Abstract
Background
Chondroitin sulfate, alone or associated with glucosamine (CS), is an effective treatment of osteoarthritis, better tolerated than non-steroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase 2 inhibitors (COXIBs) at gastrointestinal, cardiovascular and renal levels.
Objective
To estimate the health impact (toxicity by NSAIDs/COXIBs avoided with CS with or without glucosamine) and economic impact (savings due to avoided toxicities) of treatment of knee osteoarthritis with CS compared to NSAIDs/COXIBs, as a consequence of the avoidance of mild-moderate or severe gastrointestinal adverse effects (GIAE), ischaemic heart disease (IHD), acute kidney insufficiency (AKI) and chronic kidney failure (CKF).
Methods
We compared the current situation (available reimbursed prescription with CS) with a hypothetical situation without CS (treatment only with NSAIDs/COXIBs). The frequency of GIAE, IHD, AKI and CKF with CS and NSAIDs/COXIBs was obtained from published ad hoc studies. The cost of AE management and of the drugs (180 days of treatment) was obtained from Spanish sources. A probabilistic economic model was made for a 3-year period, both at national (NHS) and regional levels. Sensitivity analyses were performed for different durations of treatment (90 and 240 days).
Results
In Spain, it is estimated that 519,130, 513,616 and 507,377 patients with knee osteoarthritis will be treated with NSAIDs/COXIBs and 112,775, 114,963 and 117,262 with CS in 2020, 2021 and 2022, respectively. Due to better CS tolerability, 55,098 mild-moderate GIAE, 3060 severe GIAE, 204 IHD, 1089 AKI and 733 CKF would be avoided in 3 years. Discounting the cost of the drugs, the three-year savings for the NHS would be 21.8 (12.7–29.5) million euros.
Conclusion
Due to its better tolerability profile, CS treatment is expected to prevent thousands of AEs over the next 3 years, some of which may be life-threatening for patients, while generating considerable savings for the NHS.
Introduction
The most recent literature suggests that chondroitin sulfate, alone or associated with glucosamine (CS), is an effective treatment for osteoarthritis, according to most of the available studies published in the period 2008–2018.Citation1–Citation8 Although doubts were raised in a meta-analysis published in 2010,Citation9 the reliability of the results of this study was highly questioned due to possible methodological deficiencies.Citation10–Citation12 On the other hand, the efficacy of CS has been confirmed more recently in three randomised, double-blind clinical trials in patients with knee osteoarthritis: the MOVESCitation4 study, in which it was found that the combination of CS with glucosamine would have similar efficacy to that of celecoxib; in the MOSAICCitation5 study, in which the superiority of CS over celecoxib in reducing cartilage volume loss was demonstrated through a 2-year follow-up; and finally, in the CONCEPTCitation6 study, in which CS was superior to placebo and similar to celecoxib in reducing pain and improving joint function for 6 months in symptomatic patients. These results were also confirmed in a meta-analysis published in 2018.Citation8 CS is currently recommended for the treatment of knee osteoarthritis by, among others, the guidelines of the Spanish Society of Rheumatology,Citation13,Citation14 the European League Against RheumatismCitation15 and those published by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO).Citation16
On the other hand, as demonstrated in a recent meta-analysis,Citation17 CS is well tolerated. In fact, CS and glucosamine were not associated with an increased risk of adverse effects (AE) compared to placebo.Citation17 This good tolerability of CS contrasts with the toxicity problems described for NSAIDs/COXIBsCitation18 at gastrointestinal,Citation19 cardiovascularCitation20 and renalCitation21 levels.
