Abstract
Background
Type 2 diabetes (T2D) patients face increased risk of heart failure (HF) as they age. Sodium-glucose cotransporter 2 inhibitors (SGLT-2i) have demonstrated effectiveness in reducing HF hospitalizations in patients with T2D and HF with reduced ejection fraction (HFrEF). Diabetes guidelines recommend SGLT-2i therapy for patients with HFrEF; however, SGLT-2i cost is high.
Objective
Study objectives were to assess SGLT-2i utilization and HF hospitalization rates in commercially insured adults (age <65) with T2D and heart failure with reduced ejection fraction (HFrEF) taking metformin with/without SGLT-2i use and conduct a cost–benefit analysis of SGLT-2i use from payer and societal perspectives.
Methods
Economic models included HF hospitalization rates from real-world data (RWD) and hospitalization rate reductions from RWD and SGLT-2i clinical trials. Real-world HF hospitalization rates were obtained from claims data (MarketScan Commercial Database, years 2013–2018). Societal perspective analyses included indirect costs. Sensitivity analyses were conducted on key parameters.
Results
Among adults with T2D and HFrEF age 30–64, SGLT-2i use increased (1.1% to 17.4%) between 2013 and 2018. The HF hospitalization rate without SGLT-2i use vs with was 15.5% vs 11.0% (absolute risk reduction of 4.5%). Base case scenario net-benefit was negative across all payer perspective models, while positive for societal-perspective. Payer perspective overall net-benefit in 30–64 population: −$1,725,758 (−$4106 per person). Societal perspective net-benefit in 30–64 population: $5,996,851 ($14,269 per person). In sensitivity analyses, estimated per person base case societal net-benefit of $14,269 was most sensitive to changes in baseline HF hospitalization rates, post-discharge mortality rates, and readmission rates. Lowering SGLT-2i prescription costs 50% and 80% resulted in per person net-benefit increases of $1737 and $4004, respectively.
Conclusion
SGLT-2i utilization has steadily increased, with lower HF hospitalization rates observed among SGLT-2i users. Societal benefits of SGLT-2i use in this population are substantive; payer benefits are negative unless SGLT-2i cost is drastically reduced.
Plain Language Summary
Sodium-glucose cotransporter 2 inhibitors (SGLT-2i) are new drugs for managing type 2 diabetes (T2D). SGLT-2i use is associated with reductions in heart failure hospitalizations, but within the United States (US) SGLT-2i are expensive medications. This study compares the costs and benefits of SGLT-2i use by US individuals 30 to 64 years old with T2D and systolic heart failure. Results show insurance costs for SGLT-2i treatment exceed avoided hospitalization-related insurance costs, but positive benefits exist when costs borne by individuals and their caregivers are considered. (Current: 75 Max: 75)
Introduction
One out of every 10 US adults, representing 34.2 million individuals, has diabetes.Citation1 Type 2 diabetes (T2D) is the most prevalent form of the disease accounting for 95% of the cases.Citation1 Uncontrolled T2D can lead to complications affecting the cardiovascular, renal, and nervous systems, resulting in comorbidities such as cardiovascular disease, heart failure (HF), renal dysfunction, and retinopathy.Citation2 These comorbidities can result in costly economic impacts both for patients and health care systems. It is estimated that among patients with HF 30–40% have T2D,Citation3 and among patients with T2D, the risk of HF increases sharply as patients age, with HF prevalence estimates in T2D ranging from 10% to 30%.Citation4 HF with reduced ejection fraction (HFrEF), called systolic HF, is defined by a left ventricular ejection fraction ≤40% accompanied by signs of cardiac remodeling and dysfunction.Citation5 HFrEF is estimated to account for 50–80% of HF cases.Citation5,Citation6 For patients with T2D and HFrEF, initiation of an effective treatment plan is crucial to preventing complications from diabetes and/or heart failure, in particular, hospitalizations and premature death.
According to the American Diabetes Association (ADA) 2022 Standards of Care, metformin serves as the first-line treatment for T2D patients.Citation7 If desired HbA1C levels are not reached, adding a second-line agent to the patient’s medication regimen is recommended, with choice dependent on patient characteristics (presence of comorbidities, weight, renal function).Citation8 Two antihyperglycemic drug classes, glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter 2 inhibitors (SGLT-2i) have demonstrated cardiovascular (CV) safety and CV risk reduction among patients at high CV risk.Citation8,Citation9 The ADA 2022 Standards of Care now prefer SGLT2i for T2D management in the setting of HFrEF, regardless of HbA1C level, as clinical trials have demonstrated a significant reduction in HF hospitalization rates in T2D patients with comorbid HFrEF.Citation7,Citation10–Citation12
The first SGLT-2i was approved by the US Food and Drug Administration (FDA) in March 2013, and four SGLT-2i products are now on the market.Citation13 However, higher prescription costs of SGLT-2is compared to other second-line lower cost anti-hyperglycemic drugs may serve as a deterrent for use by both prescribers and patients. While some economic studies have predicted SGLT-2i use to be cost-effective based on clinical trial data, real-world utilization rates of SGLT-2i use in T2D and comorbid HFrEF patient populations are still largely unknown.Citation14–Citation20 Additionally, few reported studies have focused solely on the economic impact of SGLT-2i on HF hospitalization for a US T2D with comorbid HFrEF population, and few have utilized real-world data in examining the economic impact.Citation14–Citation20 Historically, HFrEF studies have typically been conducted in older populations, primarily age ≥65 years. The clinical and economic impact of SGLT-2i for younger populations is largely unreported. SGLT-2i use has been shown in clinical trials to reduce the risk of HF-related events; however, there has been no published report of an economic analysis that uses a real-world patient population data to quantify the economic impact of reduced HF hospitalization rates secondary to SGLT-2i utilization in T2D and HFrEF comorbid patients from a societal perspective.
