Abstract
Background
Boceprevir and telaprevir have recently showed dramatically better treatment outcomes than conventional PEGylated interferon plus ribavirin for the treatment of hepatitis C virus genotype 1, but the average cost per patient is unknown.
Methods
In the UK context, we performed a budget impact analysis to estimate the average per patient cost of adding boceprevir or telaprevir to PEGylated interferon plus ribavirin therapy. We considered both standard-duration therapy and response-guided therapy regimens of boceprevir and telaprevir for treatment-naïve and treatment-experienced patients. Our model utilized monthly discontinuation rates. We built a Bayesian Markov model to account for uncertainty associated with the clinical input and cost data.
Results
The total average cost of response-guided therapy with boceprevir is £22,850 and £25,060 for treatment-naïve and treatment-experienced patients, respectively. By comparison, the total average cost of response-guided therapy with telaprevir was £29,930 and £31,880 for treatment-naïve and treatment-experienced patients, respectively, whereas the total average cost of standard-duration boceprevir is £34,680 and £34,350 and for telaprevir was £32,530 and £31,680 for treatment-naïve and treatment experienced patients, respectively.
Conclusion
Our results demonstrate that response-guided therapy with boceprevir is notably less costly than response-guided therapy with telaprevir. Our results also suggest that the standard treatment duration of boceprevir is slightly more costly than the standard treatment duration of telaprevir.
Introduction
Two new direct-acting antivirals, boceprevir and telaprevir, have recently showed dramatically better treatment outcomes than conventional PEGylated interferon plus ribavirin for the treatment of hepatitis C virus (HCV) genotype 1.Citation1 Boceprevir is a protease inhibitor that binds to the HCV nonstructural 3 active site, and telaprevir is a linear peptidomimetic HCV nonstructural 3/4A serine protease inhibitor. Both treatments have recently been approved in Europe and the US for the treatment of HCV genotype 1 infection.Citation2–Citation4
The two direct-acting antivirals are both administered with the current standard of care, ie, PEGylated interferon plus ribavirin, but follow different treatment algorithms. The standard duration of boceprevir treatment is 44 weeks, where the treatment is administered from week 4 to 48 in addition to a 48-week treatment course of PEGylated interferon plus ribavirin. Recent clinical trials have also investigated the merits for response-guided therapy, in which patients receive boceprevir from week 4 to 24 (20 weeks) if HCV is undetectable at 8 weeks, and from week 4 to 36 (32 weeks) if HCV is undetectable at 24 weeks (but detectable at 8 weeks). The standard treatment duration for telaprevir is 12 weeks, where the treatment is administered from week 1 to 12 in addition to a 48-week treatment course of PEGylated interferon plus ribavirin. Recent clinical trials have also investigated the merits of telaprevir response-guided therapy, in which patients only receive PEGylated interferon plus ribavirin for the first 24 weeks if HCV is undetectable at 4 weeks and 12 weeks.Citation1,Citation5,Citation6
A recent indirect comparison meta-analysis and a recent multiple treatment comparison meta-analysis both demonstrated that boceprevir and telaprevir as standard duration are comparable in terms of achieving a sustained virological response (SVR).Citation1,Citation5 These analyses also suggested that the efficacy of response-guided therapy is comparable with the efficacy of standard duration duration.Citation1,Citation5 However, the two direct-acting antivirals are associated with exacerbations of differing adverse events that each require different types of treatment. Boceprevir is associated with an exacerbation of anemia and neutropenia,Citation1 the former of which may require blood transfusion or administration of erythropoietin until the blood cell count is back to normal. Telaprevir is associated with an exacerbation of rash and pruritus,Citation1 which requires treatments such as Atarax® or hydrocortisone until the symptoms have disappeared.