In 2010 and 2017 two economic analysesCitation22,Citation23 were published that analysed the efficiency of CS compared to NSAIDs. The VECTRACitation22 study concluded that, compared to NSAIDs, CS is a treatment with lower costs and better gastrointestinal tolerability in the management of osteoarthritis. It was estimated that for every 10,000 patients treated with CS, 2666 gastrointestinal AEs (GIAEs) would be avoided and that, over a period of 3 years, savings of 38.7 million euros would be generated for the National Health System (NHS). The study, subsequently limited to Catalonia (Spain),Citation23 concluded that treatment of 67,904 patients with CS osteoarthritis instead of NSAIDs would prevent 18,103 mild-moderate and 611 severe episodes of GIAEs annually, as well as 34 ischaemic heart disease (IHD) associated with NSAIDs. The annual savings from avoiding these episodes of GIAE and IHD were estimated at 5.8 million euros and 463,000 euros, respectively.
This study’s objective was to estimate the health impact (toxicity by NSAIDs/COXIBs avoided with CS) and economic impact (savings due to avoided toxicities) of the treatment of symptomatic knee osteoarthritis with CS compared to NSAIDs/COXIBs, as a consequence of the avoidance of mild-moderate or severe GIAEs, IHD and acute kidney injury (AKI) and chronic kidney failure (CKF).
Methods
Economic Model
A probabilistic economic model was performed, using a second-order Monte Carlo simulationCitation24–Citation28 with two objectives: (i) to explore the effect of the uncertainty of the variables in the model (population under treatment with CS or with NSAIDs/COXIBs, probability of suffering the different AEs, AE management costs), in a hypothetical cohort of 1000 patients with symptomatic knee osteoarthritis; and (ii) to calculate the 95% CI of the main results of the analysis (AEs avoided, total savings from the AEs avoided). A probabilistic analysis was performed, considering that the frequencies of the adverse effects and the probabilities are adjusted to beta distributions and the unit costs of the adverse effects are adjusted to gamma distributions.Citation29
Population
The evolution of a hypothetical cohort of patients with knee osteoarthritis was modelled. The prevalence of knee osteoarthritis in Spain, in patients over 40 years of age, was obtained from the EPISER study of the Spanish Society of RheumatologyCitation30 (). The population over 40 years of age in Spain (and the autonomous communities [regions]), projected for 2020–2022, was obtained from the National Institute of Statistics’ databaseCitation31 (). The percentage of patients with osteoarthritis following pharmacological treatment was obtained from a study on drug use in osteoarthritis in Catalonia (Spain).Citation32 The percentage of patients with symptomatic osteoarthritis treated regularly or occasionally with NSAIDs/COXIBs was obtained from the same sourceCitation32 (). “Regular” use was understood to mean a medication possession ratio (MPR) ≥50% and “occasional” use with an MPR ≥ 25% and <50%.Citation32
Table 1 Population Estimates for Spain Considered in the Study
Based on these data and the number of units sold in the period July 2018 to July 2019 of Condrosan®/Condrosulf®/CS Kern and Droglican® (IQVIA market data, 2019), the number of patients with knee osteoarthritis treated with NSAIDs/COXIBs and CS with or without glucosamine in Spain and in the autonomous communities (regions) was calculatedCitation33,Citation34 (). Sensitivity analyses were performed based on the minimum and maximum values shown in . The details of the population calculations are presented in .
Variables and Scenarios Analysed
The budgetary and health impact study was performed to estimate: (i) cost of GIAEs (mild-moderate and severe) and episodes avoided with CS; (ii) the cost of IHD and episodes avoided with CS; (iii) the cost of AKI and CKF and episodes avoided with CS. To this end, the following scenarios were compared: (i) with CS: the current scenario, in which NSAIDs/COXIBs and CS, as reimbursed prescription drugs, are used for the treatment of knee osteoarthritis; (ii) without CS: a hypothetical scenario, in which only NSAIDs/COXIBs are available for the treatment of knee osteoarthritis. We compared the current situation (of patients treated with CS or NSAIDs/COXIBs, 18% would be treated with CS and 82% with NSAIDs/COXIBs) with a hypothetical situation without CS (100% treated with NSAIDs/COXIBs). The objective of this comparison was to estimate and highlight the health and economic contribution of CS in the treatment of knee osteoarthritis.