The objectives of this study were to 1) quantify actual and potential SGLT-2i utilization rates and HF hospitalization rates in a commercially insured adult population (age <65) with T2D and comorbid HFrEF and 2) perform a cost–benefit analysis (CBA) of SGLT-2i use for the same population from a health care payer and a societal perspective.
Methods
The study used a retrospective cross-sectional research design to determine real-world SGLT-2i utilization and HF hospitalization rates in patients with T2D and HFrEF observed in a US national, commercial insurance claims database. The real-world rates, along with published SGLT-2i clinical trial event risk rates and estimates from other publicly available sources, were used to develop CBA economic models from health care payer and societal perspectives of SGLT-2i use among adults age <65 with T2D and comorbid HFrEF over a 1-year time horizon. Models assumed a large (1,000,000 members) commercially insured population. Results are stated as net-benefit and benefit-to-cost ratios. One-way sensitivity analyses of five key parameter assumptions were performed.
Data Source
Real-world data from the IBM MarketScan® Commercial database for calendar years 2013 through 2018 was used to estimate annual SGLT-2i utilization and HF hospitalization rates for the study population.Citation21 MarketScan is a claims database with medical service (eg, inpatient, outpatient) and outpatient pharmacy utilization, as well as enrollment data.Citation22 The data pertains to >40 million US individuals with employer-provided health insurance coverage from a variety of fee-for-service preferred provider organizations, and capitated health plans. Other data sources for model cost and benefit estimates included published SGLT-2i clinical trial findings and relevant peer-reviewed articles.
Study Population
MarketScan data for years 2013–2018 included adults 18–64 years old with T2D and HFrEF. Inclusion criteria for each year included continuous insurance coverage, at least one primary or secondary diagnosis of T2D and of HFrEF, and at least one prescription fill for metformin. Diagnoses were determined by ICD-9 and ICD-10 codes (Supplementary Table 1). T2D and type 1 diabetes (T1D) codes were identified using Healthcare Cost and Utilization Project (HCUP) Clinical Classification Software (CCS) classifications.Citation23,Citation24 Exclusion criteria included individuals with diagnosis of T1D, or a prescription claim for any agent within the thiazolidinedione class (thiazolidinediones are contraindicated in New York Heart Association Class III–IV HF due to risk of fluid retention and HF exacerbations).Citation25
For each year, individuals with at least one prescription fill (based on outpatient pharmacy claims) for an SGLT-2i (canagliflozin, empagliflozin, dapagliflozin) were categorized as having SGLT-2i use. Hospitalizations with a primary diagnosis of HF for individuals with and without SGLT-2i use were identified and rates calculated for each group. Summaries were by age group (18–29, 30–44, 45–54, 55–64); however, the 18–29 age group was too small to support estimates (<20 individuals with SGLT-2i use in all years), and therefore the model included individuals 30–64 years of age (Supplementary Tables 2 and 3). Trends across years 2013–2018 were reviewed and the most recent, 2018, was used for economic model parameter estimates.
Costs
Costs included the annual SGLT-2i treatment cost and treatment costs for adverse events associated with SGLT-2i use. SGLT-2i prescription costs were obtained for canagliflozin, empagliflozin, and dapagliflozin using Micromedex’s Redbook.Citation26 A once-daily regimen annual cost was estimated by multiplying the average wholesale unit price (AWP) by 365 days (adherence assumed to be 100%). In the base case scenario, AWP prices were discounted by 27% to account for standard medication discounts/rebates.Citation27 Event rates () and treatment costs () due to common and potential high-risk SGLT-2i-induced adverse events (urinary tract infections, genital mycotic infections, diabetic ketoacidosis) were from previously published literature or HCUP 2017 data. All costs were standardized to 2020 values using the annual Medical Consumer Price Index (CPI).Citation28
Table 1 Event Estimates by Age Group
Table 2 Inventory of Cost Estimates
Benefits
Benefits in the CBA model were determined from cost reductions for the SGLT-2i use population associated with decreased HF hospitalizations and post-hospitalization CV specific mortality over a 1-year period. Direct medical costs included initial HF hospitalizations and rehospitalizations, as well as post-discharge care costs. HF hospitalization costs were obtained from 2017 HCUP data using HF ICD-10 codes.Citation29 Post-discharge costs (skilled nursing facility (SNF), home-healthcare) were estimated from published studies.Citation30,Citation31
The societal perspective CBA included indirect costs. These costs were estimated by age category and were associated with lost productivity due to hospitalizations, post-discharge care, or premature mortality. Costs were calculated using the human capital method where time spent in hospital for HF or recovery post-discharge was multiplied by the estimated lost wages per respective age group. The HF hospitalization time period was calculated based on average inpatient length of stay obtained from 2017 HCUP hospital discharge data (length of stay did not differ across age groups), and published literature was used for outpatient post-hospitalization recovery time.Citation29,Citation30 Base case post-hospitalization HFrEF mortality was estimated from published literature.Citation32 Reductions in post-hospitalization CV specific mortality were obtained from the SGLT-2i clinical trials: CANVAS, EMPA-REG, and DECLARE-TIMI.Citation10–Citation12 Wage rates and losses due to premature morality were based on US annual and lifetime labor productivity estimates reported by Grosse et al, which were calculated using a 3% discount rate.Citation33
CBA Model Calculations
The CBA model insured population age distribution was determined from the 2019 US CENSUS.Citation34 Prevalence of T2D in the population was calculated using CDC estimates.Citation1 Net-benefit and benefit-to-cost ratios were estimated for each age category, and for the age 30–64 population overall.