In the UK, the weekly cost of boceprevir 800 mg three times daily is £700, and the weekly cost of telaprevir 750 mg three times daily is £1866.50. The cost of a full-length boceprevir treatment course (44 weeks) is £39,800, plus an approximate £10,000 for a 48-week course of standard of care; thus, a total of approximately £49,800. The cost of boceprevir response-guided therapy is £14,000 (20 weeks) for early responders and £22,400 (32 weeks) for late responders, plus an approximate £4000 and £6000 for standard of care for early and late responders, respectively. Thus, the total cost is approximately £18,000 for boceprevir response-guided therapy early responders and £28,400 for boceprevir response-guided therapy late responders. The cost of a full-length telaprevir treatment is £22,398 (12 weeks) plus an approximate £10,000 for a 48-week course of standard of care; thus, a total of approximately £32,398. With telaprevir response-guided therapy, early responders only receive standard of care for 24 weeks (approximately £5000), and thus, the approximate total cost for telaprevir response-guided therapy in early responders is £27,398. The total cost for telaprevir response-guided therapy in late responders is the same as for the full-course treatment.
Considering the differences in the courses of treatments with standard duration and response-guided boceprevir and telaprevir, as well as the differences in handling associated adverse events, the various treatment courses with boceprevir and telaprevir are likely to have different cost profiles. Because the current clinical evidence suggests comparability in terms of efficacy, finding the least costly of the two available treatment courses is important.
The purpose of our study was to estimate and compare the average cost per patient on boceprevir and telaprevir, and to estimate and compare the cost per SVR with boceprevir, telaprevir, and standard of care treatments. We hypothesized that: the average cost per patient for response-guided boceprevir is lower than that of response-guided telaprevir; the average cost per patient for standard-duration boceprevir and telaprevir is comparable; and the average cost per SVR achieved is comparable for boceprevir, telaprevir, and standard of care. We assess these three hypotheses for both naïve and experienced patients.
Materials and methods
We performed a budget impact analysis to estimate the average cost per patient of adding boceprevir or telaprevir to standard of care for both standard-duration and response-guided regimens.
Materials
Our models used data on 4-week discontinuation rates. We contacted the authors of each published trial and industry to obtain these data. For boceprevir, detailed 4-week discontinuation rates for standard duration therapy and response-guided therapy were made available to us from two boceprevir trials.Citation7,Citation8 For telaprevir, these data were not made available to us; however, we were able to utilize the discontinuation data in the trial publications. In most cases, data were only available at weeks 12, 24, and 48.Citation9–Citation14 We used linear interpolation to determine values at 4-week intervals. Because we were not able to retrieve any discontinuation data on response-guided telaprevir among treatment-experienced patients, we derived these data mathematically using the available response-guided discontinuation data for telaprevir naïve-patients and response-guided boceprevir patients. We assumed the ratio between response-guided therapy discontinuation rates in naïve and experienced patients were the same for boceprevir and telaprevir. Therefore, we calculated this ratio for early responders, and separately, for late responders receiving boceprevir. We subsequently multiplied the 4-week telaprevir discontinuation rates for naïve patients (early and late responders separately) with the obtained ratios between discontinuation rates for naïve and experienced patients.
Our model also utilized adverse event data, ie, the proportion of patients expected to experience an adverse event common to direct-acting antivirals and standard of care treatment (ie, anemia, neutropenia, rash, and pruritus), which was derived from the indirect comparison by Cooper et al.Citation1 Clinical data, such as the frequency of medical visits and testing required by those on treatment were also included in the model; these data were provided to us through consultations with practicing hepatologists in the US and the UK, and currently available treatment algorithms for HCV.Citation6 The costs associated with the management of adverse events and clinical monitoring were provided to us by contacting pharmacies and by interviewing practicing hepatologists and their clinic staff (data on these items were not consistently available in clinical guideline documents). MIMS UK was used to find cost data for the individual drugs (boceprevir, telaprevir, PEGylated interferon alfa-2a and alfa-2b, and ribavirin).Citation15 Finally, the rates of SVR for each treatment regimen were extracted from the indirect comparison by Cooper et alCitation1 and the Committee on Medicinal Products for Human Use database. The individual inputs and respective data sources are presented in .