An analysis was carried out for the national population and a sub-analysis at the regional level.
Time Horizon
The simulation covered a period of 3 years (2020 to 2022). Health and economic impacts were calculated for annual cycles.
Perspective of the Analysis
That of the NHS, so only direct health costs were considered.
Costs Analysed
The following costs were analysed: (i) the cost of managing AEs (mild-moderate or severe GIAE, IHD, AKI and CKF); (ii) the cost of acquiring the drugs (CS with or without glucosamine, NSAIDs/COXIBs). The costs are presented in euros (€) updated to 2019.
Costs and Probabilities of AEs
The unit costs of handling the AEs analysed are shown in . The probabilities of the appearance of the different AEs are presented in .
Figure 1 AE management costs.
Note: Data from these studies.Citation22,Citation23,Citation40
Abbreviations: AE, adverse effects; GIAE, gastrointestinal AE.
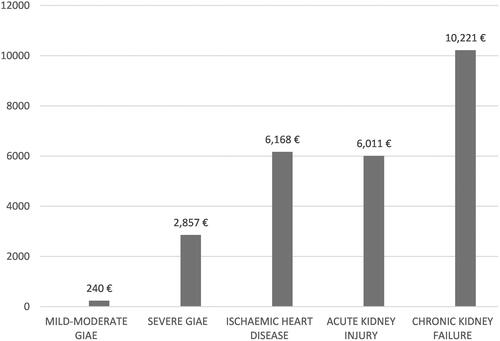
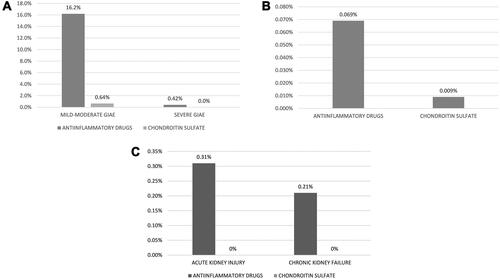
Figure 2 Probability of the appearance of AEs with CS or anti-inflammatory drugs (NSAIDs/COXIBs). (A) Gastrointestinal AE; (B) ischaemic heart disease; (C) acute kidney injury/chronic kidney failure.
Note: Data from these studies.Citation19–Citation21
Abbreviations: AE, adverse effects; GIAE, gastrointestinal AE; CS, chondroitin sulfate with or without glucosamine; IHD, ischaemic heart disease; AKI, acute kidney injury; CKF, chronic kidney failure.
GIAE
The unit costs of a mild-moderate GIAE (€240) and a severe GIAE (€2857) were obtained from the VECTRACitation22 study, whose original source was the public prices of the regions ().
The annual probabilities of suffering a mild-moderate GIAE (0.64% with CS; 16.19% with NSAIDs/COXIBs) or severe (0% with CS; 0.42% with NSAIDs/COXIBs) GIAE were obtained from the GI-REASONSCitation19,Citation35 study (). In the GI-REASONS study, 10 and 18 severe and 415 and 683 mild-moderate GIAE with celecoxib and NSAIDs, administered over 6 months, were reported in a cohort of 3970 and 3951 patients with osteoarthritis, respectively.Citation19,Citation35 On the other hand, according to the Spanish Agency of Medicines and Medical Devices,Citation36 COXIBs and NSAIDs are used in 16.08% and 83.92% of patients, respectively. Consequently, the annual probabilities of suffering from a GIAE were calculated. For example, the probability of mild-moderate GIAE with NSAIDs/COXIB would be [10.45%*16.08%] + [17.29%*83.92%] =16.19%. These assumptions are more conservative than those adopted in the previously published VECTRA study.Citation22
IHD
The economic impact of IHD was calculated from the public prices of DRG 121, 122, 123 and 140Citation23,Citation37 according to the frequency observed in the study by the Jordi Gol Primary Care Research InstituteCitation20,Citation23 (). According to this study, the probability of a patient with osteoarthritis treated with NSAIDs/COXIBs suffering from a coronary ischaemic event would be 0.120% (0.105–0.139%). In the case of treatment with CS it would be 0.070% (0.063–0.077%)Citation20,Citation23 ().