The economic impact of SGLT-2i use was modeled using two methods: real-world and trial-based. Both methods used age-group-specific real-world rates for baseline HF hospitalization rates for the “no SGLT-2i” group, and a baseline HF rehospitalization rate of 21% was applied to the “no SGLT-2i use” group based on literature examining the rates of rehospitalization in HFrEF patients.Citation35 In the real-world model, “SGLT-2i use” group HF hospitalization rates were estimated using real-world relative rates for the “SGLT-2i use” group compared to the “no SGLT-2i” group. In the trial-based models, the “SGLT-2i use” group HF hospitalization rates were estimated using relative rates reported in the CANVAS, EMPA-REG, and DAPA-HF clinical trials (each trial was modeled separately).Citation10–Citation12
An inpatient mortality rate of 3.6% for HFrEF hospitalizations was applied to both real-world and trial-based models.Citation29 Both real-world and trial-based models used a 1-year mortality estimate of 22% among individuals discharged alive from a HF hospitalization as the base case scenario for the “no SGLT-2i use” groups.Citation36 As a conservative estimate, no reductions in inpatient mortality were applied to the “SGLT-2i use” groups. One-year mortality for the “SGLT-2i use” groups was estimated using CV specific clinical trial mortality relative risk rates in trial-based models.Citation10–Citation12 The CV mortality risk rate from EMPA-REG, as a middle value between the CANVAS and DECLARE-TIMI rates, was applied to the real-world model.Citation12
Net-benefit was determined by subtracting the total SGLT-2i treatment cost (prescription and adverse event costs) from the total benefit. Benefit was calculated as the difference in HF hospitalization-related costs between the two treatment groups (SGLT-2i vs no SGLT-2i). Benefit was assessed as direct medical cost savings (hospitalization and post-discharge costs averted) for the health payer perspective analysis, and as both direct medical and indirect cost savings for the societal perspective. Net-benefit is reported for the population overall and as a net-benefit-per-person. The benefit-to-cost ratio was calculated by dividing the total benefit by the total costs of SGLT-2i treatment for both perspectives. Benefit-to-cost ratio results are reported in the supplementary material (Supplementary Table 4).
Sensitivity Analyses
One-way sensitivity analyses for the societal perspective analyses were performed varying five key parameters: hospitalization rates, readmission rates, post-discharge mortality rates, HF hospitalization costs, and SGLT-2i prescription costs. Baseline HF hospitalization rates were varied ±5%. Baseline readmission rates were varied by ±2%. The baseline post-discharge mortality rate was not specific to a population <65 years of age; using estimates assessing HF mortality in younger populations, two lower bounds were set at 12% and 5%.Citation37–Citation39 To account for the potential of rising healthcare costs in the future, the cost of a HF hospitalization was increased by 20%. Lastly, to evaluate the impact of price reductions of SGLT-2i due to release of generic alternatives or other downward pressures on price, two lower bounds at 50% and 80% reduction of the original AWP were modeled. Results of sensitivity analyses are summarized as changes to the estimated net-benefit-per-person.
Results
Population and SGLT-2i Utilization
Supplementary Table 2 provides a summary of model population estimates by age group category. For the 30–64 population, the model assumes a T2D prevalence of 7.5%, and based on MarketScan 2018 estimates, a real-world T2D with HF prevalence for age 30–64 of 3.2%, with 40.3% having HFrEF. The real-world SGLT-2i utilization trends for years 2013–2018 are presented in Supplementary Table 3. Among patients age 30–64, SGLT-2i use in eligible patients increased from 1.1% to 17.4% between 2013 and 2018. Patients aged 55–64 accounted for approximately 60% of patients with SGLT-2i use across the 5-year time period, and the mean overall age for the model population was 55.6 years.
Hospitalization Rates
The real-world hospitalization rates are shown in . Overall, the hospitalization rate for a primary HF diagnosis was 15.5% among patients without SGLT-2i use and 11.0% in those with SGLT-2i use, an absolute risk reduction (ARR) of 4.5%. SGLT-2i users’ real-world relative risk rates for HF hospitalizations and those observed in clinical trials are summarized by age group (see ). The overall real-world HF hospitalization relative risk in patients on SGLT-2i was 0.70. The greatest real-world HF hospitalization reduction was observed for the 45–54 age group, where the relative risk of 0.51 was similar to that observed in the CANVAS study (HR=0.51). Real-world relative risks for the 30–44 and 54–64 age groups, at 0.79 and 0.80, respectively, were higher than reported clinical trial rates.
Base Case
The results of the base case scenario are reported as net-benefit in and for both the real-world and trial-based models from health care payer and societal perspectives. Benefit-to-cost ratios are summarized in the Supplementary Table 4. When only direct medical benefits were examined, net-benefit and benefit-to-cost ratios did not demonstrate benefit (). Under a health care payer perspective, the average SGLT-2i treatment-related costs were $5538 per person and the real-world overall net-benefit for the 30–64 population was −$1,725,758 (−$4106 per person). Across models using clinical trial data, payer perspective net-benefit ranged from −$1,423,717 (−$3388 per person) to −$1,657,187 (−$3943 per person). However, when indirect cost savings (productivity losses averted) were included in the societal perspective analysis, both net-benefit and benefit-to-cost ratios demonstrated benefit. Under a societal perspective, real-world net-benefit for the 30–64 population was $5,996,851 ($14,269 per person) and clinical trial model benefits ranged from $6,485,361 ($15,431 per person) to $7,230,434 ($17,204 per person) ().
