?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Grass pollen-induced rhinoconjunctivitis is a common allergic respiratory disorder affecting over 20% of the UK population in terms of quality of life and sleep, work, and school patterns. The SQ-standardized grass allergy immunotherapy tablet (AIT) has been demonstrated as a disease-modifying treatment which gives a sustained effect even after completion of a treatment course. The objective of this study was to provide an economic assessment of whether treatment with the SQ-standardized grass AIT, Grazax® (Phleum pratense) in combination with symptomatic medications is preferable to the standard of care using symptomatic medications only. The analysis was performed for children with grass pollen-induced rhinoconjunctivitis, with or without concomitant asthma, in the UK.
Methods
The model evaluated the two treatment regimens in a cohort of 1,000 children from a payer’s perspective. Treatment was modeled in terms of management of symptoms, impact on resource use, and development of allergic asthma. The analysis modeled the use of SQ-standardized grass AIT and the sustained effects of treatment over a 9-year time horizon (ie, 3 years of treatment, with modeled long-term benefits). Data inputs were drawn from a recent clinical trial, published studies, and databases.
Results
SQ-standardized grass AIT improves patient outcomes, generating an incremental cost per quality-adjusted life year gained of £12,168. This is below commonly accepted thresholds in the UK.
Conclusion
The resulting incremental cost per QALY falls below commonly accepted willingness to pay thresholds. Therefore, the SQ-standardized grass AIT is a cost-effective option for the treatment of grass pollen-induced rhinoconjunctivitis in the UK pediatric population.
Introduction
Grass pollen-induced rhinoconjunctivitis is a common allergic respiratory disorder affecting over 20% of the UK population.Citation1 People of all ages are affected, and a study has estimated that allergic rhinoconjunctivitis is prevalent in 15% of 13–14-year-olds in the UK.Citation2 Quality of life for individuals with grass pollen-induced rhinoconjunctivitis may be significantly impaired, with work, school, and sleep performance being significantly affected.Citation3 Other allergic conditions, such as asthma and eczema, frequently coexist alongside grass pollen-induced rhinoconjunctivitis,Citation4–Citation6 thus adding to its societal impact and economic burden.
A variety of treatments for grass pollen-induced rhinoconjunctivitis generally aim to minimize symptoms and improve the patient’s quality of life. In order to ease symptoms, patients may need to use symptomatic medications, such as antihistamines, nasal sprays, and eye drops. However, despite use of those medications, some patients continue to experience uncontrolled symptoms. For those patients, allergen-specific immunotherapy is a potential treatment option. Grass allergen-specific immunotherapy increases the immunologic tolerance towards grass pollen by targeting the underlying cause of the allergic disease,Citation4 thereby reducing the allergic symptoms while providing a long-lasting effect. Immunotherapy has been shown to reduce the development of allergic asthma alongside allergic rhinoconjunctivitis,Citation7–Citation9 which may be particularly important to target in children, in order to prevent the potential onset of permanent damage.Citation10
The long-term effectiveness of subcutaneous immunotherapy (SCIT) has been demonstrated by several studies.Citation7,Citation11 A new, more convenient form of immunotherapy is allergy immunotherapy tablets (AIT) which can be self-administered by patients at home. Evidence has shown efficacy and a sustained effect after completion of treatment with grass AIT in the adult population,Citation12–Citation15 and efficacy in the pediatric population.Citation10 The cost-effectiveness of grass AIT has previously been demonstrated in the adult population;Citation16–Citation19 however, there is a paucity of published evidence of its economic impact in children.
Our study aimed to evaluate the costs and health outcomes from the viewpoint of the UK National Health Service associated with the use of grass AIT in combination with symptomatic medications for children with grass pollen-induced rhinoconjunctivitis in the UK compared with the use of symptomatic medications alone, which currently represents standard of care. The analysis thus attempts to answer the question: does treatment with grass AIT and symptomatic medications represent good value for money compared with treatment with symptomatic medications alone?
