Abstract
Background
Decompensated cirrhosis is a serious clinical complication of chronic hepatitis B (CHB) that places a large economic burden on the US health care system. Although entecavir has been shown to improve health outcomes in a cost-effective manner in mixed populations of CHB patients, the cost-effectiveness of entecavir has not been evaluated in CHB patients with decompensated cirrhosis.
Methods
This study assessed the cost-effectiveness of entecavir versus adefovir, from a US payer perspective, in CHB patients with decompensated cirrhosis, using a health-state transition Markov model with four health states: hepatocellular carcinoma (HCC), HCC-free survival, post-liver transplant, and death. The model considered a hypothetical patient population similar to that included in a randomized controlled trial in the target population (ETV-048): predominantly male (74%), Asian (54%), mean age 52 years, hepatic decompensation (Child–Pugh score ≥ seven), hepatitis B e antigen-positive or -negative, treatment-naïve or lamivudine-experienced, and no liver transplant history. Clinical inputs were based on cumulative safety results for ETV-048 and published literature. Costs were obtained from published literature. Costs and outcomes were discounted at 3% per annum.
Results
For 1000 patients over a 3-year time horizon, predicted overall survival and HCC-free survival were longer with entecavir than with adefovir (2.35 versus 2.30 years and 2.11 versus 2.03 years, respectively). Predicted total health care costs were $889 lower with entecavir than with adefovir ($91,878 versus $92,768). For incremental cost/life-year gained and incremental cost/HCC-free-year gained, entecavir was less costly and more effective than adefovir. Sensitivity analyses found the results to be robust to plausible variations in health-state costs and discount rate.
Conclusion
This analysis suggests that entecavir improves survival outcomes in a cost-saving manner compared with adefovir in CHB patients with hepatic decompensation.
Introduction
The availability of hepatitis B virus (HBV) vaccines has reduced the incidence of acute HBV infection in the US; however, the prevalence of chronic hepatitis B (CHB) remains high because of immigration from highly endemic countries.Citation1,Citation2 CHB affects up to 1.4 million individuals in the US and results in approximately 3000 deaths each year due to liver disease.Citation1,Citation2 The heterogeneous nature and slow progression of CHB means that it is often diagnosed later in life and at later stages of the disease. CHB often has serious clinical sequelae, including liver failure, hepatocellular carcinoma (HCC), and death, and places a large economic burden on the US health care system.Citation3,Citation4
Morbidity and mortality in CHB are linked to persistent viral replication resulting in liver injury and the development of fibrosis and eventually cirrhosis. Decompensated cirrhosis occurs in the later stages of CHB. Patients with decompensated cirrhosis have an average 5-year survival rate of 14%–35% compared with 80%–86% in patients with compensated cirrhosis.Citation5 Survival can be improved and the need for liver transplantation can be delayed or prevented by antiviral treatment that suppresses HBV viral load and stabilizes or improves disease status.Citation6–Citation8 For patients with HBV and decompensated cirrhosis, the most recent US treatment guidelines from the American Association for the Study of Liver Diseases, published in 2009, recommend prompt initiation of treatment with an oral nucleos(t)ide analog regimen, which can produce rapid viral suppression with a low risk of resistance.Citation4 Based on evidence from clinical study ETV-048,Citation9 entecavir was approved by the US Food and Drug Administration in late 2010 for the treatment of patients with decompensated cirrhosis.Citation10 Adefovir had resulted in improved clinical outcomes in a study of pre- and post-transplantation patients, including patients with decompensated liver disease,Citation6,Citation8 and was accepted by the US Food and Drug Administration as a suitable comparator to entecavir in patients with decompensated disease. The ETV-048 study is a prospective, randomized, clinical study comparing adefovir and entecavir in 191 CHB patients with hepatic decompensation (mean baseline Child–Turcotte–Pugh score: 8.59).Citation9 Over 48 weeks of treatment, entecavir demonstrated superior antiviral and biochemical activity compared with adefovir. Over a mean therapy time of 109 weeks for entecavir and 97 weeks for adefovir, cumulative numbers of patients developing HCC or dying were lower in the entecavir arm compared with the adefovir arm (12% versus 20% and 23% versus 33%, respectively). Two-thirds of patients in both groups demonstrated improvement or stabilization of Child–Turcotte–Pugh status.
