Abstract
Purpose
Non-muscle invasive bladder cancer (NMIBC) is a malignancy restricted to the inner lining of the bladder. Intravesical Bacillus Calmette-Guerin (BCG) following transurethral resection of the bladder tumor is the mainstay first-line treatment for high-risk NMIBC patients. Two systematic literature reviews (SLRs) were conducted to further assess the current evidence on BCG use in NMIBC and the humanistic and economic burden of disease.
Methods
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, Embase® and MEDLINE® were searched using the Ovid platform to identify interventional or real-world evidence studies on the health-related quality of life (HRQoL) and economic burden in NMIBC. Limited evidence was found from initial economic SLR searches in NMIBC, so additional targeted searches for bladder cancer were conducted to expand findings.
Results
Fifty-nine publications were included in the HRQoL SLR, of which 23 reported HRQoL and symptoms in NMIBC. At diagnosis, HRQoL was comparable with population norms but worsened considerably 2 years following diagnosis. Maintenance therapy with intravesical BCG was associated with reduced HRQoL, and treatment-related adverse events (AEs) resembled typical NMIBC symptoms. Twenty-two studies reported decreasing BCG compliance over time. Common AEs with BCG were frequent urination, lower urinary tract symptoms, pain, and hematuria. Forty-two publications were included in the economic SLR, of which nine assessed healthcare costs and resource use in NMIBC or bladder cancer. High-risk disease and high-intensity treatment were associated with increased healthcare costs.
Conclusion
NMIBC has a considerable symptomatic, HRQoL, and economic burden. Symptoms persisted and HRQoL worsened despite intravesical BCG treatment. NMIBC is a costly disease, with higher healthcare costs associated with increased risk of disease progression and recurrence. There is a high unmet need for safe and effective treatments that reduce the risk of disease progression and recurrence, provide symptomatic relief, and improve HRQoL for patients.
Introduction
Bladder cancer is the tenth most common cancer globally with over 500,000 new cases reported in 2018.Citation1 In the US, it is the sixth most common type of cancer overall, and the fourth most common cancer in men in 2020.Citation2,Citation3 It is estimated to account for 4.5% of all new cancer diagnoses, with 81,400 new cases estimated for 2020 in the US alone. Bladder cancer is four times more common in men than in women, and the majority of cases are seen in persons aged over 55 years.Citation2,Citation3
There are four main types of bladder cancer and five stages of disease, based on the degree of muscle involvement.Citation4,Citation5 Urothelial carcinoma (transitional cell carcinoma) is the most common type of bladder cancer, reported in up to 95% of cases. Squamous cell carcinoma, adenocarcinoma, and small cell carcinoma each account for <5% of cases.Citation4 The majority of new diagnoses of bladder cancer (75% to 80%) are non-muscle invasive bladder cancer (NMIBC), also termed superficial bladder cancer. In NMIBC, the tumor is localized to the inner lining of the bladder and does not involve the deeper muscle layers.Citation6,Citation7
Transurethral resection of bladder tumor (TURBT), followed by induction treatment with intravesical chemotherapy (ie, mitomycin C) or Bacillus Calmette-Guerin [BCG] is the first-line treatment recommended for NMIBC.Citation5 With intravesical therapy, liquid drugs are administered into the bladder through the urethra using a soft catheter. The goal of treatment is to prevent or delay disease recurrence and progression to advanced disease.Citation7 Patients can receive adjuvant induction intravesical BCG therapy for 6 weeks, followed by maintenance treatment for 1-year for those at an intermediate risk of progression or recurrence, increasing to 3 years in high-risk patients.Citation5
A patient reported outcome (PRO) is defined by the Food and Drug Administration (FDA) as any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.Citation8 PROs can reflect various outcomes, including health-related quality of life (HRQoL), and can be a measure of symptom severity, disease state, or change in a previous measure. With regards to HRQoL, the FDA defines this as a multidomain concept that represents the patient’s general perception of the effect of illness and treatment on physical, psychological, and social aspects of life.Citation8 More generally, HRQoL measures how a patient feels or functions in response to treatment and its impact on their health.
While maintenance treatment with intravesical BCG in NMIBC has shown to be effective in delaying disease progression and recurrence, due to the invasiveness of the procedure and the repeated administration involved with maintenance therapy, it is a burdensome treatment regimen associated with considerable local and systemic side effects such as pain, fatigue, cystitis, frequency, hematuria, fever, and general malaise.Citation9–Citation11 NMIBC is associated with a high symptom burden and given the adverse effects of intravesical BCG, there are issues concerning patient-reported HRQoL and treatment tolerability for patients receiving BCG. In addition, NMIBC is associated with high costs and resource use.Citation12,Citation13 The risk of tumor recurrence and disease progression necessitates long-term surveillance and repeated therapy in patients, adding to both the humanistic and economic burden of treatment.Citation5,Citation12,Citation13
A systematic literature review (SLR) was conducted to gain a comprehensive and up-to-date understanding of the clinical and HRQoL burden of NMIBC and BCG therapy. Key PROs of interest included the symptom burden of NMIBC, change in scores on disease-specific and generic PRO scales during intravesical BCG, side effects associated with therapy, and treatment compliance. During this SLR, a gap in the evidence landscape was found for the economic burden of disease and therapy. Searches were subsequently expanded to identify economic literature on the cost burden, healthcare resource use, cost-effectiveness, and budget impact of NMIBC and associated therapies. To our knowledge, a systematic review that provides a comprehensive overview of HRQoL in NMIBC, as well as the cost of disease and treatment, has not been previously conducted. The aim of this review is to present the findings on the key factors contributing to the HRQoL of patients with NMIBC, with a focus on intravesical BCG therapy, as well as to discuss the economic burden.
Methods
An SLR was conducted to assess the HRQoL and clinical burden of NMIBC (HRQoL SLR), which was expanded to conduct searches on the economic burden of bladder cancer and NMIBC (herein referred to as the economic SLR). Searches were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The scope of the SLRs were defined in terms of PICOS criteria (Population, Intervention, Comparators, Outcomes and Study Design) and followed the principles outlined in the Cochrane Handbook for Systematic Reviews of Interventions, Centre for Reviews and Dissemination (CRD)’s Guidance for Undertaking Reviews in Health Care, and Methods for the Development of National Institute for Health and Care Excellence (NICE) Public Health Guidance.Citation14–Citation16
The key biomedical literature databases (Medical Literature Analysis and Retrieval System Online [MEDLINE®], and Excerpta Medica Database [Embase®]) were searched via the Ovid platform in both the HRQoL and economic SLRs. The bibliographies of relevant meta-analyses and systematic reviews were searched to retrieve any additional literature that may have been missed from the Ovid search, and ClinicalTrials.gov was searched for additional information (see the Supplementary Materials for search strategies). The Econlit database was also searched for the economic SLR.
Searches for the HRQoL SLR were conducted between January 2009 to May 2019 and between January 2009 to June 2019 for the economic SLR. The scope of the searches were defined in terms of the PICOS Statement. In both SLRs, the target patient population was adult patients with NMIBC, early stage bladder cancer, or stage 0 or 1 bladder cancer. Interventions included any treatment including surgery (ie, TURBT, cystectomy, bladder removal) and intravesical treatment; only English-language studies were included. During initial searches for the economic SLR, limited economic evidence was found specific to NMIBC. As such, additional targeted searches were conducted using various cost terms to expand findings to include patients with bladder cancer, particularly as some papers reported NMIBC as a cost component of bladder cancer.
Studies considered in the HRQoL SLR included interventional studies (eg, randomized controlled trials [RCTs] and single-arm studies) and real-world evidence (RWE) from prospective and retrospective studies. Outcome measures included were symptoms, any HRQoL outcome, and BCG safety and compliance. Studies considered in the economic SLR included cost-effectiveness analyses (CEAs), cost-utility analyses (CUAs), cost-minimization analyses (CMAs), budget impact studies, and observational cost studies. Outcome measures were healthcare resource use (HCRU), costs, cost-effectiveness, and budget impact (see Supplementary Table 1 for the inclusion and exclusion criteria by PICOS statement and Supplementary Tables 2 and 3 for the full inclusion and exclusion criteria for both SLRs). The methodological approach for both SLRs followed three steps:
Step 1: titles and abstracts of publications identified in Ovid searches were first independently assessed by two reviewers against the pre-defined inclusion and exclusion criteria. Any discrepancies between the reviewers were reconciled by a third reviewer.
Step 2: studies selected for inclusion during the title/abstract review were then assessed based on their full-text publication.
Step 3: studies selected for inclusion following full-text review were retained for data extraction. Excluded publications were recorded with a justification in the table format as per the NICE Decision Support Unit (DSU) guidance.
Data from the studies identified in the HRQoL SLR were extracted and summarized using frequency statistics where possible. For all studies reporting HRQoL scores at a particular time point for the same PRO measure (ie, at baseline, end of induction, or maintenance), data were extracted by domain and timepoint and the weighted average calculated to derive a summary statistic. Due to the small number of studies that reported this information for each measure, other selection criteria, such as study design similarity, were not applied when selecting studies to calculate scores. Using this approach, weighted average scores were calculated for the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30), EORTC QLQ Superficial Bladder Cancer 24 (QLQ-NMIBC24, also referred to as QLQ-BLS24), and the Short Form 36 (SF-36). Results presented for the economic SLR focus on cost studies and are discussed qualitatively.