Materials and methods
Health economic evaluations
The use of grass AIT is likely to incur costs to the health care system. Although some of these costs may be offset by reductions in other resource use, the overall net change in costs may still be an increase. However, such increases in cost can be acceptable if they result in significant health gains to the patient. Cost-effectiveness analysis allows the decision-maker to assess the health gains and cost consequences associated with a health care intervention and, subsequently, make decisions about its value for money.
Cost-effectiveness analysis was used in this study to evaluate two alternative treatments in relation to their associated costs and health outcomes. The additional cost per extra unit of effect gained, demonstrated by the incremental cost-effectiveness ratio (ICER), is of key interest to policy and decision-makers because it can be used to determine whether a treatment demonstrates value for money when compared with a viable alternative:
The ICER formula determines the impact of grass AIT in combination with symptomatic medications compared with symptomatic medications alone by dividing the difference in costs of the two alternatives by the difference in the effectiveness of the two alternatives.
In this cost-effectiveness analysis, two different ICERs were calculated, ie, the incremental cost per extra quality-adjusted life year (QALY) and the incremental cost per extra “well day.” The ICER represents the amount that the payer must be willing to pay for one additional unit of health (one QALY or one “well day”) for it to be worthwhile to recommend treatment with grass AIT in combination with symptomatic treatment over symptomatic treatment alone.
Clinical data input
A recent clinical trialCitation10 compared the effects of grass AIT plus symptomatic medications versus symptomatic medications alone in children aged 5–16 years. The trial was of a randomized, double-blind, placebo-controlled design and involved 253 children with grass pollen-induced rhinoconjunctivitis, with or without asthma. Patients self-administered the SQ-standardized grass AIT (Grazax®, Phleum pratense 75,000 SQ-T/2,800 BAU; ALK-Abelló A/S, Hørsholm, Denmark) sublingually once daily.
Where possible, data from the clinical trial have been incorporated in the model, such as consumption of health care resources and the likelihood of treatment discontinuation due to adverse events.
Patients in the clinical trial were classified as having either excellent or non-excellent symptom control. Excellent control was defined in the trialCitation10 as more than 50% “well days” in the entire pollen season, whereby a “well day” was defined as a day on which a patient did not require any symptomatic medications and had a symptom score no higher than 2 (symptom score is on a scale from 0 to 18; a higher score indicates higher severity). The trial was a one-year study with patients treated prior to and throughout the pollen season. Based on a parallel immunologic response in children and adults, as measured by immunoglobulin (Ig)G4-blocking and IgE-blocking molecules, we assume for the purpose of this study that the effect in children can be extrapolated similarly to what has been observed in an adult population.Citation20
Economic model
For the current cost-effectiveness analysis, a computer-based model (Microsoft Excel) was developed to compare two treatment options for grass pollen-induced rhinoconjunctivitis: grass AIT plus symptomatic medications versus symptomatic medications alone, ie, medications taken according to symptoms and severity. The model was designed to capture the effectiveness of treatment in terms of management of symptoms, impact on resource use, and development of allergic asthma. In strict health economic terms, the present analysis is a cost-utility analysis rather than a cost-effectiveness analysis due to the measure studied being utilities (QALYs). However, we follow in the tradition of most authors and label this study a cost-effectiveness analysis.
The model follows two hypothetical cohorts of 1,000 children, aged 10 years at the beginning of treatment, with grass pollen-induced rhinoconjunctivitis, with or without concomitant asthma, who have received symptomatic medications previously. By applying data from the clinical trial and other assumptions as described below, the model calculates the costs incurred and effects experienced by the patients as a result of treatment.
At the beginning of the analysis, patients are categorized into one of eight “states” which differ according to presence of asthma, level of symptom control, and whether patients discontinue due to adverse events or continue treatment. For each of these eight states, the corresponding costs and effects are then applied.