These estimates were calculated considering that NSAIDs increase the risk of IHD only in patients at high cardiovascular risk, according to the study by De Abajo et al,Citation38 and that these patients are 50% of the total patients with vascular risk, according to the aforementioned Catalonian study.Citation20 The increased risk of ischaemic stroke associated with NSAIDs, not considered in this study, has also been highlighted in another published Spanish study.Citation39
AKI and CKF
The economic impact of AKI and CKF was calculated from public health pricesCitation40 (). The frequency of AKI (0.31%) and CKF (0.21%) associated with NSAIDs/COXIBs was obtained from the study by Nelson et al,Citation21 a retrospective study that included a large cohort of patients in the USA, treated with NSAIDs/COXIBs for at least 7 months of observation ().
Drugs Costs
The average annual cost of treatment with CS was calculated as indicated in .Citation33,Citation34,Citation41 The average cost of CS and NSAIDs/COXIBs was calculated for different durations of treatment over the period of 1 year: 180 days (6 months) in the base case of the analysis and 90 or 240 days (3 or 8 months) for the sensitivity analysis. According to its summary of product characteristics, CS treatment should be carried out for at least 3 months, although in patients with significant inflammatory symptoms, after a rest period of 2 months, treatment could be started again following the same cycle.Citation33 Therefore, over the period of 1 year, CS treatment could range from a minimum of 3 months to a maximum of 8 months. In the case of CS with glucosamine, it is advisable to administer it for a period of at least 6 months.Citation34 With respect to NSAIDs/COXIBs, the duration of the treatment is highly variable, depending on the different studies. In the GI-REASONSCitation19,Citation35 study, a randomised clinical trial aimed at analysing the GIAEs associated with celecoxib and the NSAIDs/COXIBs, treatment duration was 6 months. This study was used to obtain the average frequency of GIAEs with NSAIDs/COXIBs used in the economic model. Consequently, an average treatment duration of 180 days, between a minimum of 30 and a maximum of 240 treatment days per year, was considered in the base case.
Table 2 Average Cost per Patient of Treatment with CS
The average cost per patient treated with NSAIDs/COXIBs in the base case (180 days of treatment) was estimated at €45.68.Citation36,Citation42 It was calculated from the report on the use of non-steroidal anti-inflammatory drugs in Spain during the period 2013–2016, published by the Spanish Agency of Medicines and Medical DevicesCitation36 and from current prices by homogeneous grouping published by the Ministry of Health.Citation42 The daily doses of the various NSAIDs/COXIBs were obtained from the VECTRACitation22 study.
Base Case and Sensitivity Analysis
The base case was analysed for a treatment duration of 180 days. Sensitivity analyses were performed for treatment durations of 90 and 240 days.
Results
National Results
In Spain, it is estimated that 519,130, 513,616 and 507,377 patients will be treated with NSAIDs/COXIBs with knee osteoarthritis and 112,775, 114,963 and 117,262 with CS in 2020, 2021 and 2022, respectively.
Due to the improved tolerability of CS, 55,098 mild-moderate GIAE, 3060 severe GIAE, 204 IHD, 1089 AKI and 733 CKF would be avoided in 3 years ().
Figure 3 Estimated frequency of AE with and without CS. (A) Mild-moderate GIAE; (B) Severe GIAE; (C) Ischaemic heart disease; (D) Acute kidney injury; (E) Chronic kidney failure.
Abbreviations: AE, adverse effects; CS, chondroitin sulfate with or without glucosamine; GIAE, gastrointestinal AE.