With the increasing availability of new antiviral agents for the treatment of HBV infection, cost-effectiveness analysis serves as an aid in determining the optimal management strategies for CHB patients. Entecavir has been shown to improve health outcomes in a cost-effective manner compared with other oral antivirals and pegylated interferon in a population of patients with hepatitis B e antigen-positive CHB.Citation11 While some previous studies have examined decompensated cirrhosis as part of broader cost-effectiveness analyses of CHB management, there is a lack of economic health studies that evaluate the cost-effectiveness of antiviral treatments solely in CHB patients with decompensated liver disease in the US.Citation12–Citation17 The objective of this analysis was to evaluate the cost-effectiveness of entecavir versus adefovir from a US third-party payer perspective in treating CHB among patients with decompensated cirrhosis using published evidence and safety data extrapolated from a prospective clinical study in the target population (study ETV-048).
Methods
Model description
The model was constructed in Microsoft Excel 2007 as a four-state deterministic Markov model, defined by the clinical events of interest (). A Markov model (as opposed to a decision tree framework) was chosen to allow for the incorporation of time spent in the various health states associated with HBV-related decompensated cirrhosis. The four health states simulated in the model were HCC, HCC-free decompensated cirrhosis, survival post-liver transplant, and death. Patients entered the model with decompensated cirrhosis in the HCC-free decompensated cirrhosis state and exited the model at death or at the end of the model time horizon. The model was programed to consider a hypothetical population of 1000 patients similar to those included in the ETV-048 study, that is, adults (≥16 years) with hepatitis B e antigen-positive or -negative CHB and hepatic decompensation (Child–Turcotte–Pugh score ≥ seven).Citation9 Patients were treatment-naïve or previously treated with lamivudine (lamivudine resistance-associated mutations present or absent), and had not received any previous liver transplant.
Figure 1 Diagram of the four-state Markov model.
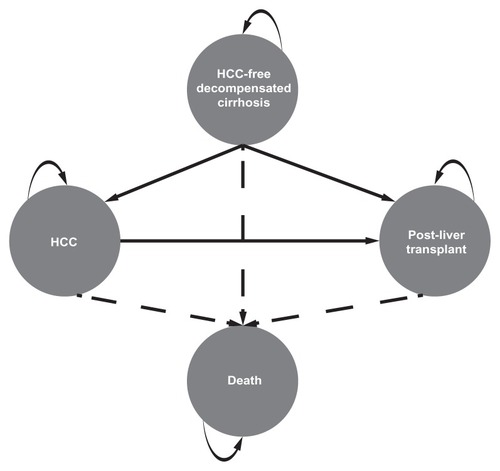
The model predicted and compared entecavir- and adefovir-specific health outcomes and associated costs over a 3-year time horizon using repeated 4-week cycles. A 3-year time horizon was chosen since this is the duration typically of interest to US third-party payers, based on the average length of enrollment in commercial plans. The cycle length was selected based on the reported data from clinical study ETV-048, which provided HCC-free survival estimates at 4-week intervals. A half-cycle correction was applied to cost and survival outputs from the i.e. cycle-specific calculations of the model. The standard discount rate of 3% (as recommended by the US Public Health Service Panel on Cost-effectiveness in Health and Medicine) was applied to costs and health outcomes every 4 weeks after the first year of treatment, subject to half-cycle correction.Citation18
Model parameters and inputs
A summary of the parameters and inputs used within the model, and the sources they are based on, is provided in .
Table 1 Model parameters and inputs used in the reference case
Modeling treatment-specific health-state transition probabilities
Following each cycle, the transition probabilities for entering the HCC disease state, remaining in the HCC-free decompensated cirrhosis state, and moving from the HCC-free decompensated cirrhosis state to the death state were based on parametric regression analysis of as-treated, cumulative HCC-free survival, and overall survival data from clinical study ETV-048 (). Overall survival for adefovir and HCC-free survival for both arms were derived from summary Kaplan–Meier data with a 240-week follow up. The Kaplan–Meier overall survival data for the entecavir group showed flattening after week 96, so the data were truncated at this point to allow for a good fit. Parametric survival techniques were used to identify the best-fitting distribution. Exponential, Weibull, and log-logistic distributions were considered and the method yielding the closest fit was chosen. To ensure that the best-fitting distribution did not deviate significantly from the observed data, the observed versus predicted distribution by treatment group for each outcome were examined.