Time horizon
In the base case, patients in the grass AIT cohort were assumed to take grass AIT for a 3-year treatment period, in line with recommendations.Citation21 Evidence has shown a sustained effect for up to 6 years after finalization of treatment with SCIT,Citation7,Citation11,Citation22 while for grass AIT so far there is evidence for a sustained effect 2 years after completion of treatment.Citation15 Although the mode of administration in the subcutaneous studies differs from that of grass AIT, the injection-based evidenceCitation11,Citation22 relates to the same active ingredient as in the grass AIT tablet used in our study (P. pratense). Therefore, the model assumes that grass AIT will have a long-lasting effect, in that the 3 years of treatment will result in an additional 6 years of sustained effect. This assumption is tested in the sensitivity analysis.
Treatment regimen
In the pediatric population, the grass AIT analyzed in this study is indicated for treatment of children aged 5–16 years and who have a clinical history of grass pollen-induced allergic rhinoconjunctivitis, with or without asthma.Citation21
It is recommended to start daily intake of grass AIT 4 months prior to the grass pollen season,Citation21 hence this was incorporated into the model. In addition to being treated with grass AIT, patients were assumed to have access to symptomatic medications when needed, based on the medications taken in the clinical trial, ie, loratadine, sodium cromoglycate, budesonide, prednisolone, salbutamol, and fluticasone, in addition to their asthma treatment. This reflects the regular practice of treatment in the UK for grass pollen-induced rhinoconjunctivitis with symptomatic medications for children.
In order to incorporate the prospect of adverse events occurring whilst on treatment, the possibility of patients discontinuing treatment due to adverse events was included in the model. The probabilities for treatment discontinuation were based on the clinical trial data, as were the majority of probabilities required for the model. Other model parameters are outlined in , including asthma prevalence and duration of the pollen season.
Table 1 Key model inputs
Unit cost data
Unit costs were identified by a search of established published sourcesCitation23–Citation25 and existing studiesCitation26,Citation27 (). All costs are reported in 2008 numbers. The model included direct costs ie, the cost of diagnosis and treatment, visits to the general practitioner, and the annual cost of asthma for those who developed the condition. The asthma cost comprised the cost of annual primary care resource use of £115, and the cost of a hospitalization (£642 per episode) due to asthma, occurring for 23% of asthma patients; generating an overall annual asthma cost of £295 after inflation of costs.Citation25–Citation27 Further resource use costs were included in the model, such as outpatient visits and nurse visits, but were not populated with corresponding resource usage data due to absence of these.
Table 2 Unit cost inputs
The cost of grass AIT used in the model was £67.50 for a 30-tablet pack (ie, £2.25 per daily dose of one tablet).Citation23 Symptomatic medication calculations used the cost per dose, according to the British National Formulary,Citation23 to generate daily costs for the different symptomatic medications. The medication costs are summarized in . Indirect costs were not included, given the National Health Service perspective of the analysis.
Utility data
The QALY is the measure of health outcome used in the model. QALYs are widely used in economic evaluations of health care because they measure individuals’ health-related quality of life as well as their length of life, and provide a generic outcome measure that facilitates comparisons between different programs.Citation28 A scale between 0 (dead) and 1 (perfect health) is used to provide a measure of a patient’s health-related quality of life.
Each of the states that individuals were categorized in had a different associated utility. These are shown in and were calculated as follows: for each of the eight states, the number of well days, as defined above, was taken from the clinical trial. Utility values on a day with no allergy (ie, a “well day”) and on a day with allergy (ie, a “non-well day”) were drawn from the study by Petersen et al,Citation29 and were 0.98 and 0.83, respectively. A well day was defined as a day with a symptom score no higher than 2 and with no rescue medication taken. The utility over the full pollen season (13 weeks’ durationCitation30) was generated by applying the utility of a non-well day for the proportion of the pollen season that the patient experienced a non-well day, and applying the utility of a well day for the remaining proportion of the season. The QALY value was then calculated using this pollen season utility and by assuming a well day utility (0.98) for the remainder of the year outside the pollen season.
