Abstract
Purpose
To assess the direct and indirect costs associated with adverse drug reactions (ADRs) in patients receiving treatment regimens for human immunodeficiency virus (HIV) infection and tuberculosis (TB) in selected Thai hospitals.
Patients and Methods
This was a retrospective study conducted between October 2014 and September 2019 at three public hospitals in Thailand. Data were obtained from a medical database and spontaneous ADR reporting system of each study site. The out-of-pocket health payments and indirect costs were determined via interviewing. All costs were updated to 2021.
Results
A total of 432 eligible patients who experienced ADRs due to HIV and TB treatment, and 93 patients were interviewed to determine direct non-medical and indirect costs. The average direct medical cost for ADR was USD 5.65 for mild cases, USD 156.54 for moderate cases, and USD 1,242.45 for severe cases. For direct non-medical costs, the average cost per episode was USD 27.29 in mild ADR, USD 70.86 in moderate ADR and USD 270.66 in severe ADR. The indirect cost incurred in each mild, moderate and severe ADR was USD 41.86, USD 89.34, and USD 552.60, respectively. The Stevens-Johnson syndrome (SJS) had the highest management costs.
Conclusion
ADRs associated with anti-tuberculosis drugs and antiretroviral drugs seem to have a substantial economic impact from a societal perspective. These findings would be useful for increasing awareness and encouraging early avoidance of ADRs.
Keywords:
Introduction
Human immunodeficiency virus (HIV) infection and tuberculosis (TB) have remained a serious public health burden worldwide, with roughly 38 million people living with HIV and 10 million contracting TB by 2020.Citation1,Citation2 These two diseases are intersected that one in 10 active TB patients also has HIV.Citation3 According to the World Health Organization (WHO), Thailand has been one of the highest rates of TB and HIV/TB in Asia.Citation2,Citation4 There were over 470,000 HIV-positive individuals, and about 84% of them received antiretroviral therapy.Citation5 As many as 105,000 cases were reported as newly diagnosed or relapsed TB, with approximately 85% successfully completing treatment.Citation6 To control infection, prevent transmission, and slow disease progression, three or four antiretroviral and/or anti-tuberculosis drugs are required for effective treatment of HIV and TB patients.Citation7–10
It has been well recognized that the use of multiple drugs over an extended period of time can lead to adverse drug reactions (ADRs), which are defined as
a toxic and unintended response to a drug that occurs at doses normally used in man either for disease prophylaxis, diagnosis, or therapy or for the modification of physiologic function.Citation11
ADRs have been shown to be associated with an increased risk of morbidity, mortality, and economic burden,Citation12,Citation13 and their severity ranges from mild to severe or life-threatening.Citation14–19 From this, ADRs have been considered a contributing factor to non-adherence, ultimately leading to treatment failure or poor prognosis.Citation20–24
In view of ongoing events, it is crucial to evaluate economic costs of ADRs for effective resource allocation, possibly leading to a more cost-effective use of antiretroviral and/or anti-tuberculosis drugs. A number of previous studies have examined the direct costs of ADRs associated with antiretroviral and/or anti-tuberculosis drugs in developing and developed countries.Citation25–29 The indirect costs caused by ADRs were identified in a few studies.Citation30,Citation31 For instance, in Sweden, Gyllensten et al estimated the average indirect costs for patients who reported ADRs to be between USD 143 and USD 200 (33% of total costs), and these costs would be increased to 6 times in long-term care.Citation30 Another study showed that the average indirect costs for patients with ADRs were USD 3,405 or 55% of the societal costs.Citation31 None of direct non-medical and indirect costs of ADRs among Thai HIV/TB patients have been determined. Accordingly, the objective of this study was to assess the costs of ADRs associated with antiretroviral and/or anti-tuberculosis drugs based on a societal perspective. These findings were essential for healthcare professionals and policymakers seeking to improve patient safety, implement economic evaluation of new interventions related to ADR prevention, and reduce healthcare and family costs.