Figure 1 Base case direct net-benefit results by age category. Net-benefit for base case scenario from health care payer perspective. Real-world (RW) and trial model results are reported for ages 30–64. Real-World – solid black, CANVAS – vertical stripe, DELCARE-TIMI – horizontal strip, EMPA-REG – diagonal stripe.
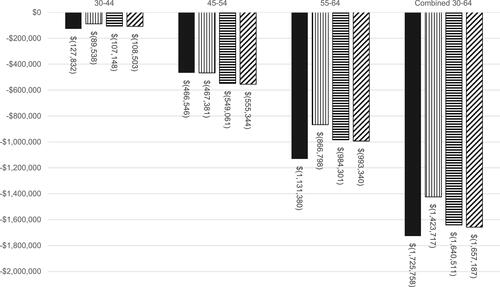
Figure 2 Base case societal net-benefit results by age category. Net-benefit for base case scenario from societal perspective. Real-world (RW) and trial model results are reported for respective age categories and for ages 30–64 combined. Real-World – Grey, CANVAS – purple, DELCARE-TIMI – pink, EMPA-REG – light blue.
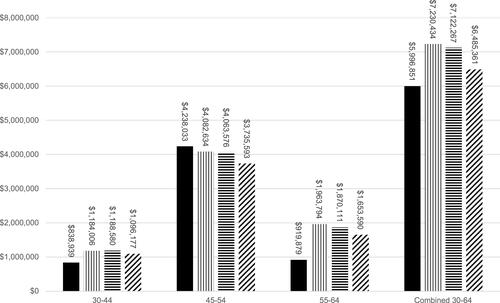
and illustrate that trends in net-benefits were consistent across age groups and model type. Under a societal perspective, the 45–54 age group derived the greatest net-benefit from SGLT-2i use across all models with net-benefits ranging from $3,735,593 to $4,238,033 (). This corresponds to $8888 and $10,084 per person. In comparison, the 30–44 age group received the least net-benefit across all societal perspective models with net-benefits ranging from $838,939 to $1,188,580 ($1996–$2828 per person) (), but also received the lowest negative net-benefits from a health care payer perspective ().
Sensitivity Analyses
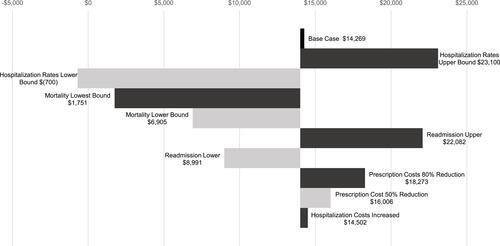
Sensitivity analysis results for the real-world societal perspective model are shown in . Results are per person net-benefit across ages 30–64. Clinical trial-based sensitivity analyses were consistent with the real-world sensitivity analyses (see Supplementary Figure 1–3). The estimated per person base case net-benefit of $14,269 was most sensitive to changes in baseline HF hospitalization rates, post-discharge mortality rates, and the readmission rates. When baseline hospitalizations were set at the lower bound (−5%), the base case net-benefit was reduced by $14,969, resulting in a net-benefit of −$700 per person; at the baseline hospitalization rate upper bound (+5%) there was an $8831 increase in net-benefit, resulting in a net-benefit of $23,100 per person. At the lowest post-discharge mortality bound (6%) the reduction of net-benefit from base case was $12,518 and at the lower post-discharge mortality bound (12%) there was a $7364 reduction of net-benefit, resulting in net-benefits of $1751 and $6905 per person, respectively. At the readmission rate upper bound (23%) there was a $7813 increase in net-benefit, and at the lower bound (19%) there was a $5278 reduction, resulting in $22,082 and $8991 per person, respectively. An 80% reduction of SGLT-2i prescription costs resulted in a $4004 net-benefit increase, and a 50% price reduction, in a $1737 net-benefit increase, resulting in a net-benefit of $18,273 and $16,006 per person, respectively. Results of the net-benefit and benefit-to-cost ratios of SGLT-2i prescription cost sensitivity analysis for real-world and trial-based models are provided in Supplementary Table 5.
Discussion
Real-world SGLT-2i utilization rates increased from 2013 to 2018 in this US population aged 30 to 64. However, in 2018 less than 20% of the eligible patients received an SGLT-2i. SGLT-2i use is now recommended by ADA Standards of Medical Care for individuals with T2D and HFrEF, and the increased utilization of SGLT-2i in this population will likely continue.
The observed real-world HF hospitalization relative rates support findings reported in the SGLT-2i clinical trials. A real-world ARR of 4.5% in HF hospitalizations was observed from the MarketScan data, a reduction comparable to the ARRs reported in the clinical trials: CANVAS (2.5%), DECLARE-TIMI (5.5%), and EMPA-REG (1.4%).Citation10–Citation12 The minimum enrollment age for the CANVAS, DECLARE-TIMI, and EMPA-REG was 30, 40, and 18, respectively, and the median age was between 63 and 65 years.Citation10–Citation12 A recent meta-analysis of RCT data for 10 studies examining SGLT-2i use among individuals with T2D and HFrEF found similar risk reductions between individuals age ≥65 and those <65 for the primary outcome of cardiovascular death or hospitalization for heart failure.Citation40 The <65 age MarketScan population (age 30–64) in this study reflects a real-world SGLT-2i user population. In this population, the relative HF hospitalization rate of 0.70 was attenuated from, but was still similar to, rates observed in the clinical trials: CANVAS (0.51), DECLARE-TIMI (0.64), and EMPA-REG (0.65).Citation10–Citation12
As in other US SGLT-2i economic evaluations, we estimated a high annual cost for SGLTI-2i use, $5515 (2021 dollars).Citation15,Citation18,Citation20 Using this cost estimate, SGLT-2i’s show a negative per person net-benefit of −$4106 for 1 year of use under the health care payer perspective when effectiveness regarding HF hospitalization and post-discharge mortality is examined. A sensitivity analysis reducing SGLT-2i cost by 80% shifted the net-benefit higher by $4004. For the payer perspective, this would essentially equalize costs and benefits. In a recent economic evaluation for dapagliflozin use among individuals with T2D and HFrEF from a UK payer perspective, the annual cost of SGLT-2i use was less than £500.Citation17 Converted to US$ using 2020 exchange rates, that SGLTI-2i cost would be less than $700, a price reduction from our base case estimate of >85%.
