictors of antipsychotic monotherapy versus polypharmacy in the stepwise logistic regression.
Early change (from baseline to the 3-month visit, the earliest postbaseline visit) in the CGI-SCH global severity was compared between the monotherapy- and polypharmacy-treated patients. Monotherapy patients experienced significantly larger improvements in CGI-SCH global severity (−0.73) compared with patients who were treated with polypharmacy (−0.57; P < 0.001).
Discussion
In this large prospective, observational study, Japanese patients with schizophrenia who were treated with olanzapine monotherapy were found to significantly differ from those treated with polypharmacy on demographics, clinical status variables, baseline symptom and functional levels, and prior medication use. In the univariate analyses, nearly all of the baseline predictors were found to be significant. However, fewer of these baseline patient characteristics independently predicted later olanzapine monotherapy use from antipsychotic polypharmacy use in the multivariate analysis. In the multivariate stepwise logistic regression model, antipsychotic monotherapy with olanzapine was significantly predicted by older age, outpatient status, switching from typical antipsychotics, switching from atypical antipsychotics, shorter illness duration, lower BMI, no prior medical comorbidities, no prior mood stabilizer use, and no prior anticholinergic use. In general, the patients receiving antipsychotic monotherapy appeared to have a somewhat simpler clinical profile.
Past research corroborated most, but not all, of these predictors of antipsychotic monotherapy. Studies from other geographies found that antipsychotic monotherapy use was predicted by older age,Citation18,Citation19 shorter duration of illness,Citation18 outpatient treatment or no prior psychiatric inpatient treatment,Citation5,Citation19 no concomitant anticholinergic use,Citation18,Citation19 and no concomitant mood stabilizer use.Citation5,Citation19 A study of Veterans Affairs schizophrenia patients in the USCitation19 found that medical comorbidities predicted lower use of antipsychotic polypharmacy, which is the opposite direction of the results found in this current research study. It is unclear whether this discrepancy is a function of different populations (the Veterans Affairs sample is almost all older males), study methods, or the restriction to olanzapine treatment in the current study.
A unique finding in this study was that switching from either typical or atypical antipsychotics to olanzapine was highly predictive of olanzapine monotherapy. By definition, switching antipsychotics limits polypharmacy because the original antipsychotic is discontinued instead of having an additional antipsychotic added to it. Similarly to past research outside of Japan, certain patients with schizophrenia had a substantially higher propensity for being treated with antipsychotic monotherapy.
Consistent with the notion that antipsychotic polypharmacy arises as a result of poor or suboptimal treatment response, this study found greater 3-month symptom improvements for patients treated with monotherapy relative to those treated with polypharmacy. Although this comparison was not adjusted for background characteristics, it suggests that the use of more effective antipsychotic treatments may improve the rate of antipsychotic monotherapy. The treatment of choice for treatment-resistant schizophrenia, clozapine, was not available in Japan until 2009 and therefore could not have been used during the study period. The polypharmacy use in this study may have been for treatment-resistant patients.
In addition to maximizing the effectiveness of antipsychotic treatments for individual patients, other approaches may also help to reduce the use of polypharmacy. A recent Japanese study found that although poor efficacy was the primary reason for initiating antipsychotic polypharmacy, many patients were started on polypharmacy prior to maximizing the dose of the initial antipsychotic.Citation28 Encouraging physicians in Japan to maximize the dose of the initial antipsychotic prior to adding a second medication may help reduce antipsychotic polypharmacy. A randomized study in the US found that many patients with schizophrenia could be effectively switched from antipsychotic polypharmacy to monotherapy.Citation29 Patients who were randomized to switch to monotherapy had similar symptom reductions during the 6-month follow-up period as those who remained on antipsychotic polypharmacy; however, a meaningful proportion (31%) of patients who were switched to antipsychotic monotherapy were restarted on polypharmacy prior to the end of the study. With the recognition that in certain situations antipsychotic polypharmacy may be appropriate,Citation10 interventions aimed at decreasing polypharmacy in Japan may be effective. The results of the current study could be used to identify patients who are at increased likelihood of polypharmacy, to more efficiently target interventions.
Limitations
This research was able to identify significant predictors of antipsychotic monotherapy. However, the OPMS study included only patients with schizophrenia initiated on olanzapine in Japan. Although some of the same characteristics have been found to be predictive of monotherapy in other geographies, the findings may not generalize to other antipsychotics or to other countries or geographic regions. Additionally, this study defined polypharmacy as 60 or more days of concomitant antipsychotic use, whereas other studies have defined polypharmacy as any concomitant antipsychotic use. Results may vary depending on the definition of antipsychotic polypharmacy that was used. Finally, although this study included a large number of predictors, there may have been some important predictors that were not available in the study dataset.
Conclusion
Consistent with past research, antipsychotic polypharmacy was common in the treatment of schizophrenia in Japan. Stepwise logistic regression revealed several significant baseline predictors of later antipsychotic monotherapy treatment, including baseline demographics (age, outpatient status), clinical status (illness duration, BMI, medical comorbidities), and prior medication use (prior mood stabilizer use, prior anticholinergic use, and switching from typical or atypical antipsychotics). In general, patients treated with monotherapy appeared to have a less complex clinical profile. The results of this study could be used to target interventions aimed at reducing polypharmacy among patients who are at an increased risk.
Acknowledgments
Funding for this study was provided by Eli Lilly and Company, Indianapolis, IN. Technical writing support was provided by Michael Stensland of Agile Outcomes Research Inc, Rochester, MN, and Susan Dennett of Strategic Health Outcomes Inc, Carmel, IN, USA.