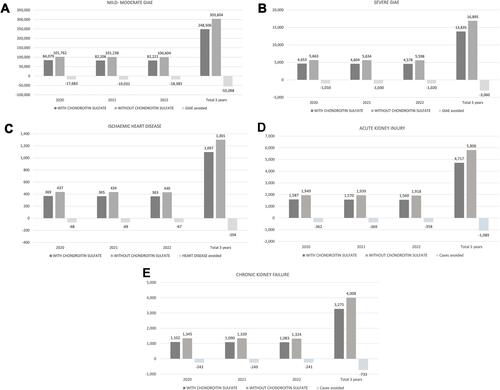
Discounting the cost of the drugs, the three-year savings for the NHS would be 21.8 (12.7–29.5) million euros. The probability of savings with CS alone or associated with glucosamine was 72.2% (74.0–68.0%) ().
Table 3 Economic Impact of Knee Osteoarthritis Treatment with/without CS
Regional Results
The regional results are presented in .
Table 4 Estimated Costs (€) of GIAE, IHD, AKI and CKF, Additional Costs and Costs Avoided with CSs. By Autonomous Community (Region)*
Discussion
According to this study, due to its improved tolerability profile, CS treatment is expected to prevent thousands of AEs over the next 3 years, some of which may put patients’ lives at risk, while generating considerable savings for the NHS.
In assessing these results, we must consider their strengths and weaknesses. The consistency of the sources used to obtain the main variables of the analysis can be considered a strength of the study. Given that the study is an economic model, the population and adverse effect frequency data were obtained, respectively, from epidemiological studies or dataCitation30,Citation31 and from clinical mega-trialsCitation19 or observational studies.Citation20,Citation21 The prevalence of knee osteoarthritis in Spain was obtained from the EPISER study of the Spanish Society of Rheumatology.Citation30 The population over 40 years of age in Spain was obtained from the National Institute of Statistics’ database.Citation31 The percentage of patients with osteoarthritis following pharmacological treatment was obtained from a study on drug use in osteoarthritis in Catalonia.Citation32 Finally, the percentage of patients with osteoarthritis treated regularly or occasionally with NSAIDs/COXIBs was obtained from the same source,Citation32 a Spanish study that included 238,536 participants, followed between 2006 and 2010.
Although it should be remembered that this is a theoretical model (which is, by definition, a simplified simulation of reality), a probabilistic model was carried out designed to explore the effect of the uncertainty of the variables in the model and calculating the 95% CI of the main results of the analysis (AEs avoided, total savings from AEs avoided). This type of model allows a better simulation of clinical reality.Citation24–Citation29
The results obtained in this study are consistent with those of two previously published Spanish economic analyses.Citation22,Citation23
With regard to the weaknesses, perhaps the most noteworthy is the calculation of the estimated number of patients treated with CS or NSAIDs/COXIBs. However, this number was calculated according to the available epidemiological and population data and was the subject of a sensitivity analysis as shown in .
The study did not consider patients receiving CS and NSAIDs concomitantly, which is estimated to be 12% of patients treated with CS according to the VECTRACitation22 study and 2.17% in the study by Wilson et al,Citation32 and should therefore also be considered a limitation of the study.
Conclusions
Due to its improved tolerability profile, CS treatment is expected to prevent thousands of AEs over the next 3 years, some of which may be life-threatening for patients, while generating considerable savings for the NHS.
Copyright/Ethics
Data accessed from the Spanish National Institute of Statistics database are freely available (www.ine.es). IQVIA market data: IQVIA is the data controller of the non-identifiable patient data used for the purposes of the study. Global sales data were managed in this study. In any case were individual patient data handled; approval by an Ethics Committee was unnecessary, in accordance with Spanish legislation (Royal Decree 1090/2015, of 4 December, regulating clinical trials with medicinal products, Ethics Committees for Investigation with medicinal products and the Spanish Clinical Studies Registry).
