Abstract
Background
The current economic recession in European countries has forced governments to design emergency measures to reduce spending on drugs, including antiretroviral therapy (ART). Switching antiretroviral drugs for others that have the same efficacy and safety profile at a lower cost (cost-reduction measures, CRM) could prove to be a valid means of generating savings.
Methods
Descriptive study of prospective consensus-based CRM undertaken in 2011 in a Catalonian hospital HIV unit among patients with prolonged plasma HIV-1 RNA <50 copies/mL.
Results
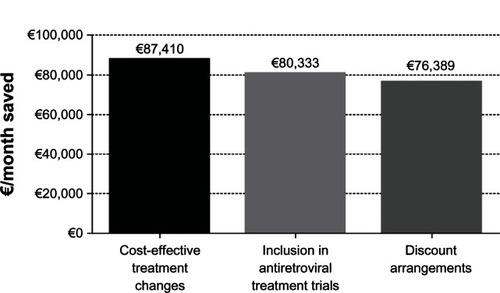
During the study period, we made 673 switches (87.5% more than the previous year), of which 378 (56.2%) were CRM (16% of all patients treated), leading to a savings of €87,410/month. Switching tenofovir/emtricitabine for abacavir/lamivudine was the most common CRM (129, 31.3%), followed by simplification to boosted protease inhibitor monotherapy (bPImono, 102, 26%). The CRM that generated the greatest saving were switching to bPImono (38%), withdrawal or replacement of raltegravir (24%), switching tenofovir/emtricitabine for abacavir/lamivudine (13%), and switching to nevirapine (5%). Cost savings with CRM were slightly higher than those achieved with medication paid for by clinical trial sponsors (€80,333/month) or through discount arrangements (€76,389/month).
Conclusion
Proactively switching antiretroviral therapy in selected treated patients with sustained virological suppression can generate significant cost savings in pharmacy spending in developed countries. These findings have implications for decision makers in designing safe strategies that maintain HIV-1 suppression at lower costs.
Introduction
The economic recession that began in 2008 has brought to light a series of structural problems in some European economies. Public debt has reached unprecedented levels that have jeopardized the sustainability of public finances and called into question the functioning of the economic systems of Greece, Ireland, Portugal, Spain, and Italy.Citation1 Recessions have significant adverse effects on health and health care.Citation2 Public financing of the national health system has been severely restricted in order to maintain universal access to public health care.Citation1,Citation3
Medication costs are the second largest component of public health spending in Spain, and hospital medication costs account for 36.5% of total spending on drugs.Citation3 Expenditure on medication in Spanish hospitals increased by 55% in 4 years, from €3.7 billion in 2006 to almost €5.8 billion in 2010.Citation3,Citation4 More than 60% of this expense is from outpatient drugs, which must be prescribed by a hospital doctor, require special follow up, and can only be dispensed by hospital pharmacy services.Citation5,Citation6 They include mainly antiretroviral drugs, cytostatic drugs, anti-TNF agents, interferon, erythropoietin, and antiviral agents that act directly against hepatitis C infection. The high price of these drugs is a key cause of the increase in total hospital expenditure, since cytostatic and antiretroviral drugs account for more than half of all spending on these products.Citation5,Citation7–Citation9
Spain’s hospital expenditure on drugs has reached a peak of €6,369,300,000, which was the debt to pharmaceutical companies in December 2011; that is 36% higher than the debt remaining at the end of 2010. The mean delay in payments from the National Health Service to the pharmaceutical industry in 2011 was 525 days, 135 more than in 2010 (annual increase of 34.6%).Citation3
Consequently, the government designed a series of emergency measures to reduce overall hospital spending by 11% in order to maintain the immediate sustainability of the health system. Clinicians at care of HIV were encouraged to accomplish the goals. These measures are based on promoting the use of generics, reducing the cost of outpatient drugs, and encouraging cost-effectiveness criteria.Citation10,Citation11
We describe the measures adopted with respect to prescription of antiretroviral drugs in a hospital HIV unit within the setting of a severe economic recession in order to reduce the cost of antiretroviral therapy (ART) in the immediate short term. We compare the impact of the savings achieved with that of other measures to reduce spending on ART.
