Abstract
Background
The safety and efficacy of racecadotril to treat acute watery diarrhea (AWD) in children is well established, however its cost effectiveness for infants and children in Europe has not yet been determined.
Objective
To evaluate the cost utility of racecadotril adjuvant with oral rehydration solution (ORS) compared to ORS alone for the treatment of AWD in children younger than 5 years old. The analysis is performed from a United Kingdom National Health Service (NHS) perspective.
Methods
A decision tree model has been developed in Microsoft® Excel. The model is populated with the best available evidence. Deterministic and probabilistic sensitivity analyses (PSA) have been performed. Health effects are measured as quality-adjusted life years (QALYs) and the model output is cost (2011 GBP) per QALY. The uncertainty in the primary outcome is explored by probabilistic analysis using 1000 iterations of a Monte Carlo simulation.
Results
Deterministic analysis results in a total incremental cost of −£379 in favor of racecadotril and a total incremental QALY gain in favor of racecadotril of +0.0008. The observed cost savings with racecadotril arise from the reduction in primary care reconsultation and secondary referral. The difference in QALYs is largely attributable to the timely resolution of symptoms in the racecadotril arm. Racecadotril remains dominant when base case parameters are varied. Monte Carlo simulation and PSA confirm that racecadotril is the dominant treatment strategy and is almost certainly cost effective, under the central assumptions of the model, at a commonly used willingness to pay proxy threshold range of £20,000–£30,000 per QALY.
Conclusion
Racecadotril as adjuvant therapy is more effective and less costly compared to ORS alone, from a UK payer perspective, for the treatment of children with acute diarrhea.
Introduction
Despite advances in medicine over the last decades, acute watery diarrhea (AWD) remains the cause of substantial morbidity in developed countries.Citation1 In England, approximately 150,000 children younger than 5 years old present with symptoms of vomiting and diarrhea (gastroenteritis) each year. In 2007–2008, approximately 37,000 children were admitted to secondary care hospitals.Citation2 Oral rehydration solution (ORS) is the standard of care to replace fluid loss, but it has no impact on the duration or severity of diarrhea. There is limited availability of alternate therapies, especially those indicated for infants and children, which reduce the duration and severity of diarrhea. Racecadotril is a nonopiate enkephalinase inhibitor with a unique mechanism of action with rapid onset.Citation3 The basally abundant enzyme enkephalinase degrades endogenous enkephalins in the intestinal mucosa that otherwise exhibit proabsorptive and antisecretory properties. Racecadotril prevents the degradation of enkephalins and thereby promotes antisecretion.Citation4 Furthermore, it has no effect on intestinal motility and therefore no enteropooling or rebound constipation is experienced. There is also no respiratory depression or other central neurotoxicity effect,Citation5–Citation7 making this a favorable drug option, especially for children.Citation4
Pediatric presentations of racecadotril were first authorized in France in 1999 and today it is approved and widely used in seven European countries (France, Spain, Italy, Portugal, Greece, Bulgaria, and Romania) and outside Europe. The efficacy of racecadotril has been well established in clinical studies and in an individual patient data meta-analysis.Citation8–Citation19 In addition, the safety of racecadotril in children has been demonstrated in clinical studies, including a large pre- and postaccess study showing that racecadotril has a favorable adverse event (AE) profile in children.Citation9,Citation12–Citation14,Citation19–Citation21 Despite its proven safety and efficacy, the cost effectiveness of racecadotril for infants and children has not yet been determined in Europe. The objective of this analysis is to evaluate the cost effectiveness of racecadotril from a UK payer perspective.
Evidence base
A systematic literature search and review identified potential clinical studies and quality of life (QoL) and resource utilization data to populate the model. Focused literature searches have been performed in databases, including PubMed, Embase, and the Cochrane library. A detailed search strategy is available upon request. Available and relevant evidence has been weighed against each other to determine the highest quality for inclusion in the model.