Patients and Methods
Study Setting and Population
Between October 2014 and September 2019, a retrospective observational study was conducted at three government hospitals: Nopparatrajathanee Hospital in Bangkok province, Buddhachinaraj Hospital in Phitsanulok province, and Queen Savang Vadhana Memorial Hospital in Chonburi province. During this time span, electronic medical records were reviewed for individuals with HIV, TB, or HIV/TB who had a history of ADRs to antiretroviral and/or anti-tuberculosis drugs. To assess eligibility, the following criteria were used: 1) Thai HIV, TB, or HIV/TB patients aged 18 years or older with either a history of ADRs due to antiretroviral and/or anti-tuberculosis drugs or receiving antiretroviral drugs and/or anti-tuberculosis drugs, 2) those who could communicate in Thai independently, and 3) those who experienced ADRs or drug toxicity to antiretroviral and/or anti-tuberculosis drugs as determined by a physician or pharmacist. Meanwhile, patients with systemic lupus erythematosus (SLE), malignancy, and pregnant women were excluded from this study. The study protocol was approved by the ethical committee of Institute for the Development of Human Research Protections, Ministry of Public Health in Thailand (COA No. IHRP2019054) and Faculty of Dentistry/Faculty of Pharmacy, Mahidol University, Institutional Review Board, Thailand (COA MU-DT/PY-IRB 2020/016.1603) and complied with the Declaration of Helsinki.
Data Collection
Patients in the database were solicited for enrollment by professional pharmacists based on their eligibility requirements. From October 2014 to September 2019, patients’ characteristics (eg age, gender, education, health scheme, occupation, and underlying disease), clinical, and financial data were all retrieved from hospital medical databases and spontaneous ADR reporting systems. Clinical data on ADRs experienced by patients comprised suspected drug, symptoms, date of adverse event onset, treatment, and causality assessment. The Naranjo’s algorithm was used to assess causality of ADR with suspected drug, categorized as definite, probable, possible, and doubtful.Citation32 Afterwards, the severity of ADR was assessed using the Hartwig’s severity assessment scale.Citation33 These were assessed and recorded by physicians and pharmacists at three study sites.
Moreover, cost statistics were divided based on a societal perspective into direct medical, direct non-medical and indirect costs. Direct medical costs covered both costs related to public health facilities and out-of-pocket payments by patients. Costs of treatment at public institutions were gathered from medical databases including medications, medical supplies, laboratory tests, medical services, and hospitalizations. Out-of-pocket health payments, direct non-medical costs (eg transportation, meal, accommodation, and caregiver time loss), and indirect costs (defined as patient time lost due to morbidity or mortality) were collected via patient interviews. After receiving written informed consent, patients were recruited for direct interviews.
Sample Size
Throughout the study period, direct medical costs for all recruited samples were collected from the hospital database. Due to limited budget and time, the estimated sample size for interviews was calculated using the single population proportion formulaCitation34 following as: n = (ZCitation2 * p * (1-p))/dCitation2 where Z = the probabilities at 95% confidence level as 1.96, p = the proportion of ADRs associated with antiretroviral drugs and anti-tuberculosis drugs at 40%Citation35,Citation36 and d = margin of error at 10%. Consequently, the required sample size for interviews was 93.
Cost Valuation
For each ADR, we calculated ADR episode-based cost of illness analysis from the first date of intending medical services associated with ADR diagnosis until the completion of treatment. The ending date of ADR episode was then the last date of the follow-up care. Unit cost of drugs and medical supplies was referred from the Drug and Medical Supply Information Center, Ministry of Public Health.Citation37 Unit cost of laboratory investigations, rooms, and medical services was mentioned from the standard cost lists for health technology assessment.Citation38 Out-of-pocket payment was obtained via patient interviews. Time costs of caregiver were calculated by multiplying the number of days spent caring for patients by their daily gross national income.Citation39 Patents’ durations were determined by the number of days lost while receiving healthcare and in-home care. All costs were adjusted to 2021 using the consumer price indexCitation40 and expressed in Thai baht (THB), which were then converted to US dollar (USD) using the Bank of Thailand’s July 2021 exchange rates (USD 1 = THB 32.61).Citation41
Data Analysis
Data were analyzed using Microsoft Excel and STATA version 15 software. Continuous variables were expressed as mean (standard deviation, SD) and median (interquartile, IQR). Categorical variables were reported as frequencies and percentages. Baseline demographics, clinical characteristics, and mean cost associated with ADRs in patients who had ADRs were all reported. Statistical differences in continuous variables across drug groups were executed using Mann–Whitney U-test (for 2 groups) or Kruskal–Wallis H-test (for >2 groups), while statistical differences in categorical variables were determined using chi-square test or Fisher’s exact test. All statistical significances were set at a p-value of less than 0.05.