CBAs unlike cost-effectiveness analyses (CEAs) allow for direct comparison between benefits and costs as both are reported in monetary units. The ability to report benefits as a monetary value (US dollars) rather than incremental health benefits or quality-adjusted life-years (QALY), as seen in CEAs, is important for use and ease of interpretation by payers and policy makers alike who may be unfamiliar with pharmacoeconomic methodologies. Often coverage decisions made by payers are centered around clinical benefits of an intervention and the associated economic impact the intervention may have. Our study reported both real-world utilization and hospitalization rates as well as the results of a model-based CBA in order to illustrate the current effects of SGLT2-i use in HFrEF and potential economic impacts.
Despite net-benefits not overcoming the prescription costs under the health payer perspective, societal net-benefits are substantial. Our CBA models calculated net-benefits (cost savings) for individuals age 30–64 ranging from $6,094,674 to $8,487,709. The real-world per person net benefit was $14,269. These societal cost savings indicate the significant cost burden due to HF hospitalizations and subsequent post-discharge care and CV-related mortality that can be averted with the use of SGLT-2i in T2D patients with HFrEF. McEwan et al used a lifetime Markov model for worsening HF (hospitalizations, urgent care, adverse events) and all-cause mortality among a population with T2D and HFrEF (average age 66).Citation17 They estimated average life-years of 6.20 for dapagliflozin users compared to 5.62 for non-SGLT-2i users.Citation17 Time spent in the hospital and recovering at post-discharge facilities or at home is time that a patient may be unable to work and/or to participate in normal activities. This is particularly impactful in a younger population, such as the one modeled in our study.
In this age 30–64 population with T2D the prevalence of HF was 3.2%, with HF prevalence 4.1% for the 55–64 age group. Few studies have examined HF prevalence in T2D populations by age group.Citation41 HF prevalence in the EMPA-REG study was 10%, and in the CANVAS study was 14–15%, rates substantively higher than in our study.Citation3,Citation41 The mean age in those clinical trial populations was 63–64 years, an older average compared to the mean age of 56 years in the MarketScan data. HF prevalence has been previously observed to increase sharply with age, and further studies examining prevalence of HF in younger populations are needed.Citation4,Citation42
Our study modeled HF hospitalization events for patients with T2D and HFrEF. More recently, similar effectiveness has been demonstrated with empagliflozin in individuals with heart failure with preserved ejection fraction (HFpEF) with or without diabetes.Citation43 The guideline for the management of HF now recommends SGLT2i use for patients with either HFrEF or HFpEF including those without diabetes.Citation44 In addition, empagliflozin and canagliflozin have demonstrated a reduction in composite major adverse cardiac events (MACE).Citation10,Citation12 The benefit of SGLT-2i’s may be underestimated when only the impact of HF hospitalization and post-discharge mortality are assessed, and only for patients with T2D and HFrEF. In comparison to McEwan et al, Kansal et al estimated the cost-effectiveness from a UK payer perspective of empagliflozin use in a population with T2D and established cardiovascular disease (average age 63) using a lifetime discrete event simulation that modeled 10 potential clinical events.Citation16 They reported average life-years for empagliflozin users was 14.0 compared to 11.9 for non-SGLTI-2i users.Citation16 SGLT-2i’s have demonstrated renoprotective effects, and these were included in the analysis by Kansal et al.Citation16,Citation45 Importantly, the renal protective effects that SGLT-2i have demonstrated in clinical trials may prove to have substantial cost savings, especially when SGLT-2i use is initiated in younger populations before significant renal dysfunction has occurred.Citation45,Citation46
The one-way sensitivity analyses’ results indicate that HF hospitalization, hospital readmission, and mortality rates have the largest impact on net-benefit determination. Results were particularly sensitive to reductions in baseline HF hospitalizations, producing a negative net-benefit in the societal perspective, real-world model. These results are expected as a decreased risk of HF hospitalization or post-discharge mortality decreases the need at a population level for high dollar treatments to prevent these events. Our HF hospitalization cost was lower than estimates in other studies.Citation18,Citation20 Increasing the HF hospitalization cost by 20% in the sensitivity analysis however had minimal impact on results. Discounts in SGLT-2i prescription costs increased the net-benefit of SGLT-2i use, suggesting price reductions or generic alternatives for SGLT-2i in the future may increase the net-benefit of SGLT-2i use from both a health care payer and societal perspective.
Limitations
This study utilized HF hospitalization events captured in a 1-year time frame, and likely underestimated the impact of SGLT-2i on HF hospitalization rates. Nguyen et al estimated the cost-effectiveness of empagliflozin at preventing CV morbidity and mortality among patients with T2D and high CV risk from a US payer perspective.Citation18 Using a lifetime Markov model they estimated an incremental cost per quality-adjusted life-year for SGLT-2i use of $76,167. Annual incidence of HF hospitalization was lower than in our study population with T2D and HFrEF, and SGLT-2i drug cost was a wholesale acquisition cost estimate and was 15% higher than our SGLT-2i cost estimate. Only direct medical costs of HF hospitalization and post-discharge care were modeled in our study. Other costs associated with HFrEF post-discharge patient care (office visits, emergency department visits, etc.) not incorporated into the model may further underestimate avoided costs associated with SGLT-2i use.