Table 1 Components and data sources for the budget impact analysis
Budget impact analysis models
Our primary model is a Bayesian Markov model that incorporates uncertainty associated with the clinical input data (eg, adverse event rates). The Bayesian analyses were carried out in WinBUGS version 1.4.3 (Cambridge, UK). Our secondary model is a simplified version of our primary model built in Microsoft Excel. The WinBUGS code for our primary model and the Excel spreadsheet for our secondary model are included in the Appendices.
We modeled the cost of patients over the course of treatment and follow-up using 4-week intervals for the first 48 weeks and considered the following 24 weeks (follow-up period) as one time interval. For each of the first 12 intervals, we estimated the 4-week cost of the intervention, the 4-week cost of general clinical monitoring and management, and the 4-week cost of treating adverse events. For clinical monitoring and management costs, we assumed that patients who discontinued were followed up for an additional 24 weeks after discontinuation, and thus, the proportion of patients requiring clinical monitoring or management were those still on treatment plus those who discontinued less than 24 weeks earlier. We also estimated the cost of clinical monitoring and management during the 24-week follow-up period following treatment. Using the 4-week estimates, we estimated the cumulative cost per every 4 weeks of treatment, as well as the total average cost per patient.
For our primary (Bayesian Markov) model, we used binomial probability distributions for the proportion of discontinuations, the proportion of adverse events, and the proportions of early and late responders to reflect the uncertainty associated with clinical input in the cost model. We also used binomial distributions for the proportion of SVRs in the standard of care group. However, we obtained the proportions of SVRs for boceprevir and telaprevir by applying the odds ratio estimates from a recent multiple treatment comparison.Citation5 In this context, we used normal distributions for the corresponding log odds ratio. For all costs related to clinical monitoring and treatment of adverse events (eg, cost of visit to the general practitioner or cost of blood transfusion), we used a normal distribution with our cost estimate as the mean standard deviation equal to approximately 20% of that estimate (ie, approximately ± 40% equals two standard deviations).
For our primary model, we conducted Markov chain simulations based on a hypothetical cohort of 1000 patients (assuming the above depicted clinical estimates), and subsequently calculated the average costs per patient as our output. For our secondary model, ie, the Microsoft Excel spreadsheet, we used all available proportion and average cost estimates as raw inputs.
Results
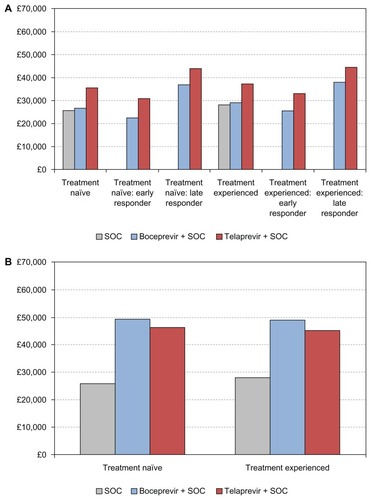
shows the total average costs and cost per SVR for response-guided therapy with boceprevir and telaprevir for both treatment-naïve and treatment-experienced patients, as derived from our primary (Bayesian Markov) model. Note that presents the same cost estimates as , but from our secondary model, the simplified Excel sheet model. shows the cumulative cost per patient over the treatment course and follow-up for those treated with response-guided therapy. Costs are presented separately for early and late responders, and as a weighted average for all patients receiving response-guided therapy. According to our primary model, the total average cost of response-guided therapy with boceprevir (all patients) is £22,850 (95% credible interval [CrI] £22,400–23,300) for treatment-naïve patients and £25,060 (95% CrI £24,320–25,770) for treatment-experienced patients. By comparison, the total average cost of response-guided therapy with telaprevir is £29,930 (95% CrI £29,560–30,280) for treatment-naïve patients and £31,880 (95% CrI £31,220–32,560) for treatment-experienced patients. The cost per SVR with boceprevir response-guided therapy is £26,748 (95% CrI £25,978–27,489) for treatment-naïve patients and £29,110 (95% CrI £27,886–30,320) for treatment-experienced patients. By comparison, the average cost per SVR with telaprevir response-guided therapy is £35,536 (95% CrI £34,760–36,237) for treatment-naïve patients and £37,194 (95% CrI £36,581–37,781) for treatment-experienced patients (see ).