Results
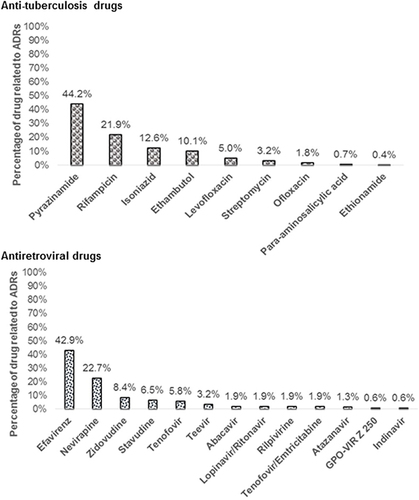
A total of 432 patients who had ADRs were included in this study. Patients’ characteristics are presented in . The mean age of the patients was 48.16 years, 60.42% were male. The majority of them were TB infections (51%). Out of 432 patients, 278 (64.35%) experienced with ADRs due to anti-tuberculosis drugs, while 154 (35.65%) had ADRs due to antiretroviral drugs. For patients hospitalized owing to ADRs, mean length of hospitalization was 5.6 days. Minimum and maximum length of stay was 2 to 26 days. According to Hartwig’s scale for assessing the severity of ADRs, 62.04% of the reported ADRs were mild, 33.33% were moderate, and 4.63% were severe. In most cases (67.36%), causality assessment was deemed probable. ADRs due to anti-tuberculosis drugs were most prevalent with pyrazinamide (44.24%), rifampicin (21.94%), and isoniazid (12.59%) (), while ADRs induced by antiretroviral drugs were most prevalent with efavirenz (42.86%), nevirapine (22.73%), and zidovudine (8.44%) ().
Table 1 Baseline and Clinical Characteristics of Patients Occurring ADRs
Direct Medical Costs
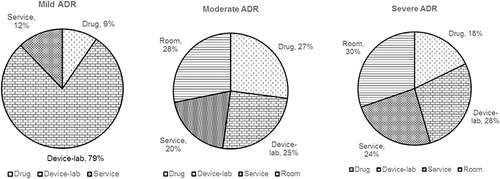
Average direct medical costs associated with management of ADRs varied significantly by severity (p = 0.0001). Average direct medical cost caused by ADRs was USD 5.65 (THB 184.23) for mild, USD 156.54 (THB 5,104.09) for moderate, and USD 1,242.45 (THB 40,511.33) for severe (). In mild cases, cost of device and laboratory testing accounted for 79% of the total (), while the room cost accounted for 28% and 30% of the total in moderate and severe cases ( and ). The average direct medical costs per episode were not significantly different for anti-tuberculosis and antiretroviral drug-induced ADRs.
Table 2 Average Direct Medical Costs of ADRs Categorized by Drug Groups (Thai Baht)
As detailed in , the average cost of ADRs per episode was lowest for others (chill and mouth ulceration) (mean: USD 2.55 (THB 83.22)) and greatest for Stevens-Johnson syndrome (SJS) (mean: USD 1,349.28 (THB 43,994.70)). The treatment cost was not significantly different between patients with ADRs due to anti-tuberculosis and antiretroviral drugs, except for SJS (p = 0.041) and hepatitis (p = 0.010). From reported ADRs, the most expensive treatment was for rifampicin-induced SJS (USD 4,805.93; THB 156,702.70), followed by pyrazinamide-induced hypersensitivity syndrome (USD 2,444.11; THB 79,692.89), efavirenz-induced DRESS (USD 1,585.86; THB 51,708.71), levofloxacin-induced SJS (USD 1,498.52; THB 48,860.80), and pyrazinamide-induced SJS (USD 1,404.67; THB 45,800.67) ().
Table 3 Reporting Top 10 Direct Medical Cost of ADRs Related to Anti-Tuberculosis Drugs and Antiretroviral Drugs
Direct Non-Medical and Indirect Costs
Results of direct non-medical cost and indirect cost analysis are summarized in and . For 93 patients interviewed with ADRs, the average direct non-medical costs differed significantly by severity. For direct non-medical costs, the average cost per episode was USD 27.29 (THB 889.88) in mild ADR, USD 70.86 (THB 2,310.46) in moderate ADR, and USD 270.66 (THB 8,825.12) in severe ADR. On average, 7.4 days were wasted due to ADRs (the minimum and maximum of 1 to 106 days). The indirect cost incurred in each mild, moderate, and severe ADR was USD 41.86 (THB 1,364.83), USD 89.34 (THB 2,913.03), and USD 552.60 (THB 18,018.05), respectively. The direct non-medical and indirect costs were highest for SJS (USD 815.80; THB 26,600) and lowest for lipoatrophy (USD 21.91; THB 714.54), as shown in .