Benefits associated with reductions in mortality were the overwhelming driver of benefits in our societal model, particularly for those with the greatest number of future productive years, those age 30–44. Avoided mortality was valued in this study using the human capital approach – the discounted sum an individual would have otherwise earned had the individual not died. While friction cost is an alternative method sometimes used in cost-effectiveness analyses, particularly analyses where a quality-adjusted life-year (QALY) is the outcome, cost–benefit analyses have traditionally utilized a human capital approach or willingness to pay approach (embodied by the value of a statistical life).Citation47 In our analysis, the lost productivity estimate was assumed to apply to all individuals in the population. While this may overstate lost productivity using a definition of foregone wages, we believe it more equitably estimates value for avoided mortality between employed and unemployed individuals.
We used MarketScan as the source for real-world SGLT-2i utilization and event rates for individuals with T2D and HFrEF. MarketScan is an administrative claims database, and diagnosis attributions for individuals may have been misclassified. Real-world SGLT-2i users were identified based on having at least one prescription claim. Including individuals with only one prescription claim however serves to underestimate benefits as reductions in costly events due to SGLT-2i use are thus attenuated. Though not truly reflective of real-world utilization trends, adherence to SGLT-2i therapy was assumed to be 100% to reflect optimal treatment. There is likely a substantive difference in adherence rates between clinical trial and real-world use. These limitations related to prescriptions may be one reason the magnitude of the overall relative risk reduction (0.70) was diminished for the MarketScan population compared to the clinical trials. Of note, however, the age 45–54 group in the MarketScan population had a larger divergence in HF hospitalization rate between SGLT-2i and no SGLT-2i use (8.8% vs 17.3%) than the age 55–64 group (11.8% vs 14.7%). While a recent meta-analysis found similar risk reduction rates for a combined outcome of HF hospitalization and cardiovascular death between individuals age ≥65 and <65, examination of potential differential effects in age groups <65 warrants further study.Citation40 Recently, the Patient-Centered Outcomes Research Institute (PCORI) funded two research studies on second-line therapies for individuals with T2D and moderate cardiovascular risk.Citation48,Citation49 These studies should provide real-world effectiveness and safety information that will be useful for both patients and providers.
This study focused on adults age <65, ultimately creating a model for individuals aged 30–64. Published peer-reviewed information on HF-related events for individuals <65 is limited; therefore, some estimates utilized in the model were not specific to a younger population. Sensitivity analyses served to account for differences in rates that may apply to a younger population. However, parameter inputs of recovery time in nursing facilities or at home were obtained from literature that focused primarily on ≥65 populations and these estimates were not altered within sensitivity analyses. Therefore, indirect cost savings may be slightly overestimated. While this study provided a unique perspective as younger adults with HFrEF are rarely the primary focus in HF studies, similar studies examining the impact of SGLT-2i among individuals age ≥65 years would be informative.
Conclusion
SGLT-2i utilization steadily increased among individuals with T2D and HFrEF from <5% in 2013 to 18% in 2018, with lower HF hospitalization rates observed among SGLT-2i users. Real-world data support a beneficial effect of SGLT-2i use on HF hospitalization rates in patients with T2D and HFrEF. RWD HF hospitalization relative risk rates were consistent with clinical trial rates from CANVAS, DECLARE-TIMI and EMPA-REG despite being representative of a younger working population. SGLT-2i utilization rates have increased over a 5-year span in patients with T2D and HFrEF and likely will continue to increase as prescribing practices align with current ADA guidelines. The current high prescription costs prevent SGLT-2i from demonstrating a positive net-benefit under a health payer perspective despite cost savings obtained from HF hospitalizations and post-discharge deaths averted. However, SGLT-2i demonstrates significant positive net benefit under a societal net-benefit when cost savings from productivity loss is incorporated. Future studies should focus on the additional economic impact of SGLT-2i’s renoprotective and composite MACE effects, and perhaps should employ a longitudinal perspective to investigate compounded benefit of SGLT-2i patients with T2D and HFrEF or HFpEF over time.
Ethics Statement
This research received exemption for the need of ethical approval by the University of New Mexico’s IRB due to its categorization as secondary research on data or specimens with no consent required.
Acknowledgments
Prior peer-reviewed presentation at a professional/scientific conference: A preliminary analysis of this research was presented as a poster presentation at the International Society for Pharmacoeconomic Outcomes Research (ISPOR) Virtual 2021 Conference and an encore presentation at Academy of Managed Care Pharmacy (AMCP) Nexus Conference 2021: Glover S, Ray G, Borrego M, Roberts M. Cost-Benefit Analysis of Sodium Glucose Cotransporter-2 Inhibitor Use for Patients <65 Years with Type 2 Diabetes and Heart Failure with Reduced Ejection Fraction. PMU12, 2021-05, ISPOR 2021, Montreal, Canada. Available at https://www.ispor.org/heor-resources/presentations-database/presentation/intl2021-3338/110539.
Disclosure
Melissa H Roberts reports grants from Sunovion Pharmaceuticals and GlaxoSmithKline and non-financial medical writing support for a journal article from AstraZeneca, outside the submitted work. The authors report no other potential conflicts of interest in relation to this work.