Model
Structure
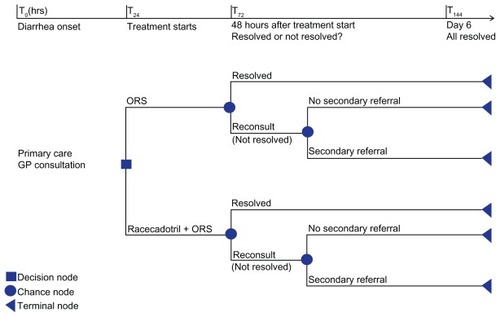
The Racecadotril in Acute Watery Diarrhea (RAWD) model is a decision tree programmed in Microsoft® Excel (Microsoft, Redmond, WA) and developed to evaluate the treatment of racecadotril as adjuvant to ORS (hereafter referred to only as racecadotril). The chosen comparator is ORS, which is the currently recommended treatment in the National Health Service (NHS) according to the National Institute for Health and Clinical Excellence (NICE).Citation2 The model performs deterministic and probabilistic sensitivity analyses and considers costs and outcomes from the United Kingdom (UK) NHS perspective. Health effects are measured as quality-adjusted life years (QALYs) and the model output is cost (GBP) per QALY. The model uses a time horizon of 6 days based on the general assumption that nonsevere acute diarrhea is self-limiting to 6 days, and therefore no discounting is applied. According to the decision tree, children with symptoms of AWD present to primary care and are prescribed racecadotril or ORS alone. Forty-eight hours after the start of treatment, children with unresolved symptoms will reconsult at primary care, whereupon they may continue treatment as before, or if symptoms have worsened, they may be referred to secondary care. The face validity of this treatment pathway has been verified by two clinical expertsCitation22,Citation23 and appears to agree with the literature reviewed and the management of diarrhea alluded to in the guidelines of the World Gastroenterology Organization, NICE, and the World Health Organization.Citation1,Citation2,Citation24 The models’ structure is illustrated in .
Base case parameters
Clinical effectiveness data is available from ten individual studies,Citation8–Citation15 two systematic reviews,Citation16,Citation17 and two meta-analyses.Citation18,Citation19 The first systematic review, published in 2007,Citation16 includes three studies in the analysis.Citation8,Citation9,Citation11 The results show that 48-hour stool output is significantly less in the racecadotril group than in the control group, with a standardized mean difference (SMD) of −0.67; 95% confidence interval [CI] (−0.9 to −0.44). The final outcome assessed is cure in 5 days or less, and pooled results show no significant difference between the racecadotril group and the control group (risk ratio [RR] 1.1; 95% CI: 0.97 to 1.21). It can, however, be argued that this endpoint is flawed because all patients recovered by day five; the difference in recovery is significant around day two, as demonstrated by Lehert et al.Citation19
The second systematic review, performed in 2008,Citation17 includes two studies in the analysis.Citation8,Citation9 It shows that stool volume at 48 hours is less in the racecadotril group than in the control group (SMD −0.65; 95% CI: −0.88 to −0.42). Again, there is no difference in the proportion of children who recover by day five (RR 0.73; 95% CI: 0.29 to 1.81).Citation17 Again, individual patient meta-analysis shows that the difference in recovery is significant around day two.Citation19
The first meta-analysisCitation18 includes four studiesCitation8,Citation9,Citation11,Citation12 and shows a significant difference in stool output at 48 hours in favor of racecadotril (P = 0.00001).Citation8,Citation9 Heterogeneity between studies was not found statistically significant (P = 0.86; I2 = 0%). The study also shows a statistically significant difference for children who revisited a pediatrician or emergency department 48 hours after treatment favoring racecadotril (RR 0.62; 95% CI: 0.40 to 0.97; P = 0.04) with homogeneity between studies (P = 0.41; I2 = 0%).Citation11,Citation12 The study further confirmed that racecadotril is comparable to ORS alone with respect to safety and tolerability.Citation20
The second meta-analysis by Lehert et al includes nine studies (one publication reports two trials)Citation8–Citation15 and aims to overcome the shortcomings of previous studies in general, which do not include all existing trials, estimate treatment effect using meta-analysis in literature, pool different endpoints, and do not explore the influence of baseline conditions on treatment effect (eg, dehydration level, rotavirus status, age, inpatient/outpatient setting, country).Citation19 The study is an individual patient (raw data) meta-analysis that includes eight out of the ten available studiesCitation19 (one excluded study compares racecadotril to loperamideCitation21 and the second was an open-label study that did not meet quality requirements).Citation25 It is considered by the model developers to be the highest quality evidence and considers all relevant studies, including those with less favorable results for racecadotril, it is therefore used for the RAWD model. Results show that twice as many patients recover at any time with the racecadotril regimen: hazard ratio (HR) 2.04 (95% CI: 1.85 to 2.32; P < 0.001).Citation19 To convert this to a proportion in the model, a Weibull distribution is fitted to the data and the proportional hazards assumption is used to vary the efficacy of the racecadotril arm with respect to the ORS arm. Using this distribution, the median for the racecadotril and ORS arms is reached at 1.75 and 2.79 days, respectively, and results in the reported HR at 2.75 days in agreement with the study data, which suggests a suitable fit. This distribution shows that after 48 hours of treatment, 58% and 26% of patients respond in the racecadotril and ORS arms, respectively. These results appear to correspond to the reductions in 48-hour stool volume and 48-hour stool output reported in other studies.Citation16–Citation19 As a conservative estimate, in the model it has been assumed that all children with unresolved symptoms at 48 hours reconsult their primary care facility.