Results
Patient-Reported Outcomes
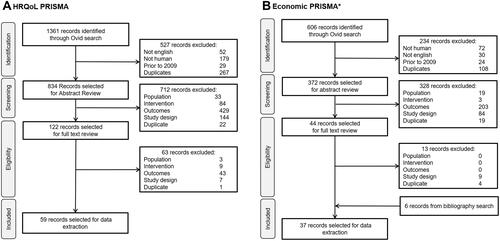
A total of 1361 publications were identified from searches for the HRQoL SLR. Of these, 834 were selected for title/abstract review, and 59 were included for data extraction ().
Figure 1 PRISMA flow diagrams for the HRQoL and economic SLRs (A and B).
Symptom Burden Prior to Treatment
Seven of the 59 studies identified in the HRQoL SLR reported the symptoms experienced by patients with NMIBC prior to receiving any treatment.Citation17–Citation23 Three were retrospective database studies,Citation19,Citation21,Citation22 two were patient surveys,Citation17,Citation23 one was a prospective observational study,Citation18 and one was an RCT.Citation20
The most frequently reported symptoms across the seven studies were hematuria, dysuria, frequent urination, and urgency ().Citation17,Citation18,Citation20–Citation24 Among patients with high-risk NMIBC disease, the overall symptom burden prior to treatment was worse for younger patients aged <65 years than those ≥65 years, and worse for women than men.Citation18
Table 1 Symptoms of NMIBC Prior to Treatment
HRQoL Over the Course of NMIBC
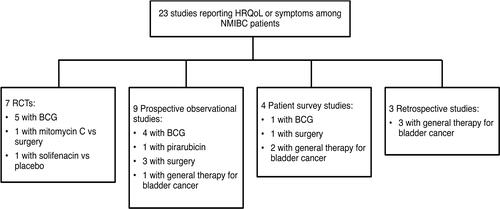
Twenty-three studies reported HRQoL outcomes or symptoms experienced by patients with NMIBC.Citation9,Citation18,Citation23,Citation25–Citation44 These were in addition to the studies reporting on symptoms prior to treatment. Seven studies were RCTs, nine were prospective observational studies, four were patient surveys, and three were retrospective database studies (). Among these studies, the EORTC QLQ-C30 scale designed for cancer patients, the tumor-specific EORTC QLQ-NMIBC24 scale, and the generic SF-36 scale were the most common PRO measures used.
Figure 2 HRQoL studies by NMIBC treatment and study type.
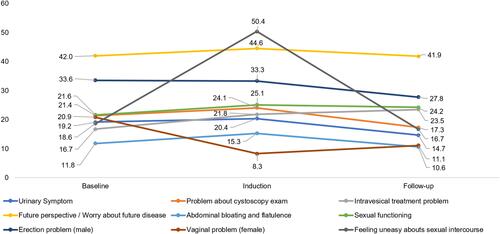
Three studies by Gontero et al, 2013, Siracusano et al, 2018, and Park et al, 2018 reported disease-specific HRQoL in patients newly diagnosed with NMIBC prior to receiving treatment (T0) using the tumor-specific EORTC QLQ-NMIBC24 scale.Citation9,Citation25,Citation33 The study by Gontero et al was a Phase 2 RCT of intravesical gemcitabine versus BCG in 120 BCG-naïve patients with intermediate-risk NMIBC. The study by Siracusano et al was a prospective observational study of intravesical BCG or mitomycin C in 103 intermediate- to high-risk treatment-naïve NMIBC patients, and the study by Park et al, was a prospective observational study of 249 NMIBC patients pre- and post-TURBT.Citation9,Citation25,Citation33 Across the studies, EORTC QLQ-NMIBC24 scores were low across problem and symptom domains at diagnosis, indicating low levels of HRQoL impairment ().Citation9,Citation25,Citation33 In particular, patients experienced low levels of urinary symptoms, abdominal bloating and flatulence, and vaginal problems, whereas worry about future disease was considered the greatest issue, with higher scores reported (ie, worse outcome) compared with other symptom/problem domains ().Citation9,Citation25,Citation33 However, relatively low scores were reported for sexual function indicating low to moderate function for this domain (score <50 out of 100, with higher scores reflecting better function).
Table 2 EORTC QLQ-NMIBC24 Scores Prior to Treatment
Low levels of HRQoL impairment, particularly for the physical and social function domains (score >90), were also reported in NMIBC patients at diagnosis on the EORTC QLQ-C30 scale by Gontero et al, 2013, Siracusano et al, 2018, and in a Chinese prospective observational study by Wei et al, 2014 ().Citation9,Citation25,Citation35 Using this generic HRQoL questionnaire used only for cancer patients, low levels of symptoms were seen across all symptom and problem domains at diagnosis (score <50, with lower score indicating fewer symptoms); overall, EORTC QLQ-C30 scores were similar to the general population.Citation9,Citation25,Citation35,Citation39 Likewise, two other studies also reported similar HRQoL scores between NMIBC patients at diagnosis and the general population on the SF-36 scale, which is a generic measure of HRQoL (see Supplementary Table 4).Citation42,Citation44
Table 3 EORTC QLQ-C30 Scores Prior to Treatment
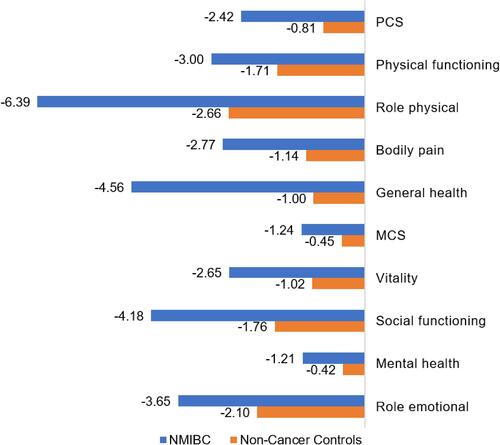
While HRQoL scores were similar to the general population at diagnosis prior to treatment, SF-36 scores from two studies by Brisbane et al, 2019 and Smith et al, 2018, which assessed HRQoL in NMIBC patients using the US Medicare Health Outcomes Survey linked to the Surveillance Epidemiology and End Results (SEER-MHOS) database, showed from baseline after 2 years following diagnosis.Citation43,Citation44 In both studies, SF-36 scores in NMIBC patients were propensity matched to the general population and showed a considerably greater deterioration in HRQoL across all SF-36 domains at 2 years following diagnosis.Citation43,Citation44 A clinically meaningful decline (≥5 points; ie, worsening)Citation44,Citation45 was seen for the role physical domain. In the study by Smith et al, recent depression was a significant predictor for decrements in Mental Component Score (MCS) and Physical Component Score (PCS) scores (p<0.01 for both). Brisbane et al, stratified results by disease risk and found significantly greater decline in PCS scores in high-risk NMIBC patients than the general population (−3.4 vs −1.4; p=0.01), which was not seen for non-high-risk patients.Citation43 Of note, Brisbane et al, excluded patients with prior cystectomy, whereas Smith et al, did not. SF-36 scores in NMIBC patients compared with the general population are presented in .
Figure 3 Decline in NMIBC HRQoL 2 years post-diagnosis on the SF-36* versus the general population**.
HRQoL Decline with BCG Intravesical Therapy
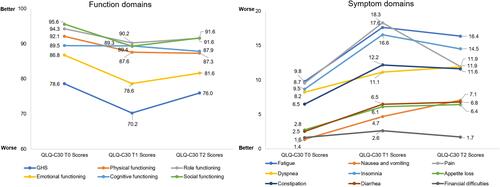
HRQoL outcomes using the EORTC QLQ-C30 scale at diagnosis (ie, baseline), during BCG induction, and during maintenance therapy were assessed by Gontero et al, and Siracusano et al, and during the induction and maintenance periods only in an open-label RCT of 84 BCG-naïve high-risk patients by Koga et al, 2010.Citation9,Citation25,Citation27 Additional contributions to the baseline (T0) dataset were provided by Wei et al, 2014.Citation35 A general worsening in the function domains and symptom scores of the EORTC QLQ-C30 was seen during the induction phase of treatment (T1). While a small improvement was seen during the maintenance phase (T1 to T2), EORTC QLQ-C30 scores did not return to baseline (T0) values ().Citation9,Citation25,Citation27,Citation35 Notably, the largest decline (ie, worsening) in function domain scores from baseline during the maintenance phase (T0 to T2) were seen for emotional (−5.2), followed by physical (−4.8), and social functioning (−4.0). For symptom domain scores, the largest increase (ie, worsening) was seen for fatigue (6.6), followed by insomnia (5.9), and nausea/vomiting (5.7).
Figure 4 EORTC QLQ-C30 during BCG treatment.*#.
EORTC QLQ-NMIBC24 scores at diagnosis, and during BCG induction and maintenance therapy were reported in the two studies by Gontero et al, and Siracusano et al, with additional baseline data provided by Park et al, 2018.Citation9,Citation25,Citation33 As seen with the EORTC QLQ-C30 scale, there was no improvement in bladder cancer symptoms despite BCG therapy, and there was a general trend towards worsening (ie, higher scores) in most symptom domains (). The greatest worsening (T0 to T2) was seen for problems related to intravesical treatment, with a score increase of 6.8. There was an improvement in all other symptom domains, as shown by a decline in scores ranging from −0.2 for worry about the future to −9.8 for vaginal side effects. A small improvement in sexual functioning was also seen across the analysis period (score increase of 2.6).