Figure 2 Regression analysis on cumulative ETV-048 data for hepatocellular carcinoma (HCC)-free survival (A), overall survival (B), and treatment duration (C) data for entecavir and adefovir.
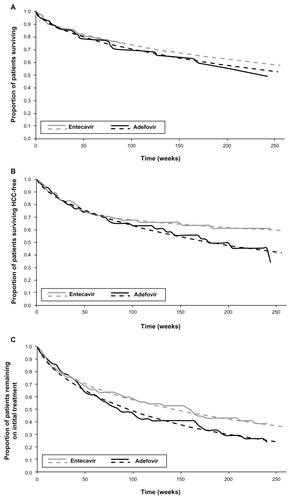
Weibull curves were used for adefovir overall survival and adefovir HCC-free survival. Sequential Weibull curves were used for entecavir overall survival and entecavir HCC-free survival data. All other health state transition probabilities in the model were estimated as being identical for each of the treatment regimens. As such, overall survival and HCC-free survival represent the primary drivers of the model.
Other health-state transition probabilities
Treatment-independent health-state transition probabilities were based on published literature. The probability of receiving a liver transplant was considered to be the same for patients with and without HCC and was based on Organ Procurement and Transplantation Network data as of March 4, 2011, which described the 2-year probability of receiving a liver transplant as 51.7% for patients with model for end-stage liver disease scores between 11 and 18.Citation19 Patients in the HCC-free decompensated cirrhosis state were assumed to only be eligible for liver transplant in the first year of disease. Patients in the HCC state were assumed to be eligible for liver transplant in the first 3 months of disease only. This assumption is consistent with previous models of HCC liver transplantationCitation28 and was based on the Milan eligibility criteria, which state that patients with HCC should only be considered for liver transplantation at an early stage of tumor development.Citation29 The probability of post-liver-transplant survival was based on a Milliman Research Report from 2008, which reported a 5-year post-transplant survival rate of 86%.Citation20 The probability of mortality from HCC was based on a retrospective analysis by Altekruse et al, who searched the Surveillance, Epidemiology, and End Results database and reported a 1-year survival rate of 47% among HCC patients.Citation21 Transition probabilities were converted into probabilities per 28-day cycle for use in the model.
Treatment duration and drug costs
Cumulative drug costs are estimated by enumerating the number of patients remaining on therapy following each cycle based on regression analyses of as-treated, cumulative entecavir and adefovir treatment duration data over 240 weeks in clinical study ETV-048 (). In both cases, Weibull curves were used to fit the data. In keeping with the third-party payer perspective of the analysis, the cost of entecavir and adefovir in the reference case were based on the US average wholesale price in October 2010 (entecavir, $28.37 per dose; adefovir, $32.52 per dose).Citation22 An entecavir dose of 1.0 mg per day was used because the patient population included patients who had been previously treated with lamivudine. Drug costs were scaled to 4 weeks to match the modeled cycle length and reduced by 16.1% to reflect the average community pharmacy reimbursement for brand name drugs as described in a 2008 survey of 223 employees.Citation23,Citation24
Disease management costs
Disease management costs were derived from published sources and inflated to 2010 US$, where necessary, using the medical care component of the US consumer price index. The cost per liver transplantation, the annual cost of patient management following liver transplantation, and the annual cost of managing patients with decompensated cirrhosis were based on an analysis of health care claims data conducted by Lee et al in which cost estimates were based on reimbursed amounts for each claim.Citation3 The annual cost of HCC was based on a previous investigation by Lang et al using the Surveillance, Epidemiology, and End Results Medicare database.Citation25 The per-event cost of death was calculated from average health care utilization statistics for end-of-life patients as described in the Dartmouth Atlas Project, and 2010 unit costs listed by the Centers for Medicare and Medicaid Services.Citation26,Citation27
Model outputs
The primary health outcomes predicted by the model were mean overall survival (years), mean HCC-free survival (years), and liver transplants (number per population). The primary economic outcomes of the model were treatment-specific total medical costs, including antiviral costs, cost of decompensated cirrhosis, cost of HCC, cost of transplant, and cost of death. Incremental cost-effectiveness ratios for HCC-free survival and overall survival were calculated as the ratio between the difference in medical care costs and the difference in the rates of respective health outcomes between entecavir and adefovir.