Methods and setting
We made a descriptive analysis of prospective data from the HIV unit of a 638-bed hospital (Hospital Universitario Germans Trias i Pujol, Barcelona, Spain). The unit comprises a team of 14 prescribing specialists attending 2577 HIV-infected patients, of whom 2401 were receiving ART during the study period (7 months, May to November 2011). Total annual consumption of medication from the hospital pharmacy is €37,463,682, of which €17,898,758 (47.78%) corresponds to antiretroviral drugs. (It is important to remember that the consumption of oncology-hematology drugs is managed externally by the Catalan Oncology Institute; therefore, these drugs are not included in the above figure.)
We studied all switches in ART made during the period of greatest economic pressure for cost reduction. In the present study, a switch in ART is defined as any of the following: prescription of any antiretroviral agent that differs in dose or frequency from that of the previous month, switching one of the drugs in the regimen with respect to the previous month, initiation or reinitiation of ART after >1 year of discontinuation, and entry to or conclusion of a clinical trial with partial or total payment of ART by the sponsor.
Cost-reduction measures (CRM) include all those switches that aim to reduce the cost of treatment in patients with virological suppression (defined as plasma HIV-1 RNA <50 copies/mL).
Definition of criteria for switching ART
Members of the medical team agreed upon the categories of CRM and treatment regimens that did not compromise the safety and efficacy profiles of previous regimens. In addition, physicians were able to initiate any of the agreed regimens or prescribe other regimens according to their individual criteria. Physicians’ criteria were consistent with those of national and European ART clinical practice guidelines.Citation12,Citation13 Trained psychologists in the HIV Unit provided psychological support in order to ensure good adherence. shows all the CRM used in this study. In the switch from tenofovir/emtricitabine (TDF/FTC) to abacavir/lamivudine (ABC/3TC), patients had to be negative for HBsAg and HLA-B*5701. Switches from triple ART to monotherapy with darunavir/ritonavir (DRV/r once daily [QD]) or lopinavir/r (LPV/r two times daily [BID]) were made in patients with no history of virological failure, HIV-1-RNA <0 copies/mL for at least 6 months before switching, good adherence to ART, and a nadir CD4 >100 cells/mm3. Raltegravir (RAL), etravirine (ETR), or maraviroc (MVC) was suspended mainly in patients who had initiated the drug in a previous clinical trial or those receiving salvage regimens with a further 3 active drugs after a prior virological failure; when necessary, RAL, ETR, or MVC was switched for another completely active drug.
Table 1 Main types of antiretroviral treatment regimens initiated in switches to a more economical regimen in individuals with suppressed plasma HIV-1 RNA (<50 copies/mL)
Data were recorded by consulting the electronic antiretroviral dispensation system of the hospital pharmacy service. The only costs included in the analysis were those of the antiretroviral combinations analyzed, since the analysis was performed from the point of view of pharmacy spending. Cost was based on the price to retailer in Euros for the year 2011 plus taxes in Spain (4% VAT) according to the antiretroviral treatment guide of GESIDA/Spanish Secretariat for the National Plan on AIDS.Citation12
For each switch in ART, the hospital pharmacist recorded the patient’s data, date of switch, reason for switch, previous ART, and new ART. When the new regimen was more economical than the previous one, the patient’s electronic clinical history was consulted to rule out clinical indications for the switch (toxicity, virological failure, drug interactions, pregnancy, and entry to or conclusion of a clinical trial with partial or total payment for medication by the trial sponsor). In cases where no clinical justification was apparent, the measure was considered a CRM. A second revision by a physician from the HIV unit served to validate the classification.
In order to calculate the monthly saving of each CRM, the monthly cost of the new ART was subtracted from the monthly cost of the previous ART, and the result was assigned to the category of switch it belonged to (). In cases where two CRMs coincided in a switch, 50% of the monthly saving was assigned to each category. For example, the switch from RAL + TDF/FTC to DRV/r was considered both a change from RAL to a more economical drug and initiation of monotherapy with DRV/r.