Figure 5 EORTC QLQ-NMIBC24 during BCG treatment.*.
The majority of studies of other therapies, including TURBT, intravesical mitomycin C, surgery, and radiation therapy, reported either a decline or no improvement in patient HRQoL outcomes following treatment.Citation23,Citation26,Citation30,Citation37,Citation39 However, one Spanish prospective cohort study reported significant improvements in mental health following diagnosis with TURBT plus mitomycin C, and in urinary symptoms with TURBT alone, after 1 year of treatment (p<0.05).Citation36
Adverse Events with BCG Therapy
Twenty studies identified in the HRQoL SLR reported adverse events (AEs) in patients treated with BCG. Ten were interventional studies (nine RCTs and one single arm study), eight were retrospective studies, and two were prospective observational studies.Citation11,Citation20,Citation27,Citation46–Citation61
AEs reported with BCG during induction and maintenance therapy were similar to the symptoms typically seen in NMIBC. Frequent urination, nocturia, hematuria, lower urinary tract symptoms, and pain on urination were the most common AEs reported in up to 97% of patients (). Other less-frequently reported AEs in descending order included cystitis, urgency, urinary retention, and difficulty urinating.
Table 4 AE Frequencies During BCG Treatment
In a separate patient survey study, the most concerning aspects of BCG treatment for NMIBC were pain related to administration/tube insertion and urinary urgency/frequency, which were both reported by 40% of patients.Citation17
Treatment Compliance
Twenty-two studies were identified in the HRQoL SLR that assessed compliance to BCG treatment across different risk categories, which included 6 studies specifically in intermediate or high-risk patients.Citation11,Citation25,Citation28,Citation31,Citation46,Citation47,Citation50,Citation53–Citation56,Citation58,Citation60,Citation62–Citation70 Across the studies, treatment compliance decreased over time. Nearly all patients completed the 6-week induction therapy period (range 85% to 98%),Citation25,Citation28,Citation46,Citation47,Citation53–Citation56,Citation60,Citation63,Citation64,Citation66,Citation67 declining over the 12-month maintenance period (53% to 83%),Citation11,Citation46,Citation50,Citation53,Citation56,Citation58,Citation63,Citation64,Citation66,Citation68,Citation69 with less than 40% compliant over 36 months of maintenance therapy (0% to 36%).Citation11,Citation46,Citation56,Citation58,Citation63,Citation67,Citation70 Intolerance to treatment and urinary AEs were cited as key reasons for discontinuation.Citation11,Citation25,Citation28,Citation31,Citation46,Citation47,Citation50,Citation53–Citation56,Citation58,Citation60,Citation62–Citation70
In high-risk NMIBC, the Spanish Phase 3 RCT conducted by Martinez-Pineiro et al, 2015 found that the majority patients completed induction BCG therapy (>95%), 70% completed 1 year of maintenance therapy, and 51.5% and 34.5% completed 2- and 3-year maintenance therapy, respectively.Citation46 Among those prematurely discontinuing treatment, 10% discontinued due to AEs or toxicities of which 2.5%, 3.5%, and 0.5% stopped treatment during the first, second, and third year, respectively.Citation46 AEs leading to treatment discontinuation commonly occurred within the first year of treatment in the phase 3 study of high-risk patients receiving 3-year BCG maintenance therapy by Brausi et al, 2014.Citation11 Reducing the dose or treatment period did not impact on reducing AE-related discontinuations.Citation11
Clinical outcomes related to the duration of maintenance therapy are mixed. Some studies have found no difference in survival, disease recurrence, or disease progression in intermediate to high-risk patients completing 1- or 3-years of BCG maintenance therapy compared with those receiving shorter regimens due to early discontinuation or receipt of induction therapy only (see Supplementary Table 5).Citation46,Citation53,Citation54,Citation70 However, improvements in 5-year recurrence-free survival rates have been reported in patients completing maintenance therapy than those stopping early.Citation53,Citation54 In general, BCG maintenance therapy is recommended for 1 to 3 years in intermediate- to high-risk patients.Citation5,Citation71 Yet there is no consensus across guidelines on the optimal duration of maintenance therapy (ie, European Association of Urology [EAU], American Urological Association [AUA], National Comprehensive Cancer Network [NCCN] and Japanese Urological Association),Citation5,Citation71–Citation73 so that some of the heterogeneity among results may relate to different treatment durations.
Cystectomy
Cystectomy is the surgical removal of the bladder.Citation74 It is a significant procedure associated with high-morbidity that can be expected to have a considerable impact on HRQoL. In a study of patients with NMIBC or bladder cancer, cystectomy was associated with a clinically meaningful impairment (change ≥10 points) on the fatigue and appetite loss symptom domains and role functioning domain of the EORTC QLQ-C30 compared with the general population.Citation39 In the retrospective study of bladder cancer patients by Smith et al, of which 86% had NMIBC, patients undergoing cystectomy experienced a significant decline 2 years post-diagnosis across a number of physical domains (PCS, physical function, role physical, and general health) and mental health domains (role emotional, vitality, and social functioning) on the SF-36.Citation44 However, a phase 2 study found no difference between NMIBC patients treated with intravesical mitomycin C or surgical management for EORTC QLQ-C30 global health status scores.Citation26
Economic Outcomes
Searches for the economic SLR identified a total of 606 publications. Of these, 372 were selected for title and abstract review, and 37 studies on NMIBC were included for data extraction (). A paucity of economic evidence specific to NMIBC was identified from the initial SLR searches. As such, additional targeted searches expanding the search criteria to include patients with bladder cancer were conducted to identify additional economic evidence. A further five studies were identified through additional targeted searches, resulting in a total of 42 economic studies selected for data extraction.
Studies reported on a range of different findings. Of the 37 studies identified from SLR searches, fifteen were cost studies, of which one evaluated total healthcare costs of bladder cancer in the US;Citation75 one was a US Markov model of healthcare costs by disease risk;Citation76 four were US database studies evaluating varying provider-level treatment intensity among Medicare beneficiaries aged ≥65 years;Citation12,Citation13,Citation77,Citation78 three assessed diagnosis and monitoring costs;Citation79–Citation81 three assessed different imaging technologies;Citation82–Citation84 and two evaluated intravesical chemotherapy post-TURBT.Citation85,Citation86 In addition, three budget impact models (BIMs) and eighteen CEAs were identified from SLR searches, of which twelve evaluated TURBT, mainly comparing diagnostic imaging during the procedure as well as comparing TURBT to other procedures or procedure setting.Citation87–Citation98 Two studies assessed intravesical therapy;Citation99,Citation100 four assessed surveillance.Citation101–Citation104 Four studies evaluated BCG and/or the impact of its shortage,Citation105–Citation108 with one being a CEA that presented AE costs associated with BCG in high-risk patients.Citation107
Of the five studies identified from additional targeted searches, two were cost studies on the economic burden of bladder cancer in Europe and Italy, respectively;Citation109,Citation110 one was a clinical model of the lifetime cost of bladder cancer;Citation111 one was a CEA of BCG versus immediate radical cystectomy;Citation112 and one was a retrospective study evaluating the impact of BCG shortage.Citation113 We herein focus on presenting findings from studies reporting healthcare costs in bladder cancer and NMIBC, with an emphasis on high-risk disease.