Table 4 Average Direct Non-Medical and Indirect Costs of ADRs Categorized by Severity and Symptoms
Table 5 Reporting Top 10 Direct Non-Medical and Indirect Costs of Adverse Drug Reactions Related to Anti-Tuberculosis Drugs and Antiretroviral Drugs
The highest cost was incurred by nevirapine-induced SJS (USD 1,220.13; THB 39,783.57), followed by amikin-induced nephrotoxicity (USD 741.98; THB 24,193.17), pyrazinamide-induced joint pain (USD 656.72; THB 21,413.17), efavirenz-induced hypersensitivity syndrome (USD 639.64; THB 20,856.09), and nevirapine-induced DRESS (USD 613.66; THB 20,009.03), as summarized in .
Discussion
This is the first cost analysis in Thailand to incorporate both direct medical, direct non-medical and indirect costs associated with managing ADRs due to anti-tuberculosis and antiretroviral drugs. During the study period, there were 432 patients with ADRs, accounting for 3% of all patients enrolled in the study setting. Compared to previous studies, ADRs were probably underreported.Citation42,Citation43 Male patients were more likely to encounter ADRs than female patients, because males were more susceptible to behavioral and social factors.Citation27,Citation44,Citation45 This resulted in an increase in transmission of disease.
Based on treatment costs of over half of patients with ADRs, mean direct medical cost caused by ADRs was USD 32 (THB 1,057) per outpatient and USD 738 (THB 24,079) per inpatient. About 17% of patients reporting ADRs were treated concurrently outside of public hospitals. The majority of high costs occurred in inpatient setting. The duration of stay was comparable to previous studiesCitation27,Citation46,Citation47, ranging from 2 to 26 days. The average length of stay in hospitals for ADR cases was 5.6 days, compared to the national average of 4.4 days.Citation48 Supporting the aforementioned findings, it has been reported that cost of ADRs was influenced by a patient’s duration of stay.Citation47,Citation49,Citation50 In both developing and developed countries, numerous previous studies have reported on direct medical costs associated with ADRsCitation25–28,Citation51,Citation52 In Thailand, Chaiyanukij et al quantified treatment costs associated with ADRs among TB patients at Tuberculosis Area Center 10 in Chiang Mai province. The costs were calculated by combining labor and material costs, and the mean ADR cost per patient was USD 7 (THB 286.44) or USD 2 (THB 83.46) per episode.Citation25 Besides this, Srimongkol (2009) conducted a cost analysis of ADRs in 136 people living with HIV at Nakornping Hospital in Chiang Mai Province (95 patients receiving GPO-VIR S and 41 patients receiving GPO-VIR Z). Direct medical costs associated with ADRs included medications, medical supplies, laboratory tests, room, food, and health care services. Mean direct medical cost of an ADR episode was USD 44 (THB 1,490) in both groups, USD 60 (THB 2,053) in the GPO-VIR S group, and USD 12 (THB 410) in the GPO-VIR Z group.Citation26 In India, Radhakrishnan et al demonstrated that direct costs of ADRs to HAART in 110 HIV-infected hospitalized patients were USD 186, including costs of medication, medical supply, laboratory investigation, and services.Citation27 In Spain, Homar et al determined costs of ADRs for 75 patients treated with fix dosage combinations and 150 patients treated with combinations of separately administered antiretroviral drugs from a single Spanish hospital. Management cost of ADRs was USD 321.Citation51 In the United States, Simpson et al examined the healthcare costs of ADRs in 2,548 HIV patients treated with NNRTIs. Mean cost of ADRs each episode ranged from USD 586 to USD 4,434.Citation52 Dekoven et al employed claims database to assess healthcare costs associated with antiretroviral drug-induced ADRs in 2,346 HIV-infected patients. Median cost per episode was USD 677, and the maximum cost was USD 12,825.Citation28 In South Africa, Schnippel et al showed that the average costs per ADR episode was USD 136 in moderate ADRs and USD 521 in serious ADRs during treatment course for multiple drug- and rifampicin –resistant tuberculosis.Citation29 However, direct comparisons between our findings and those of other studies should be made with caution due to the differences in context, such as study design, the scope of healthcare utilization, insurance systems, and county economics.