The incremental net benefit was also calculated. That is, the total cash benefit if the health outcome is converted to a dollar value by multiplying the difference in health outcome by the willingness-to-pay threshold minus the difference in total medical costs. For this purpose, a willingness-to-pay threshold of US$50,000 was used. A positive net benefit means the treatment of interest is favorable against the comparator and vice versa.
Sensitivity analysis
A univariate sensitivity analysis was undertaken to test the robustness of the model. The time horizon for follow up was varied between 1 and 5 years. The discount rate was varied from 0% to 6%. The cost of entecavir and adefovir were varied to reflect the wholesale acquisition cost ($709.23 and $813.11, respectively) without adjustment for pharmacy reimbursement.Citation22 Average wholesale price reimbursement was varied to reflect the lowest and highest discount rates reported in the survey cited for the reference case.Citation24 The annual cost of decompensated cirrhosis and the cost of liver transplant were reduced by 25% or increased to use the cost estimate based on hospital charges rather than reimbursed costs.Citation3 The annual cost of HCC was varied to reflect the lowest and highest costs reported by Lang et al for different stages of HCC (localized versus distant). The annual cost of post-liver-transplant health care was varied using alternative data sources.Citation28,Citation30 Minimum and maximum estimates of event cost for death were derived using the standard error reported for the daily hospital stay cost used to derive the estimate.Citation26,Citation27 The annual probability of post-liver-transplant survival was varied to reflect the 1-year survival rate (86%) reported in the 2008 Milliman Research Report.Citation20 Overall survival, HCC-free survival, and treatment duration for the entecavir arm were individually set to match those derived from the adefovir clinical study data. The addition of tenofovir as a salvage therapy in patients who discontinued treatment with entecavir or adefovir was also considered. Furthermore, the addition of lamivudine (100 mg daily) to the adefovir arm was considered to reflect the guideline recommendation not to use adefovir as a monotherapy in patients with decompensated cirrhosis.Citation4
Results
Health outcomes, costs, and cost effectiveness
Over the 3-year time horizon, entecavir was predicted to provide improvements over adefovir in mean overall survival (2.35 versus 2.30 years, respectively) and HCC-free survival (2.11 versus 2.03 years, respectively; ). Entecavir was also expected to provide a marginally greater impact on the number of liver transplants over the 3-year period, with six fewer transplants per 1000 population predicted under the entecavir regimen than under the adefovir regimen (). These expected improvements in HCC-free survival impacted the 3-year projected costs of patient management under the two treatment regimens (). While adefovir was predicted to offer mean savings of $1496 per patient in the cost of HCC-free decompensated cirrhosis management (due to the shorter time patients were expected to spend in this state), this saving was exceeded by greater costs in HCC patient management, liver transplantation, death, and the drug itself, relative to entecavir; for example, entecavir was predicted to provide an average saving of $1100 per patient in HCC costs compared with adefovir. As a result, over the 3-year time horizon, total medical costs were projected to be lower with entecavir ($91,878) than with adefovir ($92,768), providing a net mean saving of $889 (). Mean antiviral costs were $207 lower for entecavir than for adefovir and mean total disease state costs were $682 lower for entecavir than for adefovir.
Table 2 Survival outcomes, health care costs, and incremental cost-effectiveness ratios for entecavir versus adefovir in the treatment of decompensated chronic hepatitis B over a 3-year time horizon
The improvements in health outcomes and overall cost savings mean that entecavir was the dominant intervention (ie, was more efficacious and less costly) in terms of cost per life-year gained and cost per HCC-free life-year gained. Assuming a willingness-to-pay threshold in the US of $50,000 per year, these improvements correspond to an incremental net benefit of $3656 per patient in terms of overall survival and $5280 per patient in HCC-free survival with entecavir versus adefovir over the 3-year time horizon.