Overall Healthcare Costs
Two cost studies estimated the total healthcare costs for bladder cancer in the US and Europe to be US$4.0 billion in 2010 and €2.9 billion in 2012, respectively.Citation75,Citation109 In Europe, inpatient care accounted for over half of bladder cancer healthcare costs (€1.7 billion; 58%). Overall costs associated with bladder cancer were estimated at €4.9 billion (€2.9 billion in healthcare costs), of which indirect costs from work productivity losses and informal care accounted for 41%.Citation109
Likewise, in an Italian cost of illness study of bladder cancer, nearly half (44%) of total per patient costs were due to productivity losses.Citation110 Mean total annual per patient costs were €3,591 for all bladder cancer types and €3,252 for NMIBC, specifically.Citation110
The mean lifetime cost of bladder cancer treatment was estimated at $65,158 (USD, 2005 cost year) in a US observational medical database study of 208 patients with transitional cell bladder cancer registering at the University of Texas M.D. Anderson Cancer Center (MDACC) from 1991 to 1999.Citation111 In a scenario analysis, mean lifetime costs were higher in the best-case scenario at $120,684 due to longer survival, and lower in the worst-case scenario at $99,270.Citation111 In NMIBC specifically (Stage <1), initial and ongoing treatment for disease recurrence accounted for a large proportion of healthcare costs over a mean post-diagnosis follow-up period of 5.3 years ($6,548 [33%] and $6,401 [32%], respectively). Mean surveillance costs were $4,525 per year.Citation111 Healthcare costs were significantly higher in patients with disease complications from NMIBC than those without; this difference was consistent across all areas of disease management (p<0.05).Citation111
NMIBC Costs by Disease Risk
In the US Markov model of NMIBC, healthcare costs increased with disease risk, based on the European Organization for Research and Treatment Center (EORTC) risk tables.Citation76 Costs rose from $52,125, to $146,250, to $366,143 for low-, intermediate-, and high-risk disease, respectively, over a 5-year time horizon using a base case of a male aged 65 years (USD, 2017 cost year).Citation76 Disease progression to muscle-invasive bladder cancer (MIBC) was a primary cost driver, accounting for the majority of total costs in low-risk patients (71%), increasing to 81% and 92% in intermediate- and high-risk patients, respectively.Citation76
NMIBC Costs and Treatment Intensity
Four US studies used the SEER-Medicare database to evaluate the costs of providing different levels of treatment intensity in NMIBC among Medicare beneficiaries (ie, early Stage 0 to 2 bladder cancer) by assessing expenditures claimed within the first 2 years of diagnosis (USD, 2005 cost year in all studies).Citation12,Citation13,Citation77,Citation78 Treatment intensity was assessed by ranking per patient Medicare expenditures in the 2 years following diagnosis from low to high by either quantiles,Citation78 quartiles,Citation12,Citation77 or into three groups.Citation13
Treatment intensity was shown to vary across physicians and regions in the US and did not consistently align with disease risk.Citation12,Citation13,Citation77,Citation78 Furthermore, high-intensity treatment did not confer a survival benefit over less intense regimens and was associated with greater HCRU and patient costs ().Citation12,Citation77,Citation78 Higher rates of HCRU for disease surveillance (ie, imaging, urinalysis), physician visits, and intravesical treatment were seen in high- versus low-intensity treatment regimens. These were the key cost drivers associated with high-intensity treatment, accounting for 90% of costs.Citation12,Citation77,Citation78 By reducing the variation in treatment intensity provided by physicians, Hollingsworth et al, 2010, estimated that annual savings to Medicare of 18.6% could be achieved, equating to $18.7 million per annum.Citation78
Table 5 Per Patient Costs with High- and Low-Intensity Treatment Regimens in NMIBC
Adverse Event Costs with BCG
In a separate US CEA study, AE costs accounted for 17% of total treatment costs in high-risk patients treated with BCG compared with only 5% for mitomycin C, thereby adding to the cost-burden of disease.Citation107 The CEA model estimated that 40% of patients treated with BCG will eventually require treatment escalation to cystectomy, and 15% will progress to muscle-invasive disease.Citation107
QALYs and Utility Data
Six CEA studies from the US, UK, and Canada and the US reported quality adjusted life years (QALYs) and/or utility scores.Citation87,Citation90,Citation98,Citation99,Citation107,Citation112 Of the three studies that reported outcomes with intravesical therapy, QALYs ranged from 10.34 to 9.39 with BCG for patients with high-risk NMIBCCitation107,Citation112 to 5.15 for maintenance intravesical therapy in NMIBC patients overall.Citation99 Utility values were reported by Kulkarni et al, 2009, which were used in the study Ramamohan et al, 2014. Intravesical BCG had a utility value of −0.02 and cystectomy had a utility of 0.8.Citation112
In a CEA by Bobman et al, maintenance intravesical therapy was the most cost-effective option at US$8,862/QALY compared with other treatment approaches involving cystectomy with or without intravesical therapy.Citation99 In contrast, in the study by Kulkarni et al, in patients with high-risk NMIBC, immediate cystectomy was the more cost-effective option versus intravesical BCG, except for in older patients (>75 years) or those with high comorbidities, wherein BCG dominated.Citation112
Discussion
Findings from the HRQoL and economic literature reviews showed that NMIBC is associated with a high symptom and HRQoL burden despite current BCG therapy, and disease management incurs high costs to healthcare systems. The studies evaluated used different strains of BCG and so the findings reported for both SLRs are applicable across different strains.
Specifically, results from the HRQoL SLR showed that patients with NMIBC experience a high burden of urinary symptoms with treatment.Citation9,Citation25,Citation35,Citation39 While intravesical BCG is an effective treatment for NMIBC, it does not offer symptomatic relief to patients and the commonly reported side effects of frequent urination, hematuria, and nocturia resemble the typical disease symptoms.Citation11,Citation20,Citation27,Citation46–Citation61 Furthermore, the side effects associated with BCG intravesical therapy account for nearly 20% of total treatment costs in high-risk NMIBC patients.Citation107
Guidelines recommend maintenance therapy with BCG for up to 3 years in patients with high-risk NMIBC.Citation5 BCG compliance rates decline over time, with treatment intolerance and urinary side effects being the primary reasons for premature discontinuation.Citation11,Citation25,Citation28,Citation31,Citation46,Citation47,Citation50,Citation53–Citation56,Citation58,Citation60,Citation62–Citation70
While declining patient compliance to medication is commonly reported in other disease areas, the rate and degree of non-compliance is less severe than in NMIBC patients treated with BCG.Citation114 For example, in a real-world study of prescription data from 4,043 patients with chronic myeloid leukemia or gastrointestinal stromal tumor, the majority of patients (75% to 78%) were overall compliant to their treatment over 2 years (as assessed using medication possession ratio), with an average persistence in medication use of 255 days.Citation115 In contrast, treatment compliance rates with BCG decline from as high as 98% following 6 weeks of induction therapy, to as low as 0% after 3 years.Citation56,Citation60
The commonly used generic HRQoL (SF-36), cancer-specific (EORTC QLQ-C30), and NMIBC tumor-specific (EORTC QLQ-NMIBC24) PRO measures were examined in the HRQoL SLR. Of the five studies identified that evaluated treatment-naïve NMIBC patients at diagnosis, patients demonstrated similar HRQoL compared with the general population on the EORTC QLQ-C30 and SF-36.Citation9,Citation25,Citation35,Citation39,Citation42,Citation44 However, during induction therapy with BCG, patient HRQoL declined and then improved during the maintenance period, yet did not return to pre-treatment levels, as measured by the EORTC QLQ-C30 and the EORTC QLQ-NMIBC24 scale.Citation9,Citation25,Citation27,Citation33,Citation35 The aspects of HRQoL found to be the most negatively affected on the EORTC QLQ-C30 were the emotional, physical, and social functioning domains and the fatigue, insomnia, and nausea/vomiting symptom domains. On the EORTC QLQ-NMIBC24, the treatment-related domain of intravesical therapy was the most impacted. Likewise, NMIBC patients experienced a worsening in SF-36 scores 2 years following diagnosis, with a considerably greater decline compared with population norms across all SF-36 domains.Citation43,Citation44 Accordingly, BCG treatment did not improve the HRQoL of patients with NMIBC or provide symptomatic relief.
It is important to highlight that different PRO measures focus on different aspects of HRQoL. The SF-36 is a generic HRQoL instrument designed to measure health status against the general population and to other health conditions.Citation116 This is a well-validated and widely used PRO instrument, and was specified in our results because population norms and 2-year post-diagnosis data were available, which is valuable to ascertain the effect of BCG in patients with NMIBC compared with the general population. The EORTC QLQ-C30 is also a well-validated instrument designed for cancer patients. In terms of tumor or cancer-specificity, the EORTC QLQ-NMIBC24 is the most disease-specific scale of the three measures, which is designed and validated for use in NMIBC (measures urinary symptoms, intravesical treatment issues, future perspective, fever and general malaise, abdominal bloating and flatulence).Citation117–Citation119 A review of HRQoL instruments used in bladder cancer suggested that disease-specific scales should be administered alongside generic scales, such as the SF-36, to ensure all aspects of QoL are captured.Citation118 Therefore, findings presented in this review can be considered as broadly reflecting the key elements of HRQoL in patients with NMIBC.
As patients often require long-term BCG maintenance therapy and ongoing disease surveillance to monitor recurrence and progression, the financial burden of disease to healthcare systems is high, which was confirmed in the economic SLR. A focus of this research was on cost studies in bladder cancer and NMIBC, which estimated the total cost of bladder cancer to US and European healthcare systems to be $4.0 billion and €2.9 billion, in 2010 and 2012, respectively.Citation75,Citation109 The per-patient lifetime costs of bladder cancer were estimated at US$65,158, ranging from $99,270 to $120,684 in the worst- and best-case scenarios, respectively.Citation111 This is largely consistent with findings from a separate SLR of 44 economic studies conducted by Botteman et al, 2003, which reported the lifetime per patient costs of bladder cancer to range from $96,000 to $187,000 from the point of diagnosis to death (USD, 2001 cost year). In the review by Botteman et al, bladder cancer represented the greatest per patient lifetime cost of all cancers in the US.Citation120
In addition, healthcare costs rose substantially with increasing risk of progression to MIBC, increasing by $314,018 from low- to high-risk disease.Citation76 Healthcare costs in NMIBC vary by the intensity of treatment provided by physicians, where high-intensity regimens incur higher costs compared with low-intensity treatment, largely driven by higher rates of disease surveillance and intravesical instillation therapy procedures. Despite the additional healthcare procedures and costs involved, high-intensity treatment was not shown to provide a survival benefit.Citation12,Citation13,Citation77,Citation78
Searches of the literature revealed limited evidence on the economic cost burden of NMIBC, especially in the subgroup of patients with high-risk disease. Among the identified studies included from economic searches, a large proportion focused on the use of different imaging modalities during surgery and costs associated with routine surveillance and monitoring.