Due to lack of prior data on the costs of ADRs related to HIV/TB treatment, this study addressed this issue. In the present study, private healthcare cost, direct non-medical cost and indirect cost per patient with ADRs were USD 169 (THB 5,501), which were significantly higher than the cost per patient without ADRs. As a result, ADRs imposed a significant economic burden not only on the healthcare system but also on patients and caregivers.
The primary strength of this study was our data recruited from urban and rural regions of Thailand, which included both outpatient and inpatient care. A second strength was the fact that it incorporated detailed information on direct and indirect costs and used a consistent method of cost valuation within different settings. Despite these, this study has several limitations. First, the retrospective study was conducted using an electronic database that may include misclassified and incomplete medical information. Second, since this study was conducted at only three tertiary hospitals in Thailand, generalizability to the national level should be performed with caution. In other settings, healthcare resource used for diagnosis, testing, or treatment might be different. Given the study’s small sample size and reliance on self-reporting, the incidence findings may be underestimated. Furthermore, these study settings were involved in pharmacogenomics for rational drug use in Thailand project, which included screening for HLA-B*5701 and NAT2 before commencing abacavir and isoniazid, respectively. Consequently, the incidence and economic burden of ADRs related to them were likely misrepresented. In terms of direct non-medical and indirect costs, in particular, this research is possible owing to recollection bias. Therefore, further research is necessary to address this issue, including a prospective study and larger sample sizes.
Conclusion
Our study uncovered that ADRs related to anti-tuberculosis and antiretroviral drugs have an effect on healthcare utilization and economic burden in societal perspective. More 50% of patients with ADRs were handled in outpatient settings. Although the proportion of severe ADRs was low, the treatment costs and nonmedical costs were substantial. Our findings may serve as important criteria for future cost-effectiveness analyses of pharmacogenomics testing, thus promoting effective interventions and increasing the knowledge of healthcare professionals, policymakers, and patients towards adverse reaction avoidance.
Author Contributions
All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank the hospital staffs in Nopparatrajathanee Hospital, Bangkok province, Buddhachinaraj Hospital, Phitsanulok province and Queen Savang Vadhana Memorial Hospital, Chonburi province for helping with data collection and facilitating the study.
Additional information
Funding
References
- World Health Organiztion. HIV/AIDS; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/hiv-aids. Accessed December 6, 2021.
- World Health Organiztion. Global tuberculosis report 2021; 2021. Available from: https://www.who.int/publications/digital/global-tuberculosis-report-2021. Accessed January 31, 2022.
- Centers for Disease Control and Prevention. Global HIV and TB; 2021. Available from: https://www.cdc.gov/globalhivtb/index.html. Accessed April 20, 2021.
- Avert. HIV and AIDS in Thailand; 2020. Available from: https://www.avert.org/professionals/hiv-around-world/asia-pacific/thailand. Accessed August 5, 2021.
- United Nations Programme on HIV/AIDS. Thailand country data 2020; 2020. Available from: https://www.aidsdatahub.org/resource/thailand-country-data-2020. Accessed January 31, 2022.
- Ministry of Public Health. Tuberculosis profile: Thailand; 2021. Available from: https://www.tbthailand.org/statustb.html. Accessed January 31, 2022.
- Bureau of AIDS, Tuberculosis and Sexually Transmitted Infections, Department of Disease Control, Ministry of Public Health. Thailand national guidelines on HIV/AIDS diagnosis, treatment and prevention 2020/2021. Bangkok: Aksorn graphic and design publishing limited partnership; 2019.
- Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV; 2022. Available from: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/guidelines-adult-adolescent-arv.pdf. Accessed April 24, 2022.
- Division of Tuberculosis, Ministry of Public Health. National tuberculosis control programme guideline, Thailand 2021. Bangkok: Aksorn graphic and design publishing limited partnership; 2021.