Table 3 Utilities calculation and resource use
Resource use data
The model allows for a range of health care resources associated with the two treatment options. These were populated using data from weekly diaries during the pollen season collected from the clinical trial. Of the health care resource data monitored in the clinical trial (visits to the general practitioner, acute ward visits, use of symptomatic rescue medications, hospitalization), only general practitioner visits () and symptomatic rescue medications (not shown) were observed in the clinical trial, and hence the hospitalization and acute ward costs are not included in the calculations.
The general practitioner visits observed in the clinical trial represent the extra visits that occur additional to the standard physician visits during the pollen season. The number of standard physician visits during a pollen season for those treated with symptomatic medications only, ie, 2.4, was based on evidence from a European survey.Citation31 For the grass AIT group, one standard visit to a specialist per year was assumed.
Resource use varied according to the treatment taken, level of symptom control, and presence of asthma. It was assumed that resource use outside the pollen season was similar for the two groups, and was therefore not included in the analysis.
Compliance with prescribed grass AIT treatment was assumed to be 85% in the base case and hence only that proportion of the grass AIT medication cost is incurred. The figure of 85% was based on an assumption due to a lack of available data. However, alternative inputs were tested using sensitivity analysis.
The daily cost of symptomatic medications was multiplied by the probability of taking that particular medication, and the proportion of time the medication was taken during the pollen season, both based on the clinical trial findings.
Impact of asthma
Grass pollen-induced rhinoconjunctivitis is linked to the development of asthma, as individuals who are affected by grass pollen have an increased risk of developing asthma.Citation4,Citation5 Allergen-specific immunotherapy has been shown to be beneficial in terms of reducing the risk of developing asthma in patients with grass pollen-induced rhinoconjunctivitis. The model therefore incorporates the likelihood of developing asthma for the two treatment options, using findings from the Preventive Allergy Treatment (PAT) study.Citation7,Citation8 In the short term, the findings of 44% of immunotherapy patients and 24% of symptomatic medication patients developing asthma over 3 yearsCitation8 were converted to annual probabilities in the model. In the longer term, the 10-year PAT follow-up dataCitation7 were used, which look at development of asthma 7 years after treatment termination; asthma developed in 25% of immunotherapy patients and 45% of symptomatic medication patients. The use of PAT study data in the model assumes that the effect of grass AIT on reduction of the risk of asthma is equivalent to that of SCIT.
In addition to investigating the effect of development of asthma on patient costs, the model also includes the impact on patient health-related quality of life due to asthma; in that patients incur a loss in utility. Therefore, an annual utility loss due to asthma was included; calculated using the difference between the utility of a patient with asthma and that of a patient without asthma.Citation32,Citation33 The resulting disutility due to asthma was of magnitude 0.059 and applied to those who developed asthma during the course of the model.
Discounting
All future health outcomes (ie, QALYs) and costs were discounted at 3.5% per year, using recommendations from the UK Treasury.Citation34
Sensitivity analysis
Sensitivity analysis was conducted in order to validate the results by investigating the impact of parameter uncertainty. One-way sensitivity analysis and probabilistic sensitivity analysis were therefore conducted, and a cost-effectiveness acceptability curve was generated based on iterations from the latter. The probabilistic sensitivity analysis assumed distributions for key parameters within the model to reflect the second-order uncertainty around input values. Beta distributions, bound by zero and one, were used for probabilities and utilities, whilst cost parameters were modeled using gamma distributions (ie, producing non-negative values with no upper limit).
Results
The base case results are shown in . Patients treated with grass AIT plus symptomatic medications experienced additional QALYs, compared with patients treated with symptomatic medications alone. The use of grass AIT plus symptomatic medications resulted in each patient in the model experiencing a total of 7.37 QALYs for the 9-year time horizon, as opposed to 7.27 QALYs per patient on symptomatic medication, representing an incremental effectiveness of 0.10 QALYs per patient.