Sensitivity analysis
A series of univariate sensitivity analyses found the model to be robust to plausible variations in health-state costs, drug costs, and the discount rate, with only minor changes in net benefit observed at the upper and lower bounds of these analyses (). The model was most sensitive to changes in HCC-free survival, the time horizon of the model, and the inclusion of lamivudine as an add-on therapy to adefovir (). Using the duration of HCC-free survival predicted from the ETV-048 adefovir arm for both arms in the model negated the net benefit of entecavir over adefovir for HCC-free survival and reduced the net benefit of entecavir for overall survival. Further threshold analyses were conducted for this variable to help gain a quantitative understanding of the sensitivity. Specifically, HCC-free survival of entecavir at the end of 5 years was varied in a stepwise fashion until the net benefit over 3 years of either overall or HCC-free life -years reached zero. When the HCC-free survival at 5 years is decreased by more than 6.2%, the net benefit of HCC-free life-years will be less than zero (ie, not cost-effective at a threshold of $50,000 per year). By the same token, the reduction in HCC-free survival needed for overall life-years to reach zero was 15.4%.
Table 3 Sensitivity analysis for the estimated net benefit of entecavir versus adefovir in terms of life-years gained and HCC-free life-years gained
The net benefit of entecavir increased substantially when the time horizon was extended to 5 years and decreased when the time horizon was reduced to 1 year; however, entecavir remained the most cost-effective treatment across all time horizons in the selected range. Lastly, as expected, the inclusion of lamivudine as an add-on therapy to adefovir substantially increased the net benefit of entecavir.
Discussion
Complications of decompensated cirrhosis are key drivers of the costs associated with CHB-related health care.Citation3,Citation4,Citation17 If disease progression is allowed to continue in patients with decompensated cirrhosis, then liver transplant, at an average annual cost of more than $100,000, becomes the only effective therapeutic option.Citation3 The results of this analysis suggest that entecavir improves survival outcomes in a cost-saving manner compared with adefovir in CHB patients with hepatic decompensation. For 1000 patients over a 3-year time horizon, predicted overall and HCC-free survival were longer with entecavir than with adefovir and predicted total health care costs were lower with entecavir than with adefovir. Thus, entecavir was the dominant intervention for both incremental cost per life-year gained and incremental cost per HCC-free life-year gained.
This is the first study to focus on the cost effectiveness of antiviral treatments solely in CHB patients with decompensated liver disease. The validity of the model is strengthened by the use of extrapolated data from a prospective clinical study comparing the two interventions in the target population of patients with HBV-related decompensated liver disease. Sensitivity analyses further demonstrated the robustness of the model. Varying disease state costs to reflect the ranges reported in the literature had little impact on the net benefit of entecavir over the 3-year time horizon. However, altering the time horizon did have a profound impact on the output of the model, suggesting that the added benefit of entecavir versus adefovir is not fully realized until more than 1 year after the treatment decision. Nevertheless, entecavir did remain the most cost-effective treatment over a 1-year time horizon and the net benefit of entecavir increased substantially when the reference case time horizon was extended to 5 years.
The results of this study highlight the importance of disease stabilization in CHB patients with hepatic decompensation. The model predicted that treatment with entecavir would result in increased overall survival, increased HCC-free survival, and fewer liver transplants compared with adefovir treatment. As mortality from HCC, post-liver-transplant mortality, and the probability of liver transplant were modeled as equal for both treatment regimens, these improved health outcomes can be attributed to entecavir better maintaining patients in the HCC-free decompensated cirrhosis state than adefovir. Such disease stabilization is of particular importance for patients who are not liver transplant candidates, as prevention of further disease progression may significantly defer or prevent complete liver failure, where no further treatment options exist. In our model, reduced progression to disease states with high health care costs resulted in reduced total costs associated with entecavir treatment compared with adefovir treatment over the 3-year time horizon. Most notably, entecavir was predicted to provide an average saving of $1100 per patient in HCC costs compared with adefovir, highlighting the major financial benefit of disease stabilization in the treatment of decompensated cirrhosis.