Calculation of other CRMs
During the same period, we also counted the number of patients who were in a clinical trial whose sponsor financed all or part of ART, as well as the saving achieved with discount arrangements.
Results
During the study period, 673 of the 2401 patients treated (28.02%) received a switch of ART, that is, an increase of 87.46% with respect to the 359 switches made during the same period the previous year, which was considered the control period.
Of the 673 switches, 378 (56.17%) were due to CRM. The second most numerous group of changes were those made because of toxicity (11.29%), followed by initiation/reinitiation of therapy (9.51%) and switches due to virological failure (6.54%). The economic impact of each of these types of change is shown in . The total number of CRM represented a savings of €87,409.80/month in pharmacy spending during the study period.
Table 2 Different categories of switches in antiretroviral treatment undertaken in a cohort of 2401 HIV-1-infected patients during the study period
If we analyze the type of CRM, we see that 421 were adopted for the 378 switches in treatment. The percentage for each CRM according to the number of times it was applied is shown in and . The switch from TDF/FTC to ABC/3TC was the most common (129 times, 30.64%): it was made as the only switch on 98/129 occasions, and combined with another CRM on 31/129 occasions. The second most common CRM was simplification to monotherapy with DRV/r (63, 14.96%) and with LPV/r (41, 9.74%). In contrast, the cost savings achieved show that the CRM that generated the greatest saving was simplification to monotherapy with boosted protease inhibitors (PIs) (/r). Switches to monotherapy with DRV/r accounted for 22.7% of the total saving; switches to LPV/r accounted for 15.01%. The five most efficient types of CRM (switches to monotherapy with DRV/r or LPV/r, switches from TDF/FTC to ABC/3TC, discontinuations or replacements of RAL, and switches to NVP) account for 78.23% of the saving achieved. The most frequent switch (TDF/FTC to ABC/3TC) only generated the fourth largest saving. The correlation between the number of CRM adopted and the savings achieved with each category of switch is shown in .
Figure 1 Correlation between the number of switches identified as cost-saving measures and the costs saved with them (shown as percentages).
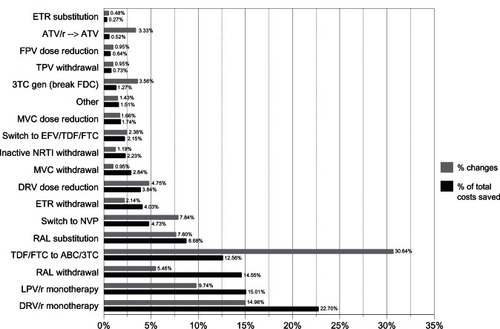
Table 3 Comparison of the number of cost-reduction measures undertaken (shown as categories) and costs saved with each one
During the same period, we identified 117 patients who participated in clinical trials with medication that was totally or partially paid for by the sponsor. This category generated a saving of €80,332.87/month.
The reduction in the purchase costs of antiretroviral drugs through discount arrangements generated a saving of €76,389.40/month during the same period ().
Figure 2 Final savings achieved during the study period with the three main cost-saving strategies: cost-effective treatment changes, inclusion in antiretroviral treatment trials (with antiretroviral medication totally or partially paid for by the sponsor of the clinical trial), and discount arrangements (shown as € per month saved).
At 48 weeks, 318 (84.1%) patients remained with the same CRM regimen. Only eight (2.1%) subjects developed virological failure (a confirmed plasma HIV-1 RNA >50 copies/mL), and the treatment was changed due to drug-related adverse events (all of them grade 1 or 2) in 26 (6.9%) subjects.