- Nahid P, Dorman SE, Alipanah N, et al. Executive summary: official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):853–867. doi:10.1093/cid/ciw566
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–1259. doi:10.1016/S0140-6736(00)02799-9
- Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013;4(Suppl 1):S73–S77. doi:10.4103/0976-500X.120957
- Giardina C, Cutroneo PM, Mocciaro E, et al. Adverse drug reactions in hospitalized patients: results of the FORWARD (Facilitation of Reporting in Hospital Ward) study. Front Pharmacol. 2018;9. doi:10.3389/fphar.2018.00350
- Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf. 2006;5(2):231–249. doi:10.1517/14740338.5.2.231
- NIH’s Office of AIDS Research. Adverse effects of antiretroviral agents; 2021. Available from: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/adverse-effects-antiretroviral-agents. Accessed June 3, 2021.
- Insani WN, Whittlesea C, Alwafi H, et al. Prevalence of adverse drug reactions in the primary care setting: a systematic review and meta-analysis. PLoS One. 2021;16(5):e0252161. doi:10.1371/journal.pone.0252161
- Teo YX, Walsh SA. Severe adverse drug reactions. Clin Med. 2016;16(1):79–83. doi:10.7861/clinmedicine.16-1-79
- Dadebo F, Wiafe E, Padayachee N, et al. Antiretroviral-related adverse reactions: a cause for concern. Sys Rev Pharm. 2021;12(9):684–690.
- Peter J, Choshi P, Lehloenya RJ. Drug hypersensitivity in HIV infection. Curr Opin Allergy Clin Immunol. 2019;19(4):272–282. doi:10.1097/ACI.0000000000000545
- Montessori V, Press N, Harris M, et al. Adverse effects of antiretroviral therapy for HIV infection. CMAJ. 2004;170(2):229–238.
- Tadesse WT, Mekonnen AB, Tesfaye WH, Tadesse YT. Self-reported adverse drug reactions and their influence on highly active antiretroviral therapy in HIV infected patients: a cross sectional study. BMC Pharmacol Toxicol. 2014;15(1):32. doi:10.1186/2050-6511-15-32
- Rajesh R, Sudha V, Varma D, Sonika S. Association between medication adherence outcomes and adverse drug reactions to highly active antiretroviral therapy in Indian human immunodeficiency virus-positive patients. J Young Pharm. 2012;4(4):250–260. doi:10.4103/0975-1483.104369
- Bea S, Lee H, Kim J, et al. Adherence and associated factors of treatment regimen in drug-susceptible tuberculosis patients. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.625078
- Wang Y, Chen H, Huang Z, et al. Drug non-adherence and reasons among multidrug-resistant tuberculosis patients In Guizhou, China: a cross-sectional study. Patient Prefer Adherence. 2019;13:1641–1653. doi:10.2147/PPA.S219920
- Chaiyanukit N. Operating cost of adverse drug reaction of antituberculosis drugs at regional tuberculosis center 10 Chiang Mai [dissertation]. Chiang Mai: Public Health, Chiang Mai University; 2001.
- Srimongkol P. Cost analysis of adverse drug reactions and effectiveness in people living with HIV/AIDS receiving GPO-Vir S or GPO-Vir Z at Nakornping Hospital, Chiang Mai Province [dissertation]. Chiang Mai: Pharmacy, Chaing Mai University; 2009.
- Rajesh R, Vidyasagar S, Varma D, et al. Evaluation of direct cost of adverse drug reactions to highly active antiretroviral therapy in Indian human immunodeficiency virus positive patients. JCRHAP. 2012;1(1):12–21. doi:10.14302/issn.2324-7339.jcrhap-12-71
- Dekoven M, Makin C, Slaff S, et al. Economic burden of HIV antiretroviral therapy adverse events in the United States. J Int Assoc Provid AIDS Care. 2015;15(1):66–76. doi:10.1177/2325957415594883
- Schnippel K, Firnhaber C, Berhanu R, et al. Direct costs of managing adverse drug reactions during rifampicin-resistant tuberculosis treatment in South Africa. Int J Tuberc Lung Dis. 2018;22(4):393–398. doi:10.5588/ijtld.17.0661
- Gyllensten H, Rehnberg C, Jönsson AK, et al. Cost of illness of patient-reported adverse drug events: a population-based cross-sectional survey. BMJ Open. 2013;3(6):e002574. doi:10.1136/bmjopen-2013-002574
- Gyllensten H, Hakkarainen KM, Hägg S, et al. Economic impact of adverse drug events–a retrospective population-based cohort study of 4970 adults. PLoS One. 2014;9(3):e92061. doi:10.1371/journal.pone.0092061
- Naranjo C, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi:10.1038/clpt.1981.154
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–2232.