There are a number of emerging therapies for NMIBC.Citation121,Citation122 Chemothermotherapy is one such treatment option for patients with NMIBC who fail intravesical BCG and develop disease recurrence.Citation121,Citation123,Citation124 It involves the intravesical application of heated chemotherapy to the bladder through use of microwaves to heat the local bladder environment or through circulation of heated fluids (hyperthermic intravesical chemotherapy [HIVEC]).Citation121,Citation122,Citation125,Citation126 In a meta-analysis of 12 studies, intravesical chemothermotherapy was associated with a significantly decreased risk of disease recurrence compared with normal-temperature chemotherapy after 2 years, with no difference in the rate of AEs.Citation125 However, AEs of hematuria, bladder spasms, and pain have been reported with chemothermotherapy.Citation122,Citation127 While no studies on chemothermotherapy were identified in our SLR, it is a treatment option that should be considered by the treating physician. Radical cystectomy is a recommended treatment following BCG failure in NMIBC, so that intravesical chemothermotherapy is an alternative bladder-sparing option to normal intravesical chemotherapy in BCG-refractory patients that may delay or avoid the need for this highly-morbid surgical procedure.Citation122,Citation123 In addition, reducing the dose of BCG to a third (1/3) is an alternative approach to alleviate the side effects of treatment. However, a study found no difference in the rate and occurrence of local or systemic side effects between patients receiving a reduced (1/3) or full BCG dose after 1 and 3 years of maintenance therapy.Citation11 The American Urological Association also advised members in 2019 to use a reduced dose of BCG for NMIBC maintenance therapy and in high-risk patients receiving induction therapy where a full dose was not possible, due to a global BCG supply shortage.Citation128 While the 1/3 BCG dose was not a focus of this review, this indicates a need for alternative therapies with a more tolerable safety profile, and in light of the ongoing shortage issues.
The methodology for the HRQoL and economic SLRs followed best practice guidelines for conducting systematic reviewsCitation14–Citation16 and included publications from both interventional and RWE studies. With variability in study methods, outcome measures, and patient characteristics, studies were heterogeneous and therefore, comparison between different studies should be interpreted with caution. Since this SLR was conducted, three additional relevant publications have become available, which expand on our findings.Citation129–Citation131 In a study by Jung et al, HRQoL was assessed using the EORTC QLQ-C30 and -NMIBC24 scales in NMIBC survivors. Among patients treated with TURBT plus intravesical immunotherapy, insomnia and fatigue were among the worst symptoms on the EORTC QLQ-C30, which corroborates with our findings. Sexual problems for NMIBC-specific domains were scored the highest on the EORTC QLQ-NMIBC24, which contrasts with our findings where an improvement in sexual function was seen.Citation129 However, the patient population assessed in this study differed as it studied HRQoL outcomes in surviving patients post-treatment as opposed to over time during treatment. In an observational study by Gozalez-Padilla et al, no clinically relevant differences in HRQoL outcomes between BCG and induction chemothermotherapy (chemohyperthermia), and mitomycin C were seen on the Functional Assessment of Cancer Therapy for Bladder Cancer patients (FACT-Bl) and International Prostate Symptom Score (IPSS). BCG appeared to have the least favorable safety profile, which is consistent with the high degree of AEs reported in our review (). Lastly, in a cost-effectiveness study by Sharma et al, maintenance treatment with full dose BCG or at a reduced 1/3 dose was not cost-effective over surveillance in patients with intermediate or high-risk NMIBC, at a willingness-to-pay threshold of US$100,000.Citation131
With regards to the economic SLR, there was a lack of economic data specific to NMIBC and targeted searches to include bladder cancer were conducted to identify additional economic evidence. As such, the economic evidence of bladder cancer may not be comprehensive. In addition, caution should be exercised when drawing comparisons between economic studies, as cost data was not adjusted to account for inflation, currency fluctuations, or currency exchange rates.
Finally, this is the first study to our knowledge that provides a comprehensive overview on HRQoL outcomes in NMIBC as well as the economic impact of disease and treatment. While effective in NMIBC, intravesical BCG therapy is an invasive procedure with resultant side effects. Unsurprisingly, findings from our study showed that HRQoL and patient compliance declines over time during BCG treatment. As NMIBC is a common and costly disease involving long-term management, particularly in high-risk disease, new cost-effective therapies are needed to improve the patient experience, enable long-term tolerability and compliance, relieve the symptom burden, and improve HRQoL outcomes. Our findings help to better understand the symptomatic burden and aspects of HRQoL most impacted by NMIBC and BCG therapy, as well as the factors contributing to the cost burden of disease. This is important to inform future research for novel interventions to address the HRQoL decrements experienced by patients and avoid progression to more advanced and costly stages of disease.
Conclusion
NMIBC is associated with a high humanistic and economic burden. Although effective in NMIBC, the current standard of care of intravesical BCG is associated with side effects and decrements in HRQoL during treatment, which impact compliance rates. NMIBC is a costly disease to manage, with higher healthcare costs associated with increased risk of disease progression. There is a high unmet need for safe and effective treatments that reduce the risk of disease progression and provide symptomatic relief and HRQoL improvements for patients.
Acknowledgments
Authors would like to acknowledge Stephanie Oldham at Purple Squirrel Economics for providing editorial support for the manuscript, which was funded by Pfizer.
Disclosure
Ira A Jacobs, Lauren J Lee, Carla M Mamolo, and Elizabeth T Masters are employees of Pfizer Inc. and own Pfizer stock. Christina S Kwon and Anna Forsythe are employees of Purple Squirrel Economics who were paid consultants to Pfizer in connection with the development of this manuscript. The authors report no other conflicts of interest in this work.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
- National Cancer Institute. Cancer of the urinary bladder - cancer stat facts. SEER. Available at: https://seer.cancer.gov/statfacts/html/urinb.html.
- American Cancer Society. Key statistics for bladder cancer. Available at: https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html.
- Martin JW, Carballido EM, Ahmed A, et al. Squamous cell carcinoma of the urinary bladder: systematic review of clinical characteristics and therapeutic approaches. Arab J Urol. 2016;14(3):183–191. doi:10.1016/j.aju.2016.07.001
- NCCN. NCCN clinical practice guidelines in oncology. Bladder Cancer. NCCN Evidence BlocksTM version 6. 2020. July 22, 2020:1–121.
- Mbeutcha A, Lucca I, Mathieu R, Lotan Y, Shariat SF. Current status of urinary biomarkers for detection and surveillance of bladder cancer. Urol Clin North Am. 2016;43(1):47–62. doi:10.1016/j.ucl.2015.08.005
- Flaig TW, Spiess PE, Agarwal N, et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. JNCCN. 2020;18(3):329–354.
- FDA. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims; 2009.
- Siracusano S, Silvestri T, Bassi S, et al. Health-related quality of life after BCG or MMC induction for non-muscle invasive bladder cancer. Can J Urol. 2018;25(5):9480–9485.
- Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus calmette-guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized southwest oncology group study. J Urology. 2000;163(4):1124–1129. doi:10.1016/S0022-5347(05)67707-5
- Brausi M, Oddens J, Sylvester R, et al. Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014;65(1):69–76.
- Skolarus TA, Ye Z, Zhang S, Hollenbeck BK. Regional differences in early stage bladder cancer care and outcomes. Urology. 2010;76(2):391–396. doi:10.1016/j.urology.2009.12.079
- Strope SA, Ye Z, Hollingsworth JM, Hollenbeck BK. Patterns of care for early stage bladder cancer. Cancer. 2010;116(11):2604–2611. doi:10.1002/cncr.25007
- Ades AE, Caldwell DM, Reken S, Welton NJ, Sutton AJ, Dias S. NICE DSU Technical Support Document 7: Evidence Synthesis of Treatment Efficacy in Decision Making: A Reviewer’s Checklist. National Institute for Health and Clinical Excellence; 2012.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). 2019.
- Centre for Reviews and Dissemination, ed. CRD’s Guidance for Undertaking Reviews in Health Care. 3rd. CRD: University of York; 2009.
- Clark M, Harris NI, Martin S, et al. The impact of non-muscle invasive bladder cancer: qualitative research with patients. Value Health. 2015;18(7):A471. doi:10.1016/j.jval.2015.09.1248
- Danielsson G, Malmstrom P-U, Jahnson S, Wijkstrom H, Nyberg T, Thulin H. Bladder health in patients treated with BCG instillations for T1G2-G3 bladder cancer-a follow-up five years after the start of treatment. Scand J Urol. 2018;52(5–6):377–384.
- Dobbs R, Petros J, Usama A-Q, Ritenour C, Issa M, Canter D. Lower urinary tract symptoms (LUTS) as a presenting symptom for bladder cancer in a veteran population. J Urol. 2013;189(4SUPPL. 1):e525. doi:10.1016/j.juro.2013.02.2637
- Fallah F, Fallah M, Sajadi Nia R. Thiotepa versus bacille calmette-guerin in non-muscle invasive bladder cancer. Curr Urol. 2012;6(3):160–164. doi:10.1159/000343532
- Hakim L, Djatisoesanto W, Soebadi M, et al. Clinical characteristics of bladder cancer in indonesia: the largest data presentation from five hospitals. Urology. 2012;80(3):S206–S207.