A study by Cojocaru et al evaluated children presenting to an emergency department (A & E) for diarrhea.Citation11 Children were admitted for 24 hours at presentation and a scheduled visit on day 2 was included in the study protocol for all patients. Additional medical visits (>day 2 ≤day 7) were required for 34% of children in the ORS arm and 18% of children in the racecadotril arm (P < 0.05).Citation11 Secondary referral was measured after day 2. The details of this study do not typically represent the scenario modeled, which is the UK primary care setting; specifically, the model does not consider children presenting to A & E instead of primary care for AWD. Only one study includes secondary referral data at 48 hours and demonstrates fewer hospital admissions for racecadotril compared to ORS at 24 hours (P < 0.005) and 48 hours (P < 0.0001); it has been included in the model.Citation13
It is difficult to measure QoL in young children, however, there are three potentially relevant publications. In one, Huppertz et al analyzes parental perception of the impact of diarrhea on the QoL of their children.Citation26 A second study evaluates the QoL in children with gastroenteritis in Canada.Citation27 A third study rates the QoL of children with acute infectious gastroenteritis.Citation28 In this study, two health state descriptions (for primary and secondary care) have been rated by general practitioners and pediatricians across five geographic areas in the UK. EuroQoL (EQ-5D) scores have been converted to utility values using UK population data and the time trade-off method.Citation28 Secondary care ratings from general practitioners are more conservative than pediatricians and are therefore used in the model to calculate an average for children younger than 5 years old. It has been assumed that children with AWD have comparable QoL to those with acute infectious diarrhea; the same utility values have been used elsewhere for rotavirus infection.Citation29 The Martin, Cottrell, and Standaert study is considered the highest quality and most relevant evidence because it includes EQ-5D data; it is based on UK population norms and utilizes the time trade-off approach and has therefore been used in the RAWD model.Citation28
For the AE data, the following frequencies for racecadotril vs ORS are reported: 12.4% vs 16.2%,Citation20 11.6% vs 10.1%,Citation19 19.1% vs 20.2%,Citation12 11.5% vs 22% (vs loperamide),Citation21 10% vs 7% (vs placebo),Citation9 5%–6% (ORS arm not reported),Citation14 and 6% (ORS arm not reported).Citation13 The highest frequency of AEs comes from a study by Santos et al, involving 84 patients.Citation12 By far the most extensive data comes from a pre-and postaccess analysis of individual case safety reports and clinical trial AEs from a total sample of 1129 records.Citation20 The Baumer and Joulin study is based on the largest sample size and is comparable and/or conservative in comparison to the other estimates listed above; it is considered fair to include it as the highest quality evidence for the frequency of AEs in the model.
The drug cost is calculated at a dose of 1.5 mg per kilogram (kg) of body weight administered three times daily. The average weight of a child is assumed to be 13.5 kg, based on an average estimate from multiple sources.Citation30–Citation33 ORS has been conservatively estimated to be administered after every stool in accordance with the definition of AWD, which states at least three watery stools are passed in 24 hours.Citation1 The weighted average cost of an ORS sachet is calculated at £0.29 per sachet, at a total daily cost of £0.88.Citation34 The drug cost of racecadotril is £0.42 per 10 mg sachetCitation35, resulting in a total daily drug cost of £3.82 (including ORS).