Discussion
In a severe economic recession with a direct impact on hospital pharmacy budgets in developed countries with free access to ART, switches aimed at reducing the cost of antiretroviral treatment agreed upon by the medical team of a hospital HIV unit led to a significant saving in total cost of therapy. These CRM led to savings similar to or higher than those achieved through clinical trials with antiretroviral medication paid for in total or in part by the sponsor. Likewise, the saving was slightly higher than that achieved through the discount arrangements in force during the same period.
The number of switches in ART during this period was almost double (+87.5%) that for the same period the previous year; more than half (56.2%) were CRM, thus highlighting the involvement of prescribing physicians from the HIV unit in achieving the priority objective of reducing the cost of ART in the immediate short term. Overall, 16% of patients treated received a change in CRM in their ART, generating a savings of €87,409.80/month in the purchase of antiretrovirals.
The most frequent CRM were switches in NRTI combinations from TDF/FTC to ABC/3TC, switches to monotherapy with PI/r (DRV or LPV), switches to NVP, and withdrawals or replacements of RAL.
In contrast, the CRM that generated the greatest savings were switches to monotherapy with PI/r (DRV or LPV, slightly more than one-third of the total saving achieved), suspension of RAL in patients with sustained virologic suppression after receiving a salvage regimen, replacement of RAL by another active drug, replacement of TDF/FTC by ABC/3TC, switches to NVP, and suspension of ETR in patients who had received salvage regimens. All of the above CRM accounted for almost 90% of the total saving achieved.
The results are consistent with mathematical models of potential cost savings generated with DRV/r in monotherapy.Citation14,Citation15 The models assumed that 15%–40% of treated patients with virological suppression would be candidates for DRV/r monotherapy. In our series, 4.2% (103 of 2401) of patients treated switched to PI/r monotherapy during the study period. Therefore, although switches to PI/r in monotherapy were important CRM, they only generated one-third of the total savings; the remaining CRM play an equally important role in achieving cost saving objectives.
The cost of antiretroviral drugs (approximately €7250/patient/year) remains the major factor contributing to treatment and care costs.Citation10–Citation12,Citation17 As HIV disease is treated earlier with more efficacious drugs, survival – and therefore costs of care – will continue to increase. Most patients treated in developed countries have complete virological suppression.Citation18–Citation20 It is necessary to find ways to lower the costs of HIV care while maintaining the highest medical standards. In Spain, ART is prescribed in reference hospital-based HIV units, thus facilitating implementation of CRM. Physicians prescribing ART should be aware that more economical options, along with equal efficacy and tolerability, may be available. They must have the full support of their hospital managers to preserve the efficacy and safety. Such cooperation would alleviate some of the conflicts involved when faced with the need to rationalize spending.
Individual components of the ART combination regimen are frequently switched for several reasons, including management of antiretroviral drug toxicity or intolerance, desire for once-daily dosing and reduced pill burden, management of potential drug interactions, patient preference, and cost.Citation13,Citation21,Citation22 National and international ART guidelines give increasing importance to cost-effectiveness criteria for prescription of ART.Citation12,Citation16–Citation18 Furthermore, earlier initiation or even universal prescription of ART to all HIV-1-infected patients increases national expenditure on ART everywhere, with the result that cost savings are increasingly relevant.Citation9,Citation17,Citation19–Citation21 Consequently, reducing the cost of ART is an immense challenge to managers and administrators at both local and national level, health care professionals, the pharmaceutical industry, and patients, particularly now that the HPTN 052 trial has proved that “treatment as prevention” works.Citation22,Citation23 It is increasingly clear that highly active antiretroviral therapy is not only a life-saving approach, but also an effective means of preventing transmission of HIV. A collaborative approach is thus required, as this target will not be reached without the profound commitment of prescribing physicians. Prescription of generics and implementation of CRM seem to be the preferred strategies for reaching this objective and maintaining free access to the health system and ART.Citation11,Citation18,Citation21
The potential reduction in costs using CRM is not indefinite, as once most of the CRM for switching ART are in place, no further cost reductions are possible with new CRM, and the strategy can only be used for maintenance. Therefore, other cost-saving strategies must be evaluated, especially price controls and replacement of brand name drugs or regimens with generics.Citation9–Citation11,Citation18,Citation24
The immediate need for cost savings in our setting prevented the performance of a prospective clinical trial to evaluate the full economic impact of the strategy, including all direct and indirect costs, and the possibility of confirming whether or not the measures were really cost-effective. The cost of potential toxicity or treatment failures resulting from these measures should be monitored in order to evaluate their impact on the final cost savings. In addition, the durability of switches should be analyzed. Such a study is already under way in our center; however, based on data from 2006 and earlier, ART already represented approximately 70% of the cost of health care in HIV-infected patients in Spain; undoubtedly, this percentage has increased owing to the continued reduction in morbidity and mortality in HIV-infected patients in developed countries.Citation9,Citation25 Therefore, it seems highly unlikely that these CRM will not prove to be cost-effective; however, a cost-effectiveness analysis is mandatory to confirm this hypothesis.