Additional information
Funding
References
- National Diabetes Statistics Report 2020. Estimates of diabetes and its burden in the United States; 2020:32.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
- Seferović PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(5):853–872. doi:10.1002/ejhf.1170
- Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24(9):1614–1619. doi:10.2337/diacare.24.9.1614
- Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324(5):488–504. doi:10.1001/jama.2020.10262
- Dulai R, Sheikh AS, Qureshi A. Prevalence, clinical characteristics and outcomes of HF with preserved versus reduced ejection fraction. Br J Cardiol. 2016;23:1–40.
- American Diabetes Association. Standards of medical care in diabetes—2022 diabetes care. Available from: https://diabetesjournals.org/care/article/45/Supplement_1/S1/138921/Introduction-Standards-of-Medical-Care-in-Diabetes. Accessed May 10, 2022.
- Association AD. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S111–S124. doi:10.2337/dc21-S009
- Standl E, Schnell O. Treatment paradigm shifting implications of recent cardiovascular outcome trials: core insights on the brink of the 2020ies. Diabetes Res Clin Pract. 2020;161:108054. doi:10.1016/j.diabres.2020.108054
- Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and heart failure in Type 2 diabetes mellitus. Circulation. 2018;138(5):458–468. doi:10.1161/CIRCULATIONAHA.118.034222
- Kato Eri T, Silverman Michael G, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in Type 2 diabetes mellitus. Circulation. 2019;139(22):2528–2536. doi:10.1161/CIRCULATIONAHA.119.040130
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Drugs@FDA: FDA-approved drugs. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=204042. Accessed March 15, 2021.
- Garry EM, Schneeweiss S, Eapen S, et al. Actionable real-world evidence to improve health outcomes and reduce medical spending among risk-stratified patients with diabetes. J Manag Care Spec Pharm. 2019;25(12):1442–1452. doi:10.18553/jmcp.2019.25.12.1442
- Arbel R, Aboalhasan E, Hammerman A, Azuri J. Sodium-glucose cotransporter 2 inhibitors for prevention of heart failure events in patients with Type 2 diabetes mellitus: a cost per outcome analysis. Clin Drug Investig. 2020;40(7):665–669. doi:10.1007/s40261-020-00929-z
- Kansal A, Reifsnider OS, Proskorovsky I, et al. Cost‐effectiveness analysis of empagliflozin treatment in people with Type 2 diabetes and established cardiovascular disease in the EMPA‐REG OUTCOME trial. Diabet Med. 2019;36(11):1494–1502. doi:10.1111/dme.14076
- McEwan P, Darlington O, McMurray JJV, et al. Cost‐effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health‐economic analysis of DAPA‐HF. Eur J Heart Fail. 2020;22(11):2147–2156. doi:10.1002/ejhf.1978
- Nguyen E, Coleman CI, Nair S, Weeda ER. Cost-utility of empagliflozin in patients with type 2 diabetes at high cardiovascular risk. J Diabetes Complications. 2018;32(2):210–215. doi:10.1016/j.jdiacomp.2017.10.006
- Kamstra R, Durkin M, Cai J, et al. Economic modelling of costs associated with outcomes reported for type 2 diabetes mellitus (T2DM) patients in the CANVAS and EMPA-REG cardiovascular outcomes trials. J Med Econ. 2019;22(3):280–287. doi:10.1080/13696998.2018.1562817
- McEwan P, Bennett H, Khunti K, Wilding J, Edmonds C, Thuresson M. Assessing the cost‐effectiveness of sodium–glucose cotransporter‐2 inhibitors in type 2 diabetes mellitus: a comprehensive economic evaluation using clinical trial and real‐world evidence. Diabetes, Obesity Metab. 2020;22:2364–2374. doi:10.1111/dom.14162
- IBM Watson Health. IBM MarketScan commercial claims and encounters database 2012–2018. Ann Arbor, United States.
- Truven Health Analytics. History and information; 2020. Available from: https://www.ibm.com/watson-health/about/truven-health-analytics. Accessed March 15, 2021.
- Clinical Classifications Software Refined (CCSR) for ICD-10-CM diagnoses. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/dxccsr.jsp. Accessed August 6, 2021.
- HCUP. Clinical Classifications Software (CCS) for ICD-9-CM. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/AppendixASingleDX.txt. Accessed August 6, 2021.
- Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Circulation. 2003;108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- RED BOOK search results - MICROMEDEX. Available from: https://www-micromedexsolutions-com.libproxy.unm.edu/micromedex2/librarian/CS/17B180/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/10A6F1/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/redbook.ShowProductSearchResults?SearchTerm=BELSOMRA&searchType=redbookProductName&searchTermId=43316&searchContent=REDBOOK&searchFilterAD=filterADActive&searchFilterRepackager=filterExcludeRepackager&searchPattern=%5Ebelsomra. Accessed April 13, 2021.
- Institute for Clinical and Economic Review. ICER’s reference case for economic evaluations: principles and rationale; 2020.
- Bureau of labor statistics data. Available from: https://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths. Accessed March 21, 2021.
- HCUPnet. HCUPnet; 2021.