The national average cost of a primary care consultation of £36 (based on an 11.7 minute consultation) and the national average cost of secondary care for a nonelective short inpatient stay of £523 are used in the model.Citation36 Hospital Episode Statistics for England (Inpatient) 2010–2011 are used to estimate the average length of stay for deterministic analysis.Citation37 Hospital Resource Group 4 (HRG4) and ICD-10Citation38 data are shown in . The value of 2.65 days (3 days) is used for deterministic analysis based on the average of ICD-10 A00-A09 (intestinal infectious diseases) and HRG4 PA21B (infectious and noninfectious gastroenteritis without complications and comorbidities) codes, those being the only codes with actual numbers of children recorded in the relevant age group (number of children aged 0–14 years: 37,889 for PA21B; 29,689 for A00-A09).Citation37,Citation38
Table 3 HRG4 and ICD-10 data for length of stay
For AEs, Baumer and Joulin report the most common AE to be vomiting (5.1% vs 5.8%), fever (2.3% vs 4.6%), and allergic AEs (1.3% vs 4.1%) for racecadotril and ORS, respectively.Citation20 Half of all AEs reported by individual case safety reports are classified as “skin and subcutaneous tissue disorders”, which are mainly rash and urticaria.Citation20 In the absence of available AE cost data, an average weighted cost for vomiting (£15), allergic AEs (£20), and fever (£50) is used in the model. A summary of the model parameters is shown in .
Table 1 Base case parameters
Model assumptions
The RAWD model assumes that children presenting with clinical dehydration at the first primary care consultation are referred immediately to secondary care in accordance with current clinical practice guidelines and are not included in the modeled population.Citation2 The treatment effect in the model is irrespective of diarrhea duration prior to the first primary care consultation and etiology of diarrhea. Also in accordance with practice guidelines and confirmed by experts in primary care, stool sampling is not routine practice and is not included in the model.Citation22,Citation23 It is assumed that when symptoms of AWD resolve, the treatment stops and the daily drug cost is no longer incurred. Furthermore, if diarrhea has not resolved at first and second primary care consultations, and if there is no secondary referral, the diarrhea will be self-limiting and resolve at day 6 (maximum treatment duration, 6 days). The model considers the drug cost of actual sachets used for each regimen and does not include the effects of wastage or compliance.
Results
The deterministic analysis calculates the cost and benefits over a six-day time horizon. The total drug cost for racecadotril vs ORS is £12.17 vs £3.03, primary care costs are £51.12 vs £62.64, secondary care costs are £40.20 vs £416.82, and AEs cost £0.35 vs £0.46 per patient. The total average cost for racecadotril and ORS treatment regimens is £103.84 vs £482.95, respectively. The most notable cost savings with racecadotril arises from the reduction in secondary care costs. The cost comparison data is summarized in . The total incremental cost is −£379 in favor of racecadotril. The total incremental QALY gain in favor of racecadotril is +0.0008 for a 6-day period. The difference in QALYs is largely attributable to the timely resolution of symptoms in the racecadotril arm. The incremental cost effectiveness ratio is −£473,750 with racecadotril being the dominant treatment strategy, which means that racecadotril (adjuvant) is more efficacious and less costly than ORS alone under the central assumptions of the model.
Table 2 Cost comparison results (deterministic)
Uncertainty
For the deterministic analysis, when the “average length of stay for secondary care” is reduced from three to one night, the incremental cost is −£128 and the incremental QALY gain is +0.0016, resulting in a dominant incremental cost effectiveness ratio of −£80,000 in favor of racecadotril. For AEs, when the least favorable estimateCitation12 is entered for both regimens, the resultant incremental cost effectiveness ratio is −£494,830.Citation12 When both parameters (LOS and AE) are varied simultaneously, the result is −£81,885, with racecadotril remaining the dominant treatment strategy. Varying deterministic base case estimates by 20% (constrained by maximum and minimum values) shows that the model is most sensitive to the QoL estimate of a “well” person. The value is taken from a UK national survey commissioned by the Department of Health and published as a working paper by the Centre for Health Economics, York University.Citation39 A total of 3395 adults in the UK were interviewed using the EQ-5D.Citation39 The base case value of 0.94 was used, which is the mean for those aged under 25 years and is the closest approximation to a child’s full health that is available, to the best of the model developers’ knowledge.