- Minana B, Cozar JM, Palou J, et al. Bladder cancer in Spain 2011: population based study. J Urol. 2014;191(2):323–328. doi:10.1016/j.juro.2013.08.049
- Mogensen K, Christensen KB, Vrang M-L, Hermann GG. Hospitalization for transurethral bladder resection reduces quality of life in Danish patients with non-muscle-invasive bladder tumour. Scand J Urol. 2016;50(3):170–174. doi:10.3109/21681805.2015.1132762
- Dobbs RW, Hugar LA, Revenig LM, et al. Incidence and clinical characteristics of lower urinary tract symptoms as a presenting symptom for patients with newly diagnosed bladder cancer. Int Braz J Urol. 2014;40(2):198–203. doi:10.1590/S1677-5538.IBJU.2014.02.09
- Gontero P, Oderda M, Mehnert A, et al. The impact of intravesical gemcitabine and 1/3 dose bacillus calmette-guérin instillation therapy on the quality of life in patients with nonmuscle invasive bladder cancer: results of a prospective, randomized, phase ii trial. J Urol. 2013;190(3):857–862. doi:10.1016/j.juro.2013.03.097
- Mostafid H, Cresswell J, Griffiths L, et al. Results of CALIBER: A phase II randomised feasibility trial of chemoablation with MMC versus surgical management in low risk non-muscle invasive bladder cancer (NMIBC). Br J Cancer. 2018;119(1):39.
- Koga H, Ozono S, Tsushima T, et al. Maintenance intravesical bacillus calmette-guérin instillation for ta, t1 cancer and carcinoma in situ of the bladder: randomized controlled trial by the BCG tokyo strain study group: maintenance intravesical BCG. International J Urology. 2010;17(9):759–766. doi:10.1111/j.1442-2042.2010.02584.x
- Yokomizo A, Kanimoto Y, Okamura T, et al. Randomized controlled study of the efficacy, safety and quality of life with low dose bacillus calmette-guerin instillation therapy for nonmuscle invasive bladder cancer. J Urol. 2016;195(1):41–46. doi:10.1016/j.juro.2015.08.075
- Zhang Z, Cao Z, Xu C, et al. Solifenacin is able to improve the irritative symptoms after transurethral resection of bladder tumors. Urology. 2014;84(1):117–121. doi:10.1016/j.urology.2014.02.034
- Michielsen D, Coomans D. Intravesical chemotherapy or immunotherapy for intermediate-risk non-muscle invasive bladder cancer: does the patient mention a different quality of life? Urology. 2013;82(3SUPPL. 1):S130–S131.
- Yoshio S, Yoshimura K, Matsui Y, et al. Assessment of lower urinary tract symptoms after bacille calmette-guerin instillation therapy for non-muscle-invasive bladder cancer. Urology. 2012;80(3SUPPL. 1):S221.
- Schmidt S, Riel R, Frances A, et al. Bladder cancer index: cross-cultural adaptation into Spanish and psychometric evaluation. Health Qual Life Outcomes. 2014;12(1):20. doi:10.1186/1477-7525-12-20
- Park J, Shin DW, Kim T-H, et al. Development and validation of the korean version of the european organization for research and treatment of cancer quality of life questionnaire for patients with non-muscle invasive bladder cancer: EORTC QLQ-NMIBC24. Cancer Res Treat. 2018;50(1):40–49. doi:10.4143/crt.2016.594
- Sapre N, Wooten A, Siddons H, et al. Tumour recurrence and intravesical BCG significantly impact upon health related quality of life in patients undergoing conservative management for superficial bladder cancer. Eur Urol Suppl. 2013;12(1):e704. doi:10.1016/S1569-9056(13)61186-0
- Wei L, Li Q, Liang H, Jianbo L. The quality of life in patients during intravesical treatment and correlation with local symptoms. J Chemotherapy. 2014;26(3):165–168. doi:10.1179/1973947813Y.0000000126
- Schmidt S, Frances A, Lorente Garin JA, et al. Quality of life in patients with non-muscle-invasive bladder cancer: one-year results of a multicentre prospective cohort study. Urol Oncol. 2015;33(1):19.e7–19.e15. doi:10.1016/j.urolonc.2014.09.012
- Krajewski W, Halska U, Poletajew S, et al. Influence of transurethral resection of bladder cancer on sexual function, anxiety, and depression. Adv Exp Med Biol. 2018;1116(0121103, 2lu):37–50.
- Steinberg RL, Thomas LJ, Mott SL, O’donnell MA. Multi-perspective tolerance evaluation of bacillus calmette-guerin with interferon in the treatment of non-muscle invasive bladder cancer. Bladder Cancer. 2019;5(1):39–49. doi:10.3233/BLC-180203
- Singer S, Ziegler C, Schwalenberg T, Hinz A, Gotze H, Schulte T. Quality of life in patients with muscle invasive and non-muscle invasive bladder cancer. Support Care Cancer. 2013;21(5):1383–1393. doi:10.1007/s00520-012-1680-8
- Ho B, Kan C, To H, et al. Health related quality of life after one-year maintenance intravesical Bacillus Calmette-Guerin for non-muscle invasive bladder: experience from a urology centre in Hong Kong. BJU Int. 2012;111(4–5):5.
- Chung J, Kulkarni GS, Morash R, et al. Assessment of quality of life, information, and supportive care needs in patients with muscle and non-muscle invasive bladder cancer across the illness trajectory. Supportive Care Cancer. 2019;27(10):3877–3885. doi:10.1007/s00520-019-4649-z
- Fung C, Pandya C, Guancial E, et al. Impact of bladder cancer on health related quality of life in 1,476 older Americans: a cross-sectional study. J Urology. 2014;192(3):690–695. doi:10.1016/j.juro.2014.03.098
- Brisbane WG, Holt SK, Winters BR, et al. Nonmuscle invasive bladder cancer influences physical health related quality of life and urinary incontinence. Urology. 2019;125:146–153. doi:10.1016/j.urology.2018.11.038
- Smith AB, Jaeger B, Pinheiro LC, et al. Impact of bladder cancer on health-related quality of life. BJU Int. 2018;121(4):549–557. doi:10.1111/bju.14047
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi:10.1097/01.MLR.0000062554.74615.4C
- Martinez-Pineiro L, Portillo JA, Fernandez JM, et al. Maintenance therapy with 3-monthly bacillus calmette-guerin for 3 years is not superior to standard induction therapy in high-risk non-muscle-invasive urothelial bladder carcinoma: final results of randomised cueto study 98013. Eur Urol. 2015;68(2):256–262. doi:10.1016/j.eururo.2015.02.040
- Tan WS, Panchal A, Buckley L, et al. Radiofrequency-induced thermo-chemotherapy effect versus a second course of bacillus calmette-guerin or institutional standard in patients with recurrence of non-muscle-invasive bladder cancer following induction or maintenance bacillus calmette-guerin therapy (hymn): a phase iii, open-label, randomised controlled trial. Eur Urol. 2019;75(1):63–71.
- Di Lorenzo G, Perdona S, Damiano R, et al. Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. 2010;116(8):1893–1900.
- Mondal HP, Yirang K, Mukhopadhyay C, Adhikary SS, Dutta B, Bhoj SS. Prospective randomized study between intravesical BCG and mitomycin-C for non-muscle-invasive urothelial carcinoma of urinary-bladder post TURBT. Bangladesh J Med Sci. 2016;15(1):74–77. doi:10.3329/bjms.v15i1.19161
- Miyazaki J, Hinotsu S, Ishizuka N, et al. Adverse reactions related to treatment compliance during BCG maintenance therapy for non-muscle-invasive bladder cancer. Jpn J Clin Oncol. 2013;43(8):827–834. doi:10.1093/jjco/hyt086
- Kamel AI, El Baz AG, Abdel Salam WT, El Din Ryad ME, Mahena AA. Low dose BCG regimen in T1 transitional cell carcinoma of the bladder: long term results. J Egypt Natl Cancer Inst. 2009;21(2):151–155.
- Wilson K, Malangone-Monaco E, Satram-Hoang S, et al. A real-world study of patterns of Bacillus Calmette-Guerin (BCG) use and associated adverse events (AEs) in non-muscle invasive bladder cancer (NMIBC) patients in the United States. Ann Oncol. 2016;27(Supplement6):vi355. doi:10.1093/annonc/mdw377.16
- Guerrero-Ramos F, Lara-Isla A, Justo-Quintas J, Duarte-Ojeda JM, de la Rosa-kehrmann F, Villacampa-Auba F. Adjuvant intravesical treatment for nonmuscle invasive bladder cancer: the importance of the strain and maintenance. Actas Urol Esp. 2017;41(9):590–595. doi:10.1016/j.acuro.2017.03.003
- Takeda T, Kikuchi E, Yuge K, et al. Discontinuance of bacille Calmette-Guerin instillation therapy for nonmuscle-invasive bladder cancer has negative effect on tumor recurrence. Urology. 2009;73(6):1318–1322. doi:10.1016/j.urology.2008.12.039
- Prasanna T, Craft P, Balasingam G, Haxhimolla H, Pranavan G. Intravesical gemcitabine versus intravesical bacillus calmette-guerin for the treatment of non-muscle invasive bladder cancer: an evaluation of efficacy and toxicity. Front Oncol. 2017;7(NOV):260. doi:10.3389/fonc.2017.00260
- Torres J, Travis D, Wong S. Standard of care for non-muscle invasive bladder carcinoma is induction followed by maintenance intra-vesical BCG therapy after trans-urethral bladder tumour resection. But how well is it tolerated? Asia-Pac J Clin Oncol. 2015;11(SUPPL.2):41.