Disclosure
Wenyu Ye is a full-time employee of Lilly Suzhou Pharmaceutical Co, Shanghai, People’s Republic of China. Haya Ascher-Svanum is a full-time employee of Eli Lilly and Company. Jennifer Flynn and Yuka Tanji are full-time employees of Eli Lilly Japan, K.K. Michihiro Takahashi is a consultant for Eli Lilly Japan, K.K. All authors are minor stockholders in Eli Lilly and Company.
References
- FalkaiPWobrockTLiebermanJWorld Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: acute treatment of schizophreniaWorld J Biol Psychiatry20056313219116173147
- LehmanAFLiebermanJADixonLBPractice guideline for the treatment of patients with schizophrenia, 2nd edAm J Psychiatry2004161Suppl 215615000267
- StahlSMAntipsychotic polypharmacy: squandering precious resources?J Clin Psychiatry2002632939411874226
- FariesDAscher-SvanumHZhuBCorrellCKaneJAntipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychoticsBMC Psychiatry200552615921508
- GangulyRKotzanJAMillerLSKennedyKMartinBCPrevalence, trends, and factors associated with antipsychotic polypharmacy among Medicaid-eligible schizophrenia patients, 1998–2000J Clin Psychiatry200465101377138815491242
- MillierASarlonEAzorinJ-MRelapse according to antipsychotic treatment in schizophrenic patients: a propensity-adjusted analysisBMC Psychiatry2011112421314943
- ClarkREBartelsSJMellmanTAPeacockWJRecent trends in antipsychotic combination therapy of schizophrenia and schizoaffective disorders: Implications for state mental health policySchizophr Bull2002281758412047024
- SernyakMRosenheckRClinician’s reasons for antipsychotic coprescribingJ Clin Psychiatry200465121597160015641863
- TappAWoodAESecrestLCombination antipsychotic therapy in clinical practicePsychiatr Serv2003541555912509667
- CorrellCURummel-KlugeCCorvesCKaneJMLeuchtSAntipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trialsSchizophr Bull200935244345718417466
- CorrellCUAntipsychotic polypharmacy, part 1: shotgun approach or targeted cotreatment?J Clin Psychiatry200869467467518507487
- BarnesTREPatonCAntipsychotic polypharmacy in schizophrenia: benefits and risksCNS Drugs201125538339921476610
- CorrellCUFredericksonAMKaneJMManuPDoes antipsychotic polypharmacy increase the risk for metabolic syndrome?Schizophr Res2007891–39110017070017
- JerrellJMCost-effectiveness of risperidone, olanzapine, and conventional antipsychotic medicationsSchizophr Bull200228458960512795493
- ProcyshynRMKennedyNBTseGThompsonBAntipsychotic polypharmacy: a survey of discharge prescriptions from a tertiary care psychiatric institutionCan J Psychiatry200146433433911387789
- YoshiTKurosawaMSugimuraKCurrent status of the psychopharmacological treatment of schizophrenic patients in Japan: From 2005 nationwide survey of 9 psychiatric hospitals in the psychiatric clinical pharmacy societyJpn J Clin Psychopharmacol20071017211731
- YoshiTUnoJNakagawaMSurvey of the prescription for psychotherapy in Japanese inpatients with schizophrenia in 2006Jpn J Clin Psychopharmacol20101315351545
- SimKSuAChanYClinical correlates of antipsychotic polytherapy in patients with schizophrenia in SingaporePsychiatry Clin Neurosci200458332432915149301
- KreyenbuhlJAValensteinMMcCarthyJFGanoczyDBlowFCLongterm antipsychotic polypharmacy in the VA health system: patient characteristics and treatment patternsPsychiatr Serv200758448917412850
- WangPFZhaoZComparison of olanzapine versus quetiapine in the treatment of hospitalized patients with schizophreniaValue Health200363355
- YuAPAtanasovPBen-HamadiRResource utilization and costs of schizophrenia patients treated with olanzapine versus quetiapine in a Medicaid populationValue Health200912570871519508658
- ZhuBAscher-SvanumHFariesDECorrellCUKaneJMCost of antipsychotic polypharmacy in the treatment of schizophreniaBMC Psychiatry200881918394168
- NovickDAscher-SvanumSBrugnoliRAntipsychotic monotherapy and polypharmacy in the treatment of outpatients with schizophrenia in the European SOHO studyJ Nerv Ment Dis2011In press
- BitterIChouJCUngvariGSPrescribing for inpatients with schizophrenia: an international multi-center comparative studyPharmacopsychiatry200336414314912905100
- ShinfukuNTanCHPharmacotherapy for schizophrenic inpatients in East Asia: changes and challengesInt Rev Psychiatry200820546046819012132
- HaroJMKamathSAOchoaSThe Clinical Global Impression- Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophreniaActa Psychiatr Scand Suppl2003416162312755850
- KaySROplerLAFiszbeinAPositive and Negative Syndrome Scale (PANSS) user’s manualNorth Tonawanda, NYMulti-Health Systems, Inc2000
- TsutsumiCUchidaHSuzukiTThe evolution of antipsychotic switch and polypharmacy in natural practice – a longitudinal perspectiveSchizophr Res20111301–3404621624824
- EssockSMSchoolerNRStroupTSEffectiveness of switching from antipsychotic polypharmacy to monotherapyAm J Psychiatry2011168770270821536693