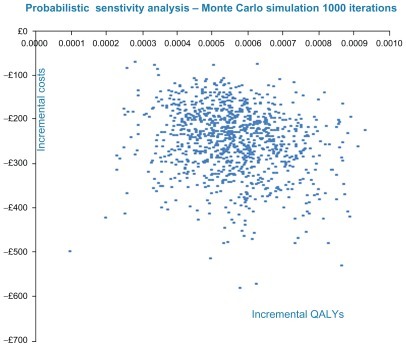
To evaluate the uncertainty in the parameter estimates, a probabilistic sensitivity analysis has been performed by fitting distributions (shown in ) to the deterministic estimates and running 1000 iterations of a Monte Carlo simulation. Probabilistic sensitivity analysis shows that racecadotril is the dominant treatment strategy, it being less costly and more effective compared to ORS alone, as shown in . The cost effectiveness acceptability curve shows that the racecadotril strategy is almost certainly cost effective at a proxy willingness-to-pay threshold range of £20,000–£30,000 per QALY.
Discussion
This model has been developed from a UK perspective and all cost and utility data are derived from British sources. The reader should consider that the available studies for the other base case parameters originate from Europe (three from FranceCitation8,Citation11,Citation21 [one study vs loperamide] and two from SpainCitation12,Citation13) and the rest of the world (Mexico,Citation14 Guatemala,Citation15 India,Citation10 and PeruCitation9). It is generally accepted that the five European studies are more closely representative of the UK health care system. Differences for the rest of the world studies are most likely to arise from differences in resource utilization, assuming that children in developing countries are more likely to have underlying conditions that predispose them to more severe diarrhea and more potential for clinical dehydration (eg, malnutrition, etc) and referral. No resource utilization (reconsultation and referral) data from the rest of world studies is used in the model.
The population modeled in this analysis is infants and children younger than 5 years old. Regarding clinical efficacy, the Lehert et al meta-analysis included nine studies for the determination of diarrhea duration (one study had two sets of results).Citation19 The maximum age in all studies is 5 years (60 months), except in the Melendez Garcia study, which is 71 months.Citation15 The authors note that results are similar for infants aged under 1 year old (n = 714; HR 2.01; 95% CI: 1.71–2.36; P < 0.001) and toddlers aged over 1 year old (n = 670; HR 2.16; 95% CI: 1.83–2.57; P < 0.001). The heterogeneity among studies is small (I2 = 0.28).Citation19 The QoL data from the Martin, Cottrell, and Standaert study includes separate scores for children under 18 months old and 18 month to 5 year olds.Citation28 Raw scores have been modified to account for inability to rate mobility and self-care in very young children. An average utility for both age groups has been calculated and used in the model.Citation28 For AEs, the Baumer and Joulin study does not specify the age range for the data included; however, the authors note that the nature and frequency of AEs does not differ significantly between children aged under 30 months and older children.Citation20 It therefore seems reasonable to assess the impact of AWD in children younger than 5 years old as a group.
In 2009, NICE issued guidance for vomiting and diarrhea in children younger than 5 years old.Citation2 The supplementary costing statement predicts that implementation of the clinical practice guideline is not likely to have significant savings at the national level.Citation40 In view of the evidence available at that time, this is a reasonable assessment; however, in the light of new evidence, there seems to be opportunity for substantial cost savings. The model shows that the first cost driver for diarrhea in the under-5 age group is the reconsultation rate for primary care (not including dehydration). For the UK, this is confirmed by experts, who report reconsultation rates as high as 30%,Citation23 with recent results from Cegedim indicating a 14.3% reconsultation rate in children 4 years old and younger.Citation41 As described, studies generally measure efficacy using stool output/48 hours, number of diarrheic stools, stool volume, and diarrhea duration. The most clinically and economically meaningful result seems to be diarrhea duration, as this has a direct influence on the reconsultation rate for children with unresolved symptoms and affects the QoL of children and their caregivers. By resolving the symptoms of diarrhea within 48 hours, the reconsultation rate can potentially be reduced, with resultant cost savings. The second cost driver identified is the secondary care admission rate, which not only contributes to cost but adds the risk of nosocomial infection.Citation42 Again, by timely resolution of diarrhea and avoidance of secondary referral costs, the burden of resource utilization is alleviated. Furthermore, the model assumes a constant secondary referral rate over time; however, it may be that as the duration of diarrhea increases, the rate of secondary referral increases accordingly. This hypothesis is not extensively explored in the current literature and may be a useful question for future research. If a differential secondary referral rate is proven, then the full benefit of racecadotril shortening disease duration in comparison to ORS aloneCitation19 is underestimated in the RAWD model. A further contributor to cost, which also has not been included in the model, arises from the impact of diarrhea in children on their parents and caregivers. Van der Wielen et al sampled 1102 parents of children younger than 5 years old seeking medical care as a result of acute gastroenteritis. The proportion requiring at least one person to be absent from work was 20%–64% for primary care setting and 39%–91% for secondary care setting.Citation43 From a broader societal perspective, the cost of children’s AWD is therefore likely to be much higher than from a payer’s perspective.