- Udovicich C, Nankivell P, Barberi A, et al. Intravesical BCG instillation for non-muscle invasive bladder cancer: prevalence of adverse effects & association with efficacy. BJU Int. 2015;115(SUPPL.4):106–107.
- Kanagawa Urological Research Group (KURG). A 2-week maintenance regimen of intravesical instillation of bacillus Calmette-Guerin is safe, adherent and effective in patients with non-muscle-invasive bladder cancer: a prospective, multicenter phase II clinical trial. Jpn J Clin Oncol. 2012;42(9):813–819. doi:10.1093/jjco/hys097
- Nepple KG, Lightfoot AJ, Rosevear HM, O’Donnell MA, Lamm DL. Bladder Cancer Genitourinary Oncology Study Group. Bacillus calmette-guérin with or without interferon α-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J Urol. 2010;184(5):1915–1919.
- Hayashi T, Yuasa T, Sano M, et al. A prospective randomized trial of intravesical bacillus calmette-guerin therapy with the Tokyo172 versus connaught strain for non-muscle invasive bladder cancer. J Urol. 2015;193(4SUPPL. 1):e297. doi:10.1016/j.juro.2015.02.1132
- Parikh A, Tuli A, Laddha P, Singh A, Mammen K. BCG immunotherapy in superficial transitional cell carcinoma of bladder-tertiary care centre experience. Indian J Urol. 2017;33(Supplement1).
- Damiano R, De Sio M, Quarto G, et al. Short-term administration of prulifloxacin in patients with nonmuscle-invasive bladder cancer: an effective option for the prevention of bacillus Calmette-Guerin-induced toxicity? BJU Int. 2009;104(5):633–639. doi:10.1111/j.1464-410X.2009.08469.x
- Othman B, Steiner D, Wallace S, Travis D. Intravesical BCG for patients with non muscle invasive bladder cancer (NMIBC): do local results reflect those in the published literature. BJU Int. 2013;111(SUPPL.1):115.
- Serretta V, Scalici Gesolfo C, Alonge V, Cicero G, Moschini M, Colombo R. Does the compliance to intravesical BCG differ between common clinical practice and international multicentric trials? Urol Int. 2016;96(1):20–24. doi:10.1159/000430501
- Witjes JA, Palou J, Soloway M, et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): results of an international individual patient data survey (IPDS). BJU Int. 2013;112(6):742–750.
- Chiapparrone G, Gesolfo CS, Solazzo A, et al. Compliance with one year maintenance intravesical BCG in patients affected by t1g3 bladder cancer. Anticancer Res. 2013;33(5):2279–2280.
- Tapiero S, Helfand A, Kedar D, et al. Patient compliance with maintenance intravesical therapy for nonmuscle invasive bladder cancer. Urology. 2018;118:107–113. doi:10.1016/j.urology.2018.04.039
- Lam S, Yang Y, Lo A, Liu M, Ng A, Tsu J. Intravesical bacillus calmette-guerin (IV BCG) treatment for bladder cancer - working toward better patient outcome. Int J Urol. 2017;24(Supplement1):63–64.
- Kan RWM, Chung VY, Fan CW. Long-term results of 1-year maintenance intravesical bacille calmette guerin (BCG) therapy with simplified schedule: less is more or less? BJU Int. 2014;113(SUPPL.1):4.
- Grant T, Donaldson I, Kadi N, Thomas S, Ratan H. Natural history and outcome of patients with high risk non-muscle invasive bladder cancer treated with Bacille Calmette Guerin: A contemporary UK series. J Urol. 2012;187(4SUPPL. 1):e676. doi:10.1016/j.juro.2012.02.1531
- Babjuk M, Burger M, Comperat E, et al. EAU Guidelines: Non-Muscle-Invasive Bladder Cancer. EAU Guidelines Office; 2017.
- Chang SS, Bochner BH, Chou R, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urology. 2017;198(3):552–559. doi:10.1016/j.juro.2017.04.086
- Kubota Y, Nakaigawa N. Committee for establishment of the clinical practice guideline for the management of bladder cancer and the japanese urological association. essential content of evidence-based clinical practice guidelines for bladder cancer: the japanese urological association 2015 update. Int J Urol. 2016;23(8):640–645.
- National Cancer Institute. Definition of cystectomy. Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cystectomy.
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi:10.1093/jnci/djq495
- Mossanen M, Wang Y, Szymaniak J, et al. Evaluating the cost of surveillance for non-muscle-invasive bladder cancer: an analysis based on risk categories. World J Urol. 2019;37(10):2059–2065.
- Hollenbeck BK, Ye Z, Dunn RL, Montie JE, Birkmeyer JD. Provider treatment intensity and outcomes for patients with early-stage bladder cancer. J Natl Cancer Inst. 2009;101(8):571–580. doi:10.1093/jnci/djp039
- Hollingsworth JM, Zhang Y, Krein SL, Ye Z, Hollenbeck BK. Understanding the variation in treatment intensity among patients with early stage bladder cancer. Cancer. 2010;116(15):3587–3594. doi:10.1002/cncr.25221
- De Jong IJ, Jonker L, Weerink M, Cornel E, Luijendijk D, Leliveld A. The clinical impact of routine bladder biopsy after BCG treatment of high grade non muscle invasive bladder cancer. J Clinical Oncol. 2016;2 SUPPL. 1.
- Udell I, Kurpad R, Angela B, et al. The cost impact of routine addition of urovysion FISH early postoperative to non-muscle-invasive bladder cancer surveillance. J Am Coll Surg. 2012;215(3SUPPL. 1):S145–S146.
- Vecino-Oritz AI, Glover R, Adams EJ. Modelling the burden of bladder cancer and monitoring cystoscopies in Europe. European Urology Supplements. 2016;15(3):e203. doi:10.1016/S1569-9056(16)60205-1
- Dansk V, Malmstrom P-U, Blackberg M, Malmenas M. Hexaminolevulinate hydrochloride blue-light flexible cystoscopy in the detection and follow-up of nonmuscle-invasive bladder cancer: cost consequences during outpatient surveillance in Sweden. Future Oncology. 2016;12(8):1025–1038. doi:10.2217/fon-2015-0021
- Ihara Z, Furneri G, Haycock L, Chowdhury CA, Besser V. Economic value of narrow band imaging versus white light endoscopy for the management of non-muscle invasive bladder cancer: cost-consequence model. Value Health. 2018;Supplement 3:S253–S254. doi:10.1016/j.jval.2018.09.1514
- Klaassen Z, Li K, Kassouf WW, Black PC, Dragomir A, Kulkarni GS. Contemporary cost-consequences analysis of blue light cystoscopy with hexaminolevulinate in non-muscle invasive bladder cancer (CUA prize winner). Canadian Urological Association J. 2017;6 Supplement 4:S248.
- Feifer A, Xie X, Brophy JM, Segal R, Kassouf W. Contemporary cost analysis of single instillation of mitomycin after transurethral resection of bladder tumor in a universal health care system. Urology. 2010;76(3):652–656. doi:10.1016/j.urology.2009.12.070
- Lee CT, Globe D, Colayco D, Gilmore A, Bramley T. Economic consequences of preventable bladder tumor recurrences in non-muscle invasive bladder cancer. Value Health. 2011;3:A179. doi:10.1016/j.jval.2011.02.989
- Al Hussein Al Awamlh B, Lee R, Chughtai B, Donat SM, Sandhu JS, Herr HW. A cost-effectiveness analysis of management of low-risk non-muscle-invasive bladder cancer using office-based fulguration. Urology. 2015;85(2):381–386. doi:10.1016/j.urology.2014.09.041
- Moltke A-L, Drejer D, Jensen JB. Photodynamic diagnosis (PDD) in flexible cystoscopy-impact on effectiveness and costs. A randomized controlled trial. J Urology. 2017;220:37–38.
- Garfield SS, Gavaghan MB, Armstrong SO, Jones JS. The cost-effectiveness of blue light cystoscopy in bladder cancer detection: united States projections based on clinical data showing 4.5 years of follow up after a single hexaminolevulinate hydrochloride instillation. J Urology. 2013;20(2):6682–6689.
- Green DA, Rink M, Cha EK, et al. Cost-effective treatment of low-risk carcinoma not invading bladder muscle. BJU Int. 2013;111(3Pt B):E78–84. doi:10.1111/j.1464-410X.2012.11454.x
- Hermann GG, Mogensen K, Rosthoj S. Outpatient diode laser treatment of intermediate-risk non-invasive bladder tumors without sedation: efficacy, safety and economic analysis. Scand J Urol. 2018;52(3):194–198. doi:10.1080/21681805.2018.1450782
- Jablonowski Z, Konecki T, Ziobro M, et al. Cost-utility of hexaminolevulinate blue light cystoscopy (HAL) assisted transurethral resection of the bladder tumour (TURB) compared to TURB with white light cystoscopy (WLC) alone in patients with non-muscle invasive bladder cancer (NIMBC) in Poland. Value Health. 2015;18(7):A360. doi:10.1016/j.jval.2015.09.694
- James P, Pai A, Chetwood A, et al. Financial benefit of tula (transurethral laser ablation) for recurrent non-muscle invasive bladder cancer. J Endourol. 2017;Supplement 2:A396–A397.