There are a number of limitations to this study. Firstly, using adefovir as a comparator, although appropriate at the time of the ETV-048 study, does not necessarily reflect the current treatment practice in the US since tenofovir is now also approved for the treatment of CHB patients with decompensated cirrhosis. Results for entecavir and tenofovir might be expected to be comparable, since studies have shown that the mean total health care costs associated with each agent when used as a monotherapy are comparableCitation31 and that the efficacy of these agents in this patient population is similar.Citation32 In addition, guidelines issued before the approval of tenofovir recommended that adefovir be used in combination with lamivudine, not as a monotherapy, in patients with decompensated cirrhosis.Citation4 The inclusion of costs for add-on lamivudine in the adefovir arm goes some way to address this limitation; however, the potential clinical benefits that might be derived from this addition are not considered. Secondly, the emergence of a resistant virus was not specifically included in the model. The patient population included patients with previous lamivudine treatment, in whom the risk of emergence of entecavir resistance is greater than in treatment-naïve patients.Citation10 In the ETV-048 study, entecavir-resistant virus variants were detected in three patients, all of whom carried lamivudine-resistant variants at baseline, and adefovir-resistant virus variants were detected in six patients, two of whom carried lamivudine-resistant variants at baseline. Citation9 The development of drug resistance can lead to hepatic flares or even death, as well as a loss of efficacy, requiring discontinuation of the treatment in question. However, since the treatment duration and survival probabilities included in the model were derived from clinical data from the ETV-048 study, any deaths or treatment discontinuations resulting from the emergence of a resistant virus would already have been taken into consideration. Thirdly, switching to an alternative antiviral therapy following treatment failure was not explicitly considered in the model. According to treatment guidelines, patients who failed treatment on adefovir could theoretically have added entecavir, telbivudine, or lamivudine (if they had no previous lamivudine exposure), or switched to tenofovir plus lamivudine, emtricitabine, or entecavir; patients failing on entecavir could have switched to tenofovir.Citation4,Citation5 A switch to tenofovir in patients discontinuing treatment in either arm was included in the sensitivity analysis and had little impact on the resulting net benefit of entecavir over adefovir, although it should be noted that any potential clinical benefit resulting from this switch was not considered. Lastly, it should be noted that this analysis applies to a specific patient population (those with HBV-associated decompensated cirrhosis) and to a specific health care setting (with a US third-party payer perspective), thus the results may not be applicable to other patients with CHB or to other health care settings.
Treatment guidelines recommend treating HBV infection before the development of liver cirrhosis based on viral load and serum alanine aminotransferase levels.Citation4,Citation5 This approach can significantly delay, or even reverse, the progression of disease and is clearly more cost effective than waiting to initiate treatment only at the onset of serious illness.Citation11,Citation33 However, early treatment is often not possible as it is estimated that 65% of the HBV-infected population in the US are unaware of their infection, meaning that initial diagnosis frequently occurs at a late stage of disease progression, when symptoms of decompensated cirrhosis are apparent.Citation2 Despite the preferential outcomes of early treatment, it is still beneficial to treat HBV with decompensated cirrhosis, as demonstrated in the ETV-048 study. The results of this analysis suggest that for HBV-infected patients who have progressed to decompensated cirrhosis, entecavir improves survival outcomes in a cost-saving manner compared with adefovir.
Acknowledgments
This study was funded by Bristol-Myers Squibb. Editorial assistance for preparation of the manuscript was provided by ArticulateScience, funded by Bristol-Myers Squibb.
Disclosure
The authors report no conflicts of interest in this work.
References
- Centers for Disease Control and PreventionRecommendations for identification and public health management of persons with chronic hepatitis B virus infectionMMWR Recomm Rep200857RR-8120
- MitchellAEColvinHMPalmer BeasleyRInstitute of Medicine recommendations for the prevention and control of hepatitis B and CHepatology20105172973320186842
- LeeTAVeenstraDLIloejeUHSullivanSDCost of chronic hepatitis B infection in the United StatesJ Clin Gastroenterol20043810 Suppl 3S144S14715602162