Table 4 Incremental cost per QALY
The total cost of treatment for those treated with symptomatic medications alone was estimated to be £2,247 for each patient over the 9 years featured in the base case. The treatment cost for each patient treated with grass AIT and symptomatic medications in combination was estimated to be £3,449 per patient over this period of time, representing an additional cost of £1,202.
The result of the cost-effectiveness analysis is that the additional benefits of grass AIT therapy for children with grass pollen-induced rhinoconjunctivitis can be acquired at a cost of £1,202/0.10 QALY = £12,168 per QALY. This number is the ICER, and we note that it falls below commonly accepted willingness to pay thresholds (which range between £20,000 and £30,000 per QALY in the UKCitation35). The interpretation is that the benefits of grass AIT can be acquired at a cost that is considered by UK authorities as being reasonable and thus the treatment should be made available to relevant patients. In addition, the analysis demonstrated a cost per additional well day of £12.42, as shown in .
Table 5 Incremental cost per well day
Sensitivity analysis
The results of the one-way sensitivity analyses indicate that varying parameters by ±20% in most cases generated ICERs that reached a maximum of approximately £18,000 per QALY. demonstrates the sensitivity analysis results; for each parameter that was varied the value in the base case is shown, in addition to the resulting ICERs when the base case value was either increased or decreased by 20%. When the time horizon of the model was varied (2–30 years), the ICER remained below the £30,000 per QALY threshold for all time horizons above 5 years (ie, 2 years of sustained effect).
Table 6 Sensitivity analysis
The use of different discount rates also had an impact on the results. An increase in the discount rates to 6% for both costs and benefits led to a rise in the ICER, whilst discount rates of 6% for costs and 1.5% for benefits had a lower associated ICER than the base case. When costs and benefits were undiscounted, the ICER fell by 19%. This is intuitive because, whilst the costs of grass AIT are incurred in the short term, the benefits become apparent over a greater number of years. Also shown in is the impact of varying grass AIT compliance, the price of grass AIT, asthma prevalence, and the difference between the utility of a well day and a non-well day. The latter two variables were not influential towards the results, whilst compliance and the price of grass AIT were found to have a larger impact.
When the assumption of 85% compliance was dropped (ie, assumed 100% compliance), the ICER increased to £15,695. If compliance was assumed to be 70%, the ICER was £8,642. In the model, compliance affects only the cost of treatment.
Probabilistic sensitivity analysis
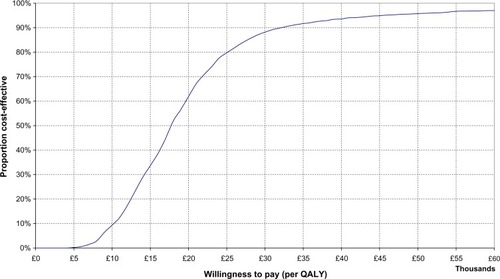
The cost-effectiveness acceptability curve depicted in summarizes the probability that the analysis will show that grass AIT is cost-effective for different willingness to pay threshold values. indicates that for a threshold of £30,000 per QALY, given the assumptions applied, there is a 90% chance that this cost-effectiveness analysis would produce the result that grass AIT is cost-effective compared with symptomatic medications alone.
Discussion
The finding of long-term cost-effectiveness of grass AIT in children is similar to the previously reported result that grass AIT is a cost-effective treatment for the adult population. Nasser et alCitation19 reported an ICER of £8,816 (2005 prices) for the UK patients involved in their study who had coexisting asthma, when productivity loss was excluded from the analysis. The cost-effectiveness of grass AIT in adults has been explored for seven Northern European countries from a societal perspective.Citation16 The resulting cost per QALY was identified as ranging between €12,930 and €18,263 (£8,847 and £12,496, 2005 prices). The adult-based grass AIT cost-effectiveness studies adopted a similar approach to our study, using the same time horizon and length of sustained effect. Factors behind our ICER results exceeding those found by the other studies are the perspectives taken for the analysis, the utilities, discount rates, and the resource use items included.