- Klaassen Z, Black PC, Kulkarni GS. A cost-effectiveness analysis of hexaminolevulinate blue light-assisted transurethral resection of bladder tumours in a universal healthcare system. Canadian Urological Association J. 2017;11(6Supplement 4):S212–S213.
- Malmstrom P-U, Hedelin H, Thomas YK, Thompson GJ, Durrant H, Furniss J. Fluorescence-guided transurethral resection of bladder cancer using hexaminolevulinate: analysis of health economic impact in Sweden. J Urology. 2009;43(3):192–198.
- Marteau F, Kornowski A, Bennison C, Tempest MJ, Mariappan P, Witjes JA. Cost-effectiveness of the optical imaging agent hexaminolevulina te for patients with non-muscle invasive bladder cancer. Value Health. 2013;16(7):A408–A409. doi:10.1016/j.jval.2013.08.493
- Rose JB, Armstrong S, Hermann GG, Kjellberg J, Malmstrom P-U. Budget impact of incorporating one instillation of hexaminolevulinate hydrochloride blue-light cytoscopy in transurethral bladder tumour resection for patients with non-muscle-invasive bladder cancer in Sweden. BJU Int. 2016;1(6B):E102–113.
- Wong KA, Zisengwe G, Athanasiou T, O’Brien T, Thomas K. Outpatient laser ablation of non-muscle-invasive bladder cancer: is it safe, tolerable and cost-effective? BJU Int. 2013;112(5):561–567. doi:10.1111/bju.12216
- Bobman J, Deibert C, Ahn J, Stevenson S, Benson M, McKiernan J. Evaluating cost and quality of life in non-muscle invasive bladder cancer. J Urol. 2013;189(4SUPPL. 1):e174. doi:10.1016/j.juro.2013.02.1817
- Lee CT, Barocas D, Globe DR, et al. Economic and humanistic consequences of preventable bladder tumor recurrences in nonmuscle invasive bladder cancer cases. J Urol. 2012;188(6):2114–2119. doi:10.1016/j.juro.2012.08.005
- de Bekker-grob EW, van der Aa MNM, Zwarthoff EC, et al. Non-muscle-invasive bladder cancer surveillance for which cystoscopy is partly replaced by microsatellite analysis of urine: a cost-effective alternative? BJU Int. 2009;104(1):41–47. doi:10.1111/j.1464-410X.2008.08323.x
- Fujita N, Momota M, Tobisawa Y, et al. Risk-stratified surveillance and cost effectiveness of follow-up after trans-urethral resection of bladder tumor in patients with primary non-muscle-invasive bladder cancer. European Urology Supplements. 2019;18(1):e951–e952. doi:10.1016/S1569-9056(19)30691-8
- Heijnsdijk EAM, Nieboer D, Garg T, Lansdorp-Vogelaar I, de Koning HJ, Nielsen ME. Cost-effectiveness of surveillance schedules in older adults with non-muscle-invasive bladder cancer. BJU Int. 2019;123(2):307–312. doi:10.1111/bju.14502
- Ok BG, Ji YS, Ko YH, Song PH. Usefulness of urine cytology as a routine work-up in the detection of recurrence in patients with prior non-muscle-invasive bladder cancer: practicality and cost-effectiveness. J Urology. 2014;55(10):650–655.
- Bachir BG, Dragomir A, Aprikian AG, et al. Contemporary cost-effectiveness analysis comparing sequential bacillus Calmette-Guerin and electromotive mitomycin versus bacillus Calmette-Guerin alone for patients with high-risk non-muscle-invasive bladder cancer. Cancer. 2014;120(16):2424–2431. doi:10.1002/cncr.28731
- Bekcic S, Mitrovic L, Baltezarevic D, Radojevic V, Samardzic J, Milenkovic V. Pricing and reimbursement analysis of bacillus calmette-guerin (BCG) immunotherapy for bladder cancer. Value Health. 2014;7:A654.
- Ramamohan V, Mladsi DM, Boey W, Pozzi R, Kaye JA. An outcomes model for high-risk non-muscle-invasive bladder cancer treatment options. Value Health. 2014;3:A87. doi:10.1016/j.jval.2014.03.505
- Ourfali S, Ohannessian R, Fassi-Fehri H, Pages A, Badet L, Colombel M. Recurrence rate and cost consequence of the shortage of bacillus calmette-guerin connaught strain for bladder cancer patients. Eur Urol Focus. 2019. doi:10.1016/j.euf.2019.04.002
- Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA. Economic burden of bladder cancer across the European Union. Eur Urol. 2016;69(3):438–447. doi:10.1016/j.eururo.2015.10.024
- Gerace C, Montorsi F, Tambaro R, et al. Cost of illness of urothelial bladder cancer in Italy. Clinicoecon Outcomes Res. 2017;9:433–442. doi:10.2147/CEOR.S135065
- Avritscher EBC, Cooksley CD, Grossman HB, et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68(3):549–553.
- Kulkarni GS, Alibhai SMH, Finelli A, et al. Cost-effectiveness analysis of immediate radical cystectomy versus intravesical Bacillus Calmette-Guerin therapy for high-risk, high-grade (T1G3) bladder cancer. Cancer. 2009;115(23):5450–5459. doi:10.1002/cncr.24634
- Cascone V, Rizza G, Smeriglio A, Tomaino A. Lack of BCG for the treatment of bladder cancer: pharmacoeconomic and clinical impact. Eur J Hosp Pharm. 2014;21(Suppl1):A78.2–A78. doi:10.1136/ejhpharm-2013-000436.192
- Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. doi:10.4065/mcp.2010.0575
- Tsang J, Rudychev I, Pescatore SL. Prescription compliance and persistency in chronic myelogenous leukemia (CML) and gastrointestinal stromal tumor (GIST) patients (pts) on imatinib (IM). JCO. 2006;24(18_suppl):6119. doi:10.1200/jco.2006.24.18_suppl.6119
- RAND Healthcare. 36-Item Short Form Survey Instrument (SF-36). Available at: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/survey-instrument.html.
- Blazeby JM, Hall E, Aaronson NK, et al. Validation and reliability testing of the EORTC QLQ-NMIBC24 questionnaire module to assess patient-reported outcomes in non-muscle-invasive bladder cancer. Eur Urol. 2014;66(6):1148–1156. doi:10.1016/j.eururo.2014.02.034
- Danna BJ, Metcalfe MJ, Wood EL, Shah JB. Assessing symptom burden in bladder cancer: an overview of bladder cancer specific health-related quality of life instruments. Bladder Cancer. 2016;2(3):329–340. doi:10.3233/BLC-160057
- EORTC. EORTC questionnaires: quality of life of cancer patients. EORTC – Quality of Life.
- Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer. Pharmaco Economics. 2003;21(18):1315–1330. doi:10.1007/BF03262330
- Han KS, Hong SJ. Management of BCG failures in non-muscle-invasive bladder cancer. Korean J Urol. 2009;50(11):1037–1047. doi:10.4111/kju.2009.50.11.1037
- Şanlı Ö, Lotan Y. Alternative therapies in patients with non-muscle invasive bladder cancer. Turk J Urol. 2017;43(4):414–424. doi:10.5152/tud.2017.64624
- de Jong JJ, Hendricksen K, Rosier M, Mostafid H, Boormans JL. Hyperthermic intravesical chemotherapy for BCG unresponsive non-muscle invasive bladder cancer patients. BLC. 2018;4(4):395–401. doi:10.3233/BLC-180191
- Nativ O, Witjes JA, Hendricksen K, et al. Combined thermo-chemotherapy for recurrent bladder cancer after bacillus calmette-guerin. J Urology. 2009;182(4):1313–1317. doi:10.1016/j.juro.2009.06.017
- Liu K, Zhu J, Song Y-X, et al. Thermal intravesical chemotherapy reduce recurrence rate for non-muscle invasive bladder cancer patients: a meta-analysis. Front Oncol. 2020;10:29. doi:10.3389/fonc.2020.00029
- Sousa-Escandon MA, Flores Carbajal J, Sousa-Gonzalez D, Rodriguez-Gomez S. Intravesical chemohyperthermia for nmibc: rationale and results of this developing treatment. In: Ather MH, editor. Bladder Cancer - Management of NMI and Muscle-Invasive Cancer. InTech; 2017.
- Sousa A, Inman BA, Pineiro I, et al. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. Int J Hyperthermia. 2014;30(3):166–170. doi:10.3109/02656736.2014.900194
- American Urological Association. American Urological Association BCG shortage notice. Available at: https://www.auanet.org/practice-resources/BCG-info/BCG-shortage-notice.
- Jung A, Nielsen ME, Crandell JL, et al. Health-related quality of life among non-muscle-invasive bladder cancer survivors: a population-based study. BJU Int. 2020;125(1):38–48. doi:10.1111/bju.14888
- González-Padilla DA, González-Díaz A, Guerrero-Ramos F, et al. Quality of life and adverse events in patients with nonmuscle invasive bladder cancer receiving adjuvant treatment with BCG, MMC, or chemohyperthermia. Urol Oncol. 2020. doi:10.1016/j.urolonc.2020.07.003
- Sharma V, Wymer KM, Borah BJ, et al. Cost-effectiveness of maintenance bacillus calmette-guérin for intermediate and high risk nonmuscle invasive bladder cancer. J Urol. 2020;204(3):442–449. doi:10.1097/JU.0000000000001023