Disclosure
CRT and DRR are employees of Health Value, who were paid consultants to Reig Jofre, SA, in connection with the conduct of this study and development of the manuscript. MH and CN work at Reig Jofre, SA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Hochberg M, Zhan M, Langenberg P. The rate of decline of joint space width in patients with osteoarthritis of the knee: a systematic review and meta-analysis of randomized placebo-controlled trials of chondroitin sulfate. Curr Med Res Opin. 2008;24:3029–3035. doi:10.1185/03007990802434932
- Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int. 2010;30:357–363. doi:10.1007/s00296-009-0969-5
- Zeng C, Wei J, Li H, et al. Effectiveness and safety of Glucosamine, chondroitin, the two in combination, or celecoxib in the treatment of osteoarthritis of the knee. Sci Rep. 2015;5:16827. doi:10.1038/srep16827
- Hochberg MC, Martel-Pelletier J, Monfort J, et al.; MOVES Investigation Group. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2016;75:37–44. doi:10.1136/annrheumdis-2014-206792
- Pelletier JP, Raynauld JP, Beaulieu AD, et al. Chondroitin sulfate efficacy versus celecoxib on knee osteoarthritis structural changes using magnetic resonance imaging: a 2-year multicentre exploratory study. Arthritis Res Ther. 2016;18:256. doi:10.1186/s13075-016-1149-0
- Reginster JY, Dudler J, Blicharski T, Pavelka K. Pharmaceutical-grade Chondroitin sulfate is as effective as celecoxib and superior to placebo in symptomatic knee osteoarthritis: the ChONdroitin versus CElecoxib versus Placebo Trial (CONCEPT). Ann Rheum Dis. 2017;76:1537–1543. doi:10.1136/annrheumdis-2016-210860
- Simental-Mendía M, Sánchez-García A, Vilchez-Cavazos F, Acosta-Olivo CA, Peña-Martínez VM, Simental-Mendía LE. Effect of glucosamine and chondroitin sulfate in symptomatic knee osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Rheumatol Int. 2018;38:1413–1428. doi:10.1007/s00296-018-4077-2
- Zhu X, Sang L, Wu D, Rong J, Jiang L. Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2018;13:170. doi:10.1186/s13018-018-0871-5
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. doi:10.1136/bmj.c4675
- Giacovelli G, Rovati LC. Glucosamine and osteoarthritis. Conclusions not supported by methods and results. BMJ. 2010;341:c6338.
- Pelletier JP, Hochberg MC, Du Souich P, Kahan A, Michel BA. Glucosamine and osteoarthritis. Effect size is encouraging. BMJ. 2010;341:c6328. doi:10.1136/bmj.c6328
- Reginster JY, Altman RD, Hochberg MC. Glucosamine and osteoarthritis. Prescribed regimen is effective. BMJ. 2010;341:c6335. doi:10.1136/bmj.c6335
- Panel of Experts of the Spanish Society of Rheumatology (SER). First consensus document of the Spanish Society of Rheumatology on the pharmacological treatment of knee osteoarthritis. Reumatol Clin. 2005;1:38–48. doi:10.1016/S1699-258X(05)72711-X
- Benito-Ruiz P. Guías y recomendaciones para el diagnóstico y tratamiento de la artrosis: en busca del consenso. [Guidelines and recommendations for the diagnosis and treatment of osteoarthritis: in search of consensus]. Sem Fund Esp Reumatol. 2012;13(Suppl 1):1–32.