Our cost-effectiveness findings for AIT are consistent with those of pharmacoeconomic studies for SCIT. In particular, the cost savings demonstrated by our model in the pediatric population are in line with those found by Hankin et al in 2010,Citation36 where children who received SCIT incurred lower health care costs in comparison with matched controls. In the adult population, an economic model comparing SCIT combined with symptomatic therapy versus symptomatic therapy alone demonstrated cost-effective results in a German setting; an ICER of €8,308 per QALY was found, taking the third-party payer perspective.Citation37 Keiding and JorgensenCitation38 also found SCIT to be cost-effective in a range of European countries. When compared with SCIT, the study conducted by Pokladnikova et al in 2008 found AIT to be less expensive from the third-party payer perspective and societal perspective, although highlighted that the patient costs can differ depending on the type of allergen extract required.Citation39
The relevance of the data presented in this paper rather depends on whether the clinical trial population that was included matches with patients treated outside a clinical trial setting. Interestingly, the inclusion criteria, selection of patients, and treatment pattern corresponded closely with what is commonly applied and with guideline recommendations in daily routine practice for allergen immunotherapy. Further, the 23 investigators in the clinical trial on which the economic study is based represented a broad selection of those doctors usually treating rhinoconjunctivitis and asthma in the area under analysis.
The asthma cost included in the model is higher than the asthma cost based on the clinical trial. The cost in the model incorporates all primary care resource use, such as general practitioner visits, nurse visits, and medications, which were not all collected in the clinical trial. In addition, it is difficult to determine whether the clinical trial resource use should be attributed to allergic rhinoconjunctivitis or asthma. Hence, the asthma cost was sourced from a comprehensive primary care cost study,Citation26 in addition to also incorporating hospitalization costs.
An important input was the time horizon used for the analysis. Grass AIT stayed below the cost-effectiveness threshold of £30,000 even when the time horizon was reduced to 5 years (ie, only 2 years of sustained effect assumed). Data have been published showing that the effect of grass AIT treatment is sustained over 2 years following 3 years of treatmentCitation15 and data for SCIT suggest a sustained effect over 6 yearsCitation7,Citation11,Citation22 following 3 years of treatment. The base case analysis of 9 years and the limit of 5 years for cost-effectiveness thus do not seem unrealistic.
The usage of symptomatic medications for both treatment arms was based on the clinical trial. However, medications were used as required rather than on a prophylactic basis, which may mean that fewer medications were used and may, therefore, impact on the quality of life outputs.Citation16
In order to be able to utilize data relating to the proportion of well days experienced by children in the clinical trial, we applied utilities for a “well day” and “non-well day” as previously explained, rather than applying available EQ-5D values. This was due to the pediatric version of the EQ-5D not being available at the time of the clinical trial, hence the standard EQ-5D questionnaire was completed by or with the help of the children’s parents, which is perhaps not as reflective of children’s quality of life as the approach taken in our study. We investigated the impact of instead using the EQ-5D values in our analysis, which led to an ICER of £16,642. Hence the results remained within the cost-effective range when the EQ-5D data were included.
Data on health-related quality of life in children are typically less abundant than similar data for the adult population, and we were not able to find data on the health states of a “well day” and “non-well day” for children suffering from allergic rhinitis. We therefore chose to apply utilities for a “well day” and “non-well day”Citation29 taken from adults. Similarly, the studies used to calculate the asthma disutilityCitation32,Citation33 did not relate specifically to children. To our knowledge, there is no evidence to suggest that the health states of children could be expected to differ substantially from the adult values used, but this is an obvious drawback of our analysis which should lead the way for further research.
Our analysis identified a cost per well day of approximately £12. This is higher than the cost per symptom-free day, similar to the cost per well day found in studies relating to asthma treatment. The ICER in terms of cost per symptom-free day ranged from £1.47 to £6.92 for studies investigating the impact of inhaled corticosteroids, early asthma intervention, and also an education program for children with asthma.Citation40–Citation43 The decision as to whether the cost per symptom-free day provides value for money will be dependent on the local situation.Citation40 Due to the disease-specific nature of the cost per symptom-free day measure, the cost per QALY is a more useful measure in decision-making across disease areas.