We did not analyze switches to more expensive ART regimens made during the study, as these were not considered CRM. In some cases, a more economical ART regimen might have been chosen; therefore, such switches could have the potential for further savings, but they were not evaluated in the present series.
In conclusion, our findings suggest that switching ART could generate significant cost savings during a severe economic recession. The main strategies for reducing the cost of ART and guaranteeing free health care in developed countries in recession are as follows: CRM combined with prescription of generic drugs, savings from clinical trials in which the sponsor pays for treatment, and savings generated by discount arrangements. The cost-effectiveness of CRM should be thoroughly evaluated by including direct and indirect costs. Similarly, strategies that involve prescribing physicians in cost savings in ART should be developed. Finally, patients who are candidates for CRM should be identified using strict inclusion criteria to ensure adequate safety and efficacy and taking the relevant ethical conditions into account.
Acknowledgments
This work was supported by CHAIN, Collaborative HIV and Anti-HIV Drug Resistance Network (Integrated Project no 223131, funded by the European Commission Framework 7 Program) and by Gala contra la Sida. Barcelona 2011. We are grateful to Thomas O’Boyle for editorial assistance.
Disclosure
Josep M Llibre has received funding for research or payment for conferences or participation on advisory boards from Abbott, Boehringer-Ingelheim, Bristol–Myers Squibb, Gilead Sciences, Jansen-Cilag, Merck Sharp and Dohme, Tibotec, and ViiV Healthcare. José R Santos has received research funding, consultancy fees, and lecture sponsorships from and has served on advisory boards for Abbott, Boehringer Ingelheim, Gilead Sciences, Glaxo Smith-Kline, Janssen-Cilag, Bristol–Myers Squibb, ViiV Healthcare, and Pfizer. Bonaventura Clotet has served during the past 2 years as a consultant on advisory boards or participated in speakers’ bureaus or conducted clinical trials for BMS, Abbott, Gilead, Janssen, Merck, Siemens, and ViiV. Xavier Bonafont has received payment for conferences from Abbott and Gilead Sciences. Angels Andreu has received payment for conferences from Abbott and Janssen-CiIag.
The other authors report no conflicts of interest in this work.