- Jordan KM, Arden, NK, Doherty M, et al. EULAR recommendations 2003: an evidenced based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145–1155. doi:10.1136/ard.2003.011742
- Bruyere O, Reginster J-Y. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs Aging. 2007;24(7):573–580. doi:10.2165/00002512-200724070-00005
- Honvo G, Reginster JY, Rabenda V, et al. Safety of symptomatic slow-acting drugs for osteoarthritis: outcomes of a systematic review and meta-analysis. Drugs Aging. 2019;36(Suppl 1):65–99. doi:10.1007/s40266-019-00662-z
- Bernad M. Situación actual de los SYSADOA en España. [Current situation of the SYSADOA in Spain]. (Editorial). Reumatol Clin. 2016;12:181–183. doi:10.1016/j.reuma.2016.03.012
- Cryer B, Li C, Simon LS, Singh G, Stillman MJ, Berger MF. GI-REASONS: a novel 6-month, prospective, randomized, open-label, blinded endpoint (PROBE) trial. Am J Gastroenterol. 2013;108:392–400. doi:10.1038/ajg.2012.467
- Instituto de Investigación en Atención Primaria Jordi Gol. Riesgo cardiovascular en pacientes con osteoartritis: estudio de casos y controles. [Cardiovascular risk in patients with osteoarthritis: case-control study]. Code: IJG-M01-2012-01. November 18, 2014.
- Nelson DA, Marks ES, Deuster PA, O’Connor FG, Kurina LM. Association of nonsteroidal anti-inflammatory drug prescriptions with kidney disease among active young and middle-aged adults. JAMA Netw Open. 2019;2:e187896. doi:10.1001/jamanetworkopen.2018.7896
- Rubio-Terrés C; Grupo del estudio VECTRA. Evaluación económica del uso de CS y antiinflamatorios no esteroideos en el tratamiento de la artrosis. Datos del estudio VECTRA. [Economic evaluation of the use of CS and non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis. Data from the VECTRA study]. Reumatol Clin. 2010;6:187–195. doi:10.1016/j.reuma.2009.12.009
- Rubio-Terrés C, Rubio-Rodríguez D, Möller I. Aproximación al impacto presupuestario y sanitario de la prescripción de CS en el tratamiento de la artrosis de rodilla y manos en comparación con los antiinflamatorios no esteroideos en Cataluña. [Approach to the budgetary and health impact of the prescription of SC in the treatment of osteoarthritis of the knee and hands in comparison with non-steroidal anti-inflammatory drugs in Catalonia]. Pharmacoecon Span Res Artic. 2017;14(1):19–25. doi:10.1007/s40277-016-0066-6
- Anguita P, González C, Cañete M, Rubio-Rodríguez D, Rubio-Terrés C. Coste de los efectos adversos asociados a enzalutamida o apalutamida en el tratamiento del cáncer de próstata resistente a la castración no metastásico en España. [Cost of adverse effects associated with enzalutamide or apalutamide in the treatment of non-metastatic castration-resistant prostate cancer in Spain]. Rev Esp Econ Salud. 2019;14:794–805.
- Manito N, Rubio-Rodríguez D, González J, et al. Análisis económico del tratamiento ambulatorio intermitente con levosimendán de la insuficiencia cardiaca en España. [Economic analysis of intermittent outpatient treatment with levosimendan for heart failure in Spain]. Rev Esp Cardiol. 2019. doi:10.1016/j.recesp.2019.06.019
- Rubio-Terrés C, Rubio-Rodríguez D. Probabilistic analysis: sensitivity analysis or main result? (Editorial). Pharmacoeconomics. 2016;1:2. doi:10.4172/pe.1000e102
- Rubio-Terrés C, Rubio-Rodríguez D. Costs of adverse events associated with erlotinib or afatinib in first-line treatment of advanced EGFR-positive non-small cell lung cancer. Clinicoecon Outcomes Res. 2017;9:31–38.
- Turnes J, García F, Diago M, et al. Impacto económico de la optimización de los recursos asistenciales en el abordaje del paciente con hepatitis C. Gastroenterol Hepatol. 2019;42(Suppl 1):26–33. doi:10.1016/S0210-5705(20)30185-0
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford, UK: Oxford University Press; 2006.