Figure 1 Average cumulative cost per patient treated with response-guided therapy.
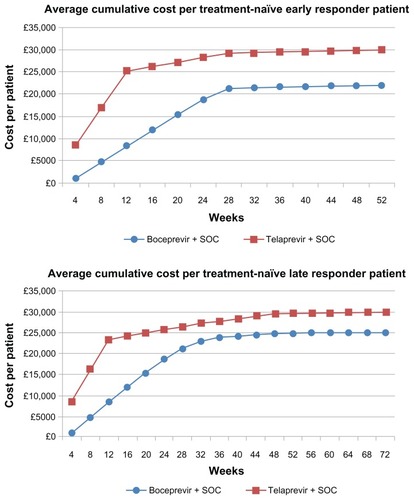
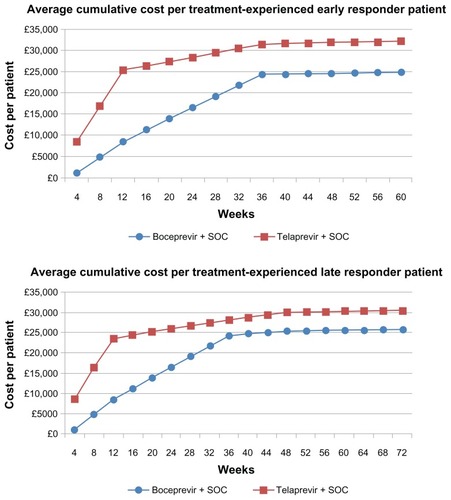
Figure 2 Average cumulative cost per patient treated with standard-duration therapy.
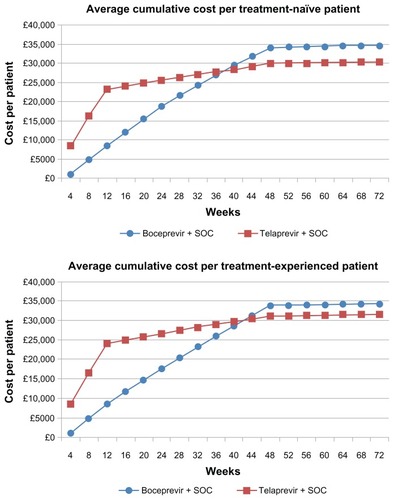
Figure 3 Cost per sustained virologic response for response-guided therapy (A) and standard-duration therapy (B).
Appendix 1 Average cost estimates from the secondary (Microsoft Excel) budget impact analysis for response-guided therapy
Table 2 Median cost estimates and 95% credible intervals from the primary (Bayesian) budget impact analysis for response-guided therapy
shows the total costs and cost per SVR for standard-duration boceprevir and telaprevir for both treatment-naïve and experienced patients, as derived from our primary (Bayesian Markov) model. Note that shows the same cost estimates as , but from our secondary model, the simplified Excel sheet model. presents the cumulative cost per patient over the treatment course and follow-up for those treated with a standard duration of therapy. According to our primary model, the total average cost of standard duration boceprevir is £34,680 (95% CrI £33,850–35,490) for treatment-naïve patients and 34,350 (95% CrI £33,390–35,260) for treatment-experienced patients. By comparison, the total average cost of standard duration telaprevir is £32,530 (95% CrI £32,160–32,880) for treatment-naïve patients and £31,680 (95% CrI £31,200–32,450) for treatment-experienced patients. The cost per SVR with standard duration boceprevir is £49,540 (95% CrI £48,350–50,700) for treatment-naïve patients and £49,070 (95% CrI £47,700–50,510) for treatment-experienced patients. By comparison, the total average cost of SVR with a standard duration of telaprevir is £46,471 (95% CrI £45,943–46,971) for treatment-naïve patients and £45,260 (95% CrI £44,580–46,360) for treatment-experienced patients.