- Lemeshow S, Hosmer DW Jr., Klar J, Lwanga SK. Adequacy of Sample Size in Health Studies. Chichester: John Wiley & Sons Ltd; 1990.
- Nuntasaen T, Soontornpas R, Mootsikapan P, Soontornpas C. Management of adverse reaction in patients with HIV-infection at a university hospital of Thailand. IJPS. 2016;12:29–33.
- Thongrayng W, Kasinwat N, Limcharoen N, Nooratkaew K. Adverse reactions during the use of anti-tuberculosis drugs and treatment failure. Thai Pharm Health Sci J. 2008;4(1):46–51.
- Drug and Medical Supply Information, Ministry of Public Health. Drug and medical supply reference price; 2021. Available from: http://dmsic.moph.go.th/#. Accessed April 4, 2021.
- Riewpaiboon A. Standard cost lists for health economic evaluation in Thailand. J Med Assoc Thai. 2014;97(Suppl 5):S127–134.
- Office of the national economic and social development board. National Income of Thailand 2020; 2021. Available from: https://www.nesdc.go.th/nesdb_en/more_news.php?cid=154&filename=national_account. Accessed June 21, 2021.
- Bureau of Trade and Economic Indices. Report for consumer price index of Thailand year 2021; 2021. Available from: http://www.price.moc.go.th/price/cpi/index_new_all.asp. Accessed June 20, 2021.
- Bank of Thailand. Rates of exchange of commercial banks in Bangkok metropolis; 2021. Available from: https://www.bot.or.th/App/BTWS_STAT/statistics/ReportPage.aspx?reportID=123&language=eng. Accessed July 21, 2021.
- Mudbouch N. Antiretroviral therapy: the incidence of the adverse drug reaction (ADR) among HIV infected adult patients. SCNJ. 2014;1(2):1–16.
- Thongraung W, Kasinwat N, Limcharoen N, Nuratkaew K. Adverse reactions during using antituberculosis drugs and treatment failure. Thai Pharm Health Sci J. 2008;4(1):46–51.
- Imam F, Sharma M, Khayyam KU, et al. Adverse drug reaction prevalence and mechanisms of action of first-line anti-tubercular drugs. Saudi Pharm J. 2020;28(3):316–324. doi:10.1016/j.jsps.2020.01.011
- Thontham A, Polsook R. Symptom experience of adverse drug reaction among male and female patients with newly diagnosed pulmonary tuberculosis in Thailand. BNJ. 2021;7(3):195–202. doi:10.33546/bnj.1337
- Suh DC, Woodall B, Shin S, Hermes-De Santis ER. Clinical and economic impact of adverse drug reactions in hospitalized patients. Ann Pharmacother. 2000;34(12):1373–1379. doi:10.1345/aph.10094
- Bordet R, Gautier S, Le Louet H, et al. Analysis of the direct cost of adverse drug reactions in hospitalised patients. Eur J Clin Pharmacol. 2001;56(12):935–941. doi:10.1007/s002280000260
- Strategy and planning division of Office of the Permanent Secretary Ministry of Public Health. Summary of illness report 2020; 2021. Available from: https://bps.moph.go.th/new_bps/sites/default/files/ill_2020_full_27092021%20v2.pdf. Accessed January 25, 2022.
- Yang MS, Kim JY, Kang MG, et al. Direct costs of severe cutaneous adverse reactions in a tertiary hospital in Korea. Korean J Intern Med. 2019;34(1):195–201. doi:10.3904/kjim.2015.365
- Formica D, Sultana J, Cutroneo PM, et al. The economic burden of preventable adverse drug reactions: a systematic review of observational studies. Expert Opin Drug Saf. 2018;17(7):681–695. doi:10.1080/14740338.2018.1491547
- Homar F, Lozano V, Martínez-Gómez J, et al. Cost analysis of HIV treatment and drug-related adverse events when fixed-dose combinations of antiretrovirals (FDCs) were stopped, versus continuation with FDCs. Health Econ Rev. 2012;2(1):16. doi:10.1186/2191-1991-2-16
- Simpson KN, Chen SY, Wu AW, et al. Costs of adverse events among patients with HIV infection treated with nonnucleoside reverse transcriptase inhibitors. HIV Med. 2014;15(8):488–498. doi:10.1111/hiv.12145