The assumptions underlying the cost-effectiveness model were made using the most relevant and robust evidence available. A key assumption of the model relates to the continuing effectiveness of grass AIT in the long term, after treatment has ended, which has been demonstrated for both sublingual and subcutaneous forms of immunotherapy.Citation7,Citation11–Citation13 An effective treatment is likely to significantly reduce health care expenditure on management of asthma, which may develop in the long term if symptoms are not well managed. The efficacy from the treatment period was therefore extrapolated for the remaining years of the model, and discounted.
Due to the lack of evidence relating to grass AIT in relation to development of asthma, data for SCIT were used.Citation7,Citation8 However, the raw material in the subcutaneous formulation used in these studies is the same as that in grass AIT, so it was considered reasonable to use these findings for our model.
The clinical trial collected data on physician visits but no other use of health care resources, such as emergency room visits and hospitalizations. As such, it is likely that successful management of grass pollen-induced rhinoconjunctivitis would result in savings in use of other health care resources that were not included in the trial. Therefore, this analysis may have underestimated the cost savings associated with the use of grass AIT. In addition, a reduction in medication use has been demonstrated following AIT;Citation44 hence if our model were to incorporate this possibility, further cost savings could be seen.
This study demonstrates that SQ-standardized grass AIT is effective and increases patient quality of life. Additional costs are incurred in achieving these additional health benefits compared with current standard of care. The ICER of £12,168 per QALY falls below the commonly used threshold of £20,000–£30,000 per QALY as used by the UK National Institute for Health and Care Excellence. Therefore, AIT (Grazax®) is a cost-effective treatment option compared with symptomatic medications alone for grass pollen-induced rhinoconjunctivitis in children in the UK.
Disclosure
This research was supported with funding from ALK-Abelló A/S, Denmark.
References
- BauchauVDurhamSPrevalence and rate of diagnosis of allergic rhinitis in EuropeEur Respir J20042475876415516669
- AsherMIMontefortSBjorkstenBWorldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveysLancet200636873374316935684
- BousquetJKhaltaevNCruzAAAllergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen)Allergy200863Suppl 86816018331513
- BousquetJVan CauwenbergePKhaltaevNAllergic rhinitis and its impact on asthmaJ Allergy Clin Immunol2001108S147S33411707753
- LinnebergAHenrik NielsenNFrolundLThe link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy StudyAllergy2002571048105212359002
- Royal College of PhysiciansAllergy: the unmet need A blueprint for better patient careA report of the Royal College of Physicians Working Party on the provision of allergy services in the UKLondon, UKRoyal College of Physicians2003 Available from: http://www.bsaci.org/pdf/allergy_the_unmet_need.pdfAccessed October 3, 2013
- JacobsenLNiggemannBDreborgSSpecific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT studyAllergy20076294394817620073
- MollerCDreborgSFerdousiHAPollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study)The J Allergy Clin Immunol2002109251256
- NiggemannBJacobsenLDreborgSFive-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in childrenAllergy20066185585916792584
- BufeAEberlePFranke-BeckmannESafety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapyJ Allergy Clin Immunol2009123167173. e719130937
- DurhamSRWalkerSMVargaEMLong-term clinical efficacy of grass-pollen immunotherapyN Engl J Med199934146847510441602
- DahlRKappAColomboGSublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 yearsJ Allergy Clin Immunol2008121512518. e218155284
- Di RienzoVMarcucciFPuccinelliPLong-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10-year prospective studyClin Exp Allergy20033320621012580913
- DurhamSRYangWHPedersenMRSublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitisJ Allergy Clin Immunol200611780280916630937
- DurhamSRSustained efficacy of the SQ-standardized grass allergy immunotherapy tablet during the peak grass pollen season 2 years after treatmentAllergy2012651