- Voigt J, John MS, Taylor A, Krucoff M, Reynolds MR, Michael Gibson C. A reevaluation of the costs of heart failure and its implications for allocation of health resources in the United States. Clin Cardiol. 2014;37(5):312–321. doi:10.1002/clc.22260
- Echouffo-Tcheugui JB, Bishu KG, Fonarow GC, Egede LE. Trends in health care expenditure among US adults with heart failure: the medical expenditure panel survey 2002–2011. Am Heart J. 2017;186:63–72. doi:10.1016/j.ahj.2017.01.003
- Schmier JK, Ong KL, Fonarow GC. Cost-effectiveness of remote cardiac monitoring with the CardioMEMS heart failure system. Clin Cardiol. 2017;40(7):430–436. doi:10.1002/clc.22696
- Grosse SD, Krueger KV, Pike J. Estimated annual and lifetime labor productivity in the United States, 2016: implications for economic evaluations. J Med Econ. 2019;22(6):501–508. doi:10.1080/13696998.2018.1542520
- Bureau UC. Age & sex tables. The United States Census Bureau. Available from: https://www.census.gov/topics/population/age-and-sex/data/tables.html. Accessed March 15, 2021.
- Lee DS, Johansen H, Gong Y, et al. Regional outcomes of heart failure in Canada. Can J Cardiol. 2004;20(6):599–607.
- Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA. 2007;297(17):1883–1891. doi:10.1001/jama.297.17.1883
- Wong CM, Hawkins NM, Jhund PS, et al. Clinical characteristics and outcomes of young and very young adults with heart failure: the CHARM programme (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity). J Am Coll Cardiol. 2013;62(20):1845–1854. doi:10.1016/j.jacc.2013.05.072
- Basic C, Rosengren A, Alehagen U, et al. Young patients with heart failure: clinical characteristics and outcomes. Data from the Swedish heart failure, national patient, population and cause of death registers. Eur J Heart Fail. 2020;22(7):1125–1132. doi:10.1002/ejhf.1952
- Taylor CJ, Ordóñez-Mena JM, Roalfe AK, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. BMJ. 2019;364:l223. doi:10.1136/bmj.l223
- Bhattarai M, Salih M, Regmi M, et al. Association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in patients with Type 2 diabetes and other risk factors for cardiovascular disease: a meta-analysis. JAMA Netw Open. 2022;5(1):e2142078. doi:10.1001/jamanetworkopen.2021.42078
- Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. doi:10.1186/s12933-018-0728-6
- Zhou L, Deng W, Zhou L, et al. Prevalence, incidence and risk factors of chronic heart failure in the Type 2 diabetic population: systematic review. Curr Diabetes Rev. 2009;5(3):171–184. doi:10.2174/157339909788920938
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi:10.1056/NEJMoa2107038
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–e1032. doi:10.1161/CIR.0000000000001063
- Kluger AY, Tecson KM, Lee AY, et al. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc Diabetol. 2019;18:99. doi:10.1186/s12933-019-0903-4
- Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39.
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Guidelines for regulatory impact analysis. ASPE. Available from: https://aspe.hhs.gov/reports/guidelines-regulatory-impact-analysis. Accessed May 10, 2022.
- Patient Centered Outcomes Research. Second-line therapies for patients with Type 2 diabetes and moderate cardiovascular disease risk. second-line therapies for patients with Type 2 diabetes and moderate cardiovascular disease risk. PCORI; 2021. Available from: https://www.pcori.org/research-results/2021/second-line-therapies-patients-type-2-diabetes-and-moderate-cardiovascular-disease-risk. Accessed May 10, 2022.
- Patient Centered Outcomes Research. The comparative effectiveness and safety of four second-line pharmacological strategies in a Type 2 diabetes study (The CER-4-T2D Study). The comparative effectiveness and safety of four second-line pharmacological strategies in a Type 2 diabetes study (The CER-4-T2D Study). PCORI; 2021. Available from: https://www.pcori.org/research-results/2021/comparative-effectiveness-and-safety-four-second-line-pharmacological-strategies-type-2-diabetes-study-cer-4-t2d-study. Accessed May 10, 2022.
- MedLibrary.org. FARXIGA (AstraZeneca Pharmaceuticals LP): FDA package insert. Available from: https://medlibrary.org/lib/rx/meds/farxiga-2/.Accessed August 9, 2021.
- Pharmaceuticals BI, Inc. Jardiance (Boehringer Ingelheim Pharmaceuticals, Inc.): FDA package insert. MedLibrary.org. Available from: https://medlibrary.org/lib/rx/meds/jardiance-3/. Accessed August 9, 2021.
- Pharmaceuticals J, Inc. INVOKANA (Janssen Pharmaceuticals, Inc.): FDA package insert. MedLibrary.org. Available from: https://medlibrary.org/lib/rx/meds/invokana-2/. Accessed August 9, 2021.
- Boehringer Ingelheim. A Phase III, multicentre, international, randomised, parallel group, double blind cardiovascular safety study of BI 10773 (10 Mg and 25 Mg administered orally once daily) compared to usual care in Type 2 diabetes mellitus patients with increased cardiovascular risk. clinicaltrials.gov; 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT01131676. Accessed August 8, 2021.
- Janssen Research & Development, LLC. A randomized, multicenter, double-blind, parallel, placebo-controlled study of the effects of JNJ-28431754 on cardiovascular outcomes in adult subjects with Type 2 diabetes mellitus. clinicaltrials.gov; 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT01032629. Accessed August 8, 2021.
- AstraZeneca. Dapagliflozin effect on cardiovascular events A multicenter, randomized, double-blind, placebo-controlled trial to evaluate the effect of dapagliflozin 10 Mg once daily on the incidence of cardiovascular death, myocardial infarction or ischemic stroke in patients with Type 2 diabetes. clinicaltrials.gov; 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT01730534. Accessed August 8, 2021.
- Amos TB, Montejano L, Juneau P, Bolge SC. Healthcare costs of urinary tract infections and genital mycotic infections among patients with type 2 diabetes mellitus initiated on canagliflozin: a retrospective cohort study. J Med Econ. 2017;20(3):303–313. doi:10.1080/13696998.2016.1259167