Conclusion
The RAWD model demonstrates that, considering the best available evidence, racecadotril is cost effective in the treatment of AWD in children. The model highlights the potential cost savings arising from reduction in diarrhea duration and avoidance of reconsultation and referral rates in children with diarrhea.
Acknowledgments/disclosures
Doctor Lisa Silver and Doctor Sarah Hall provided expert opinion from the UK NHS. Ute Zerwes and Christoph Schmidt performed literature searches in addition to Tamlyn Rautenberg. Kurt Banz from Outcomes International performed independent external model validation. Rick Aultman provided independent statistical consultation and validation with no compensation from any party. Douglas Foerster is employed by Abbott. The model development was sponsored by Abbott. No medical writing services have been used in the production of this manuscript.
References
- World Gastroenterology Organisation (WGO)World Gastroenterology Organisation practice guideline: Acute diarrhea2008 Available from: http://www.omge.org/assets/downloads/en/pdf/guidelines/01_acute_diarrhea.pdfAccessed on August 12, 2011
- National Collaborating Centre for Women’s and Children’s Health (NCCWCH)Diarrhoea and vomiting diagnosis, assessment and management in children younger than 5 years caused by gastroenteritis2009
- LecomteJMAn overview of clinical studies with racecadotril in adultsInt J Antimicrob Agents2000141818710717506
- FarthingMJAntisecretory drugs for diarrheal diseaseDig Dis2006241–2475816699263
- SpillantiniMGGeppettiPFanciullacciMMichelacciSLecomteJMSicuteriFIn vivo ‘enkephalinase’ inhibition by acetorphan in human plasma and CSFEur J Pharmacol198612511471503015640
- KniselyJSBeardsleyPMAcetoMDBalsterRLHarrisLSAssessment of the abuse potential of acetorphan, an enkephalinase inhibitorDrug Alcohol Depend19892321431512702924
- Duval-IflahYBerardHBaumerPEffects of racecadotril and loperamide on bacterial proliferation and on the central nervous system of the newborn gnotobiotic pigletAliment Pharmacol Ther199913Suppl 691410646046
- CezardJPDuhamelJFMeyerMEfficacy and tolerability of racecadotril in acute diarrhea in childrenGastroenterology2001120479980511231932
- Salazar-LindoESantisteban-PonceJChea-WooEGutierrezMRacecadotril in the treatment of acute watery diarrhea in childrenN Engl J Med2000343746346710944563
- SavitaMRMysoreGMCRacecadotril – a novel drug for the treatment of acute watery diarrhea in Indian children2nd Pediatric Infectious Diseases Conference2006 Available from: http://www.pediatriconcall.com/fordoctor/conference_abstracts/racecadotrial.aspAccessed on September 2, 2011
- CojocaruBBocquetNTimsitSEffect of racecadotril in the management of acute diarrhea in infants and childrenArch Pediatr20029877477912205786
- SantosMMaranonRMiguezCVazquezPSanchezCUse of racecadotril as outpatient treatment for acute gastroenteritis: a prospective, randomized, parallel studyJ Pediatr20091551626719394033
- Alvarez CalatayudEPinei SimonGTaboada CastroLEfectividad de racecadotrilo en el tratamiento de la gastroenteritis aguda [The effectiveness of racecadotril in the treatment of acute gastroenteritis.]Acta Pediatr Esp2009673117122
- Gutierrez-CastrellonPAcosta-BastidasMLlamosas GallardoBEnsayo clinico aleatorizado y analisis farmacoeconomico del impacto de racecadotrilo (Hidrasec) como coadyuvante en el tratamiento de la gastroenetritis aguda sobre la reduccion de los gastos hospitalierios relacionados en lactantes menores de 24 meses en MexicoRev Invest Clin2011In press
- Melendez GarciaJMRodriguezJTRacecadotril en el tratamiento de la diarrea agudo en ninosRev Facultad Med (Guatemala)200742528
- SzajewskaHRuszczynskiMChmielewskaAWieczorekJSystematic review: racecadotril in the treatment of acute diarrhoea in childrenAliment Pharmacol Ther200726680781317767464