Appendix 2 Average cost estimates from the secondary (Microsoft Excel) budget impact analysis for standard-duration therapy
Table 3 Median costs estimates and 95% credible intervals from the primary (Bayesian) budget impact analysis for standard-duration therapy
also presents the cost estimates and cost per SVR for standard of care. The total average cost of standard of care is £10,010 (95% CrI £9920–10,360) for treatment-naïve patients and £6730 (95% CrI £6610–6850) for treatment-experienced patients. The cost per SVR is £25,740 (95% CrI £24,670–26,860) for treatment-naïve patients and £28,040 (95% CrI £27,540–28,540) for treatment-experienced patients (see also ).
Interpretation
In summary, the total average cost of a standard duration of treatment with boceprevir is slightly more costly than a standard duration of treatment with telaprevir, and so is the average cost per SVR. However, the total average cost of response-guided therapy with boceprevir is notably smaller than response-guided therapy with telaprevir, and so is the average cost per SVR. Moreover, the cost per SVR is comparable for response-guided therapy with boceprevir and standard of care, while the cost per SVR is more costly with telaprevir response-guided therapy.
The observed differences can be explained by the differing rates with which costs are accumulated under the different treatments. With telaprevir (both standard-duration and response-guided therapy), all patients receive the costly component of treatment, telaprevir, within the first 12 weeks. With boceprevir, patients are only given standard of care for the first 4 weeks of the treatment course, whereas the most costly component of treatment, boceprevir, is administered throughout the remaining 44 weeks (or less with response-guided therapy). With response-guided therapy in particular, the cost of boceprevir is cut dramatically, whereas the cost of telaprevir remains the same. The discontinuation rates, and thus the proportion of patients remaining on treatment, also play a role in the cost of boceprevir and telaprevir treatment. Most patients continue for 12 weeks of the full 48-week treatment course, and therefore all receive a full course of telaprevir. By contrast, patients who discontinue between weeks 12 and 48 will only receive part of the full course of boceprevir. Thus, the total average costs are typically pulled towards favoring boceprevir, because a full course of treatment is not wasted on those patients who discontinue.
The costs associated with clinical monitoring and treatment of adverse events are both relatively small compared with the costs of standard of care and boceprevir/telaprevir, and thus have little relative impact on total average cost estimates.
Discussion
We have estimated the total average cost and cost per SVR for standard duration and response-guided therapy with boceprevir and telaprevir added to standard of care, and for standard of care alone. Our results demonstrate that response-guided therapy with boceprevir is notably less costly than response-guided therapy with telaprevir, and that the cost per SVR for response-guided therapy with boceprevir is comparable with the cost per SVR for standard of care. Our results also suggest that standard-duration boceprevir is slightly more costly than standard-duration telaprevir.
Our analyses have several strengths and limitations. We have made use of the strongest clinical evidence in the form of recent results from indirect and multiple treatment comparison meta-analyses. We have also made use of detailed discontinuation data from clinical trials. Further, we interviewed several practicing hepatologists and their clinic staff members to get realistic estimates of costs, standards for adverse event treatments, and standards for clinical monitoring and management, and incorporated uncertainty surrounding our estimates through realistic probability distributions in our Bayesian model. However, the current clinical evidence remains limited because only a few trials for each of the considered treatment regimens (standard-duration and response-guided therapy) and patient groups (naïve and experienced) exist. Thus, our assumptions concerning comparable SVR and discontinuation rates may be challenged with emerging evidence from future clinical trials. In addition, we had access to detailed discontinuation rates only for boceprevir trials. For the modeling of adverse events, we assumed a constant relative risk within each 4-week interval. However, it is possible that this occurs with differing risks throughout the full course of treatment. For example, one could argue patients are more likely to suffer from adverse events at an early stage. However, the costs of adverse events are comparably small. Thus, it is unlikely that our results would be sensitive to deviations from the currently assumed adverse event rates.