- LokASMcMahonBJChronic hepatitis B: update 2009Hepatology20095066166219714720
- European Association for the Study of the LiverEASL clinical practice guidelines: management of chronic hepatitis BJ Hepatol2012In press
- FontanaRJManagement of patients with decompensated HBV cirrhosisSemin Liver Dis2003238910012616454
- LiawYFSungJJChowWCLamivudine for patients with chronic hepatitis B and advanced liver diseaseN Engl J Med20043511521153115470215
- SchiffELaiCLHadziyannisSAdefovir dipivoxil for waitlisted and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term resultsLiver Transpl20071334936017326221
- LiawYFRaptopoulou-GigiMCheinquerHEfficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with evidence of hepatic decompensationHepatology2011549110021503940
- Baraclude® (entecavir) US prescribing informationBristol-Myers Squibb CompanyPrinceton, NJ 08543122010
- SpackmanDEVeenstraDLA cost-effectiveness analysis of currently approved treatments for HBeAg-positive chronic hepatitis BPharmacoeconomics20082693794918850763
- ArnoldEYuanYIloejeUCookGCost-effectiveness analysis of entecavir versus lamivudine in the first-line treatment of Australian patients with chronic hepatitis BAppl Health Econ Health Policy2008623124619382822
- ButiMBrosaMCasadoMARuedaMEstebanRModeling the cost-effectiveness of different oral antiviral therapies in patients with chronic hepatitis BJ Hepatol20095164064619576651
- KanwalFGralnekIMMartinPDulaiGSFaridMSpiegelBMTreatment alternatives for chronic hepatitis B virus infection: a cost-effectiveness analysisAnn Intern Med200514282183115897532
- KanwalFFaridMMartinPTreatment alternatives for hepatitis B cirrhosis: a cost-effectiveness analysisAm J Gastroenterol20061012076208916968510
- VeenstraDLSullivanSDClarkeLCost effectiveness of entecavir versus lamivudine with adefovir salvage in HBeAg-positive chronic hepatitis BPharmacoeconomics20072596397717960954
- YuanYIloejeUHHayJSaabSEvaluation of the cost-effectiveness of entecavir versus lamivudine in hepatitis BeAg-positive chronic hepatitis B patientsJ Manag Care Pharm200814213318240879
- SiegelJETorranceGWRussellLBLuceBRWeinsteinMCGoldMRGuidelines for pharmacoeconomic studies. Recommendations from the panel on cost effectiveness in health and medicine. Panel on cost effectiveness in health and medicinePharmacoeconomics19971115916810172935
- Organ Procurement and Transplantation Network. [website on the Internet]Liver Kaplan-Meier percentage transplanted at specific time points for registrations listed: 1999–2004 Available from: http://optn.transplant.hrsa.govAccessed July 15, 2011
- HauboldtRHHansonSG2008US organ and tissue transplant cost estimates and discussionMilliman Research Report42008
- AltekruseSFMcGlynnKAReichmanMEHepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005J Clin Oncol2009271485149119224838
- Red Book 2008Red Book for Windows Version 6112750Montvale, New JerseyThomson PDR2008
- TagTRubensteinEAMCP guide to pharmaceutical payment methods, 2009 updates (version 2.0)J Manag Care Pharm200915Suppl 6aS1S61
- The Pharmacy Benefit Management Institute. [website on the Internet]Prescription drug benefit cost and plan design report, 2008–2009 edition. Takeda Pharmaceuticals North America, Inc. November 2008The Pharmacy Benefit Management Institute2011 Available from: http://www.pbmi.com/2008_report/pdfs/Revised_Report_20112009.pdfAccessed July 27, 2011
- LangKDanchenkoNGondekKShahSThompsonDThe burden of illness associated with hepatocellular carcinoma in the United StatesJ Hepatol200950899918977551
- Centres for Medicare and Medicaid Services. [website on the Internet]CMS Medicare and Medicaid Statistical Supplement 2010 Edition Available from: http://www.cms.gov/MedicareMedicaidStatSupp/09_2010.aspAccessed July 15, 2011
- GoodmanDCFisherESChangC-HQuality of end-of-life cancer care for Medicare beneficiaries: regional and hospital-specific analyses. A report of the Dartmouth Atlas ProjectLebanon, New HampshireDartmouth Institute for Health Policy and Clinical Practice2010
- NauglerWESonnenbergASurvival and cost-effectiveness analysis of competing strategies in the management of small hepatocellular carcinomaLiver Transpl2010161186119420879017
- MazzaferroVRegaliaEDociRLiver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosisN Eng J Med1996334693699
- BestJHVeenstraDLGeppertJTrends in expenditures for Medicare liver transplant recipientsLiver Transpl2001785886211679983
- JingWMenaELiMTreatment patterns, healthcare use and costs associated with first-line treatment for chronic hepatitis B with entecavir versus tenofovirHepatology201154Suppl 1 Abstract 481
- LiawY-FSheenI-SLeeC-MTenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver diseaseHepatology201153627221254162
- PostSESodhiNKPengCHWanKPollackHJA simulation shows that early treatment of chronic hepatitis B infection can cut deaths and be cost-effectiveHealth Aff (Millwood)20113034034821289356