- Emparanza KnörrJIOzcoidi ErroIMartínez AnduezaMCCallén BlecuaMTAlústiza MartínezEAseguinolaza IparraguirreIRevisión sistemática sobre la eficacia de racecadotrilo en el tratamiento de la diarrea aguda [Systematic review of the efficacy of racecadotril in the treatment of acute diarrhea.]An Pediatr (Barc)200869543243819128744
- HaoRDe VeraMResurreccionERacecadotril in the treatment of acute diarrhea in children: a meta-analysisPIDSP Journal20101121932
- LehertPCheronGCalatayudGARacecadotril for childhood gastroenteritis: an individual patient data meta-analysisDig Liver Dis201143970771321514257
- BaumerPJoulinYPre- and postmarketing safety profiles of Racecadotril sachets, a “new” antidiarrhoeal drugJ Pediatr Gastroenterol Nutr200948Suppl 3E99
- TurckDBerardHFretaultNLecomteJMComparison of racecadotril and loperamide in children with acute diarrhoeaAliment Pharmacol Ther199913Suppl 6273210646049
- HallSExpert Interview Primary Care Physician United Kingdom13112012
- SilverLExpert Interview Primary Care Physician United Kingdom13122011
- World Health Organization (WHO)The treatment of diarrhoea – A manual for physicians and other senior health workers2005 Available from: http://www.who.int/maternal_child_adolescent/documents/9241593180/en/Accessed on February 16, 2012
- ChaconJAnalysis of factors influencing the overall effect of racecadotril on childhood acute diarrhea. Results from a real-world and post-authorization surveillance study in VenezuelaTher Clin Risk Manag2010629329920668711
- HuppertzHIForsterJHeiningerURoosRNeumannHUHammerschmidtTThe parental appraisal of the morbidity of diarrhea in infants and toddlers (PAMODI) surveyClin Pediatr (Phila)200847436337118270310
- BrissonMSenecalMDroletMMansiJAHealth-related quality of life lost to rotavirus-associated gastroenteritis in children and their parents: a Canadian prospective studyPediatr Infect Dis J2010291737519907361
- MartinACottrellSStandaertBEstimating utility scores in young children with acute rotavirus gastroenteritis in the UKJ Med Econ200811347148419450099
- GoossensLMStandaertBHartwigNHovelsAMAlMJThe cost-utility of rotavirus vaccination with Rotarix (RIX4414) in the NetherlandsVaccine20082681118112718215445
- World Health Organization (WHO)The WHO Child Growth Standards2012 Available from: http://www.who.int/childgrowth/en/Accessed on February 16, 2012
- Buzzle comAverage Weight for Children by Age2012 Available from: http://www.buzzle.com/articles/average-weight-for-children-by-age.htmlAccessed on February 16, 2012
- Royal College of Paediatrics and Child Health (RCPCH)UK-WHO growth charts2012 Available from: http://www.rcpch.ac.uk/Research/UK-WHO-Growth-ChartsAccessed on February 16, 2012
- World Health Organization (WHO)Growth reference data for 5–19 years2012 Available from: http://www.who.int/growthref/en/Accessed on February 16, 2012
- Joint Formulary CommitteeBritish National Formulary2008
- AbbottProductsOperationsAGUnited Kingdom Drug Cost Estimate2012
- Personal Social Services Research UnitUnit Costs of Health and Social Care 2010 PSSRUPersonal Social Services Research Unit Kent2010
- Primary diagnosis: summaryHospital Episodes Statistics – HESonline2012 Available from: http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=202Accessed on February 16, 2012
- Healthcare Resource GroupsHospital Episodes Statistics – HESonline2012 Available from: URL: http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=206Accessed on February 16, 2012
- KindPHardmanGMacranSUK Population Norms for EQ-5D, Discussion Paper 172Centre for Health Economics York University1999
- National Institute for Health and Clinical Excellence (NICE)Costing statement: diarrhoea and vomiting in children2009
- Cegedim Strategic Data UK LimitedCegedim Strategic Data2011
- KoletzkoSOsterriederSAcute infectious diarrhea in childrenDtsch Arztebl Int20091063353954719738921
- Van der WielenMGiaquintoCGotheforsLImpact of community- acquired paediatric rotavirus gastroenteritis on family life: data from the REVEAL studyBMC Fam Pract2010112220230601