Our results come with straightforward implications for clinical practice. Assuming that response-guided therapy is as effective as a standard duration of treatment, boceprevir added to standard of care appears to be advantageous both from a patient and societal perspective. Patients will typically prefer the most effective treatments, ie, boceprevir or telaprevir over standard of care. However, funding agencies must make decisions as to whether the additional gain in effectiveness is worth paying the additional money. In this situation, the cost of curing one patient (obtaining an SVR) is comparable with standard of care as it is with boceprevir added to standard of care. This is the case for both treatment-naïve and treatment-experienced patients. At the same time, the resources wasted on providing discontinuing patients with telaprevir renders this agent an economically nonpreferable option.
Disclosure
Merck and Co funded this research based on a submitted protocol. ACEK was an employee of Merck Sharp and Dohme Corp at the time of this project and assisted with content expertise and access to data.
References
- CooperCDruytsEThorlundKBoceprevir and telaprevir for the treatment of chronic hepatitis C genotype 1 infection: an indirect comparison meta-analysisTher Clin Risk Manag2012810513022442631
- PoordadFKhungarVEmerging therapeutic options in hepatitis C virus infectionAm J Manag Care201117 Suppl 4S123S13021767068
- HofmannWPZeuzemSA new standard of care for the treatment of chronic HCV infectionNat Rev Gastroenterol Hepatol2011825726421468124
- CiesekSMannsMPHepatitis in 2010: the dawn of a new era in HCV therapyNat Rev Gastroenterol Hepatol20118697121293503
- CooperCLesterRThorlundKDirect-acting antiviral therapies for hepatitis C genotype 1 infection: a multiple treatment comparison meta-analysisQJM2012In press
- NelsonDJensenDSulkowskiMHCV clinical management resource2011 Available from: img.medscape.com/article/746/388/HCV_746388.pdfAccessed October 8, 2012
- PoordadFMcConeJBaconBBoceprevir for untreated chronic HCV genotype 1 InfectionN Engl J Med2011364131195120621449783
- BaconBRGordonSCLawitzEBoceprevir for previously treated chronic HCV genotype 1 infectionN Engl J Med2011364131207121721449784
- JacobsonIMMcHutchisonJGDusheikoGTelaprevir for previously untreated chronic hepatitis C virus infectionN Engl J Med2011364252405241621696307
- KumadaHToyotaJOkanoueTChayamaKTsubouchiHHayashiNTelaprevir with PEGinterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in JapanJ Hepatol2011561788421827730
- HezodeCForestierNDusheikoGTelaprevir and PEGinterferon with or without ribavirin for chronic HCV infectionN Engl J Med2009360181839185019403903
- McHutchisonJGEversonGTGordonSCTelaprevir with PEGinterferon and ribavirin for chronic HCV genotype 1 infectionN Engl J Med2009360181827183819403902
- McHutchisonJGMannsMPMuirAJTelaprevir for previously treated chronic HCV infectionN Engl J Med2010362141292130320375406
- ZeuzemSAndreonePPolSTelaprevir for retreatment of HCV infectionN Engl J Med2011364252417242821696308
- MIMSMIMS: Prescription drug database and drug prescribing guide2012 Available at: http://www.mims.co.uk/
- ShermanKFlammSAfdhalNResponse-guided telaprevir combination treatment for hepatitis C virus infectionN Engl J Med2011365111014102421916639
- MarcellinPFornsXGoeserTTelaprevir is effective given every 8 or 12 hours with ribavirin and PEGinterferon alfa-2a or-2b to patients with chronic hepatitis CGastroenterology2011140245921034744
- DruytsEMillsENachegaJO’ReganCCooperCDifferences in clinical outcomes among hepatitis C genotype 1-infected patients treated with PEGinterferon alpha-2a or PEGinterferon alpha-2b plus ribavirin: a meta-analysisClin Exp Gastroenterol20125112122427726