References
- KentikelenisAKaranikolosMPapanicolasIBasuSMcKeeMStucklerDHealth effects of fiancial crisis: omens of a Greek tragedyLancet201137898011457145821988763
- StucklerDBasuSSuhrckeMCouttsAMcKeeMThe public health effect of economic crises and alternative policy responses in Europe: an empirical analysisLancet2009374968631532319589588
- Farmaindustria [press release]Madrid, SpainFarmaindustria1262012 Available from: http://www.farmaindustria.es/Prensa_Farma/NotasDePrensa/FARMA_113933?idDoc=FARMA_113933Accessed August 30, 2012 Spanish
- SahuquilloMREl gasto farmacéutico hospitalario crece un 55% en cuatro anos. [The hospital pharmaceutical budget increases 55% in 4 years]. El País2011 Available from: http://elpais.com/diario/2011/11/10/sociedad/1320879603_850215.htmlAccessed August 30, 2012 Spanish
- Hospital pharmacyAmbulatory Spending Products in Spain 2007Spanish Working Group for the Analysis of Health Investments Available from: http://www.msps.es/estadEstudios/estadisticas/sisInfSanSNS/pdf/grupodeTrabajoSanitario2007.pdfAccessed July 27, 200720540552 Spanish
- Hospital pharmacyAmbulatory Spending Products in Spain 2006Spanish Working Group for the Analysis of Health Investments2007 Available from: http://www.msps.es/estadEstudios/estadisticas/sisInfSanSNS/pdf/grupodeTrabajoSanitario2007.pdfAccessed July 27, 2012540552 Spanish
- MariottoABYabroffKRShaoYFeuerEJBrownMLProjections of the cost of cancer care in the United States: 2010–2020J Natl Cancer Inst2011103211712821228314
- WalenskyRPPaltielADLosinaEThe survival benefits of AIDS treatment in the United StatesJ Infect Dis20061941111916741877
- MandaliaSMandaliaRLoGRising population cost for treating people living with HIV in the UK, 1997–2013PLoS One2010512e1567721209893
- Generalitat de Catalunya [homepage on the Internet]Action plan for the sustainability of the Health System 20112011 Available from: http://www10.gencat.cat/gencat/AppJava/cat/actualitat2/2011/10302plademesuresurgentsperalasostenibilitatdelsistemasanitari2011.jspAccessed July 27, 2012 Spanish
- GazzardBMoecklinghoffCHillANew strategies for lowering the costs of antiretroviral treatment and care for people with HIV/AIDS in the United KingdomClinicoecon Outcomes Res2012419320022888265
- Executive summary. Consensus document of GESIDA and SPNS (Spanish Secretariat for the National Plan on AIDS) regarding combined antiretroviral treatment in adults infected by the human immunodeficiency virus (Jan 2012)Enferm Infecc Microbiol Clin2012306315324
- European AIDS Clinical Society [Homepage on the Internet]Guidelines version 6.0 – Oct 2011 Available from: http://www.europeanaidsclinicalsociety.org/Accessed July 28, 2012
- PasquauJGostkorzewiczJLedesmaFAnceauAHillAMoecklinghoffCBudget impact analysis of switching to darunavir/ritonavir monotherapy for HIV-infected people in SpainAppl Health Econ Health Policy201210213914122293019
- GazzardBHillAAnceauACost-efficacy analysis of the MONET trial using UK antiretroviral drug pricesAppl Health Econ Health Policy20119421722321682350
- Panel on Antiretroviral Guidelines for Adults and AdolescentsGuidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescentsDepartment of Health and Human Services Available form: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdfAccessed September 4, 20121239
- ThompsonMAAbergJAHoyJFAntiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panelJAMA2012308438740222820792
- British HIV Association [Homepage on the Internet]BHIVA guidelines for the treatment of HIV-1 positive adults with antiretroviral therapy 2012 Available from: http://www.bhiva.org/Accessed September 4, 2012
- BeckEJMandaliaSSanghaRThe cost-effectiveness of early access to HIV services and starting cART in the UK 1996–2008PLoS One2011612e2783022194795
- NosykBMontanerJSThe evolving landscape of the economics of HIV treatment and preventionPLoS Med201292e100117422347816
- SloanCEChampenoisKChoisyPNewer drugs and earlier treatment: impact on lifetime cost of care for HIV-infected adultsAIDS2012261455622008655
- CohenMSChenYQMcCauleyMPrevention of HIV-1 infection with early antiretroviral therapyN Engl J Med2011365649350521767103
- CohenMSMuessigKESmithMKPowersKAKashubaADAntiviral agents and HIV prevention: controversies, conflicts, and consensusAIDS201226131585159822507927
- CallahanDCost control – time to get seriousN Engl J Med20093617e1019641194
- GonzaloTGarciaGMMuñoz-FernándezMASocio-economic impact of antiretroviral treatment in HIV patients. An economic review of cost savings after introduction of HAARTAIDS Rev2009112799019529748