- Seoane-Mato D, Sánchez-Piedra C, Díaz-González F, Bustabad S; EPISER 2016 working group. Prevalence of rheumatic disease in adult population in Spain. EPISER 2016 study. Ann Rheum Dis. 2018;77.
- INE [Spanish National Statistics Institute]. Población y fenómenos demográficos nacionales. Población residente en España a 1 de enero, por sexo, edad y año. [Population and national demographic phenomena. Population resident in Spain by sex, age and year]. Available from: http://www.ine.es/. Accessed October 14, 2019.
- Wilson N, Sanchez-Riera L, Morros R, et al. Drug utilization in patients with OA: a population-based study. Rheumatol (Oxf). 2015;54:860–867. doi:10.1093/rheumatology/keu403
- CONDROSAN 400 mg hard capsules. CONDROSAN 400 mg granulate for oral solution. Summary of product characteristics. Available from: http://www.aemps.gob.es/cima/pdfs/es/ft/64549/FT_64549.pdf. Accessed September 17, 2019.
- DROGLICAN 200 mg/250 mg hard capsules. Summary of product characteristics. Available from: http://www.aemps.gob.es/cima/pdfs/es/ft/71394/FT_71394.pdf. Accessed September 17, 2019.
- A trial of GI safety of celecoxib compared with non-selective nonsteroidal antiinflammatory drugs (NSAIDS) (GI-REASONS). Available from: https://clinicaltrials.gov/ct2/show/results/NCT00373685?term=GI-REASONS&draw=2&rank=1. Accessed November 21, 2019.
- AEMPS [Spanish Agency of Medcines and Medical Devices]. Utilización de medicamentos antiinflamatorios no esteroideos en España durante el periodo 2013–2016. Informe de utilización de medicamentos U/AIN/V1/11/09/2017. [Use of non-steroidal anti-inflammatory drugs in Spain during the 2013-2016 period. Drug use report U / AIN / V1 / 11/09/2017]. Available from: https://www.aemps.gob.es/medicamentosUsoHumano/observatorio/docs/antiinflamatorios-AINEs-periodo-2013-2016.pdf. Accessed September 17, 2019.
- Catalan Institute of Health. Resolution SLT/353/2013 dated 13 February on the revision of public prices corresponding to the health services provided by the Catalan Institute of Health. Official Gazette of the Government of Catalonia; March 1, 2013: 6326.
- De Abajo FJ, Gil MJ, García Poza P, et al. Risk of nonfatal acute myocardial infarction associated with non-steroidal antiinflammatory drugs, non-narcotic analgesics and other drugs used in osteoarthritis: a nested case-control study. Pharmacoepidemiol Drug Saf. 2014;23:1128–1138. doi:10.1002/pds.3617
- García-Poza P, de Abajo FJ, Gil MJ, Chacón A, Bryant V, García-Rodríguez LA. Risk of ischemic stroke associated with nonsteroidal anti-inflammatory drugs and paracetamol: a population-based case-control study. J Thromb Haemost. 2015;13:708–718. doi:10.1111/jth.12855
- ORDER 727/2017, dated 7 August, of the Regional Minister of Health, setting the public prices for the provision of health services and activities of the network of centres in the Community of Madrid. Official Gazette of the Community of Madrid No. 198;August 21, 2017.
- BotPlus. Available from: https://botplusweb.portalfarma.com/. Accessed November 21, 2019.
- Agrupación homogénea de las presentaciones de los medicamentos para pacientes no hospitalizados que requieren para su dispensación receta médica oficial u orden de dispensación, junto con el precio vigente [Homogeneous grouping of the presentations of outpatient medicines that require an official prescription or dispensing order for their dispensing, together with the current price]. Available from: https://www.mscbs.gob.es/profesionales/farmacia/pdf/AGRUPACIONES_HOMOGENEAS_MEDICAMENTOS.pdf. Accessed September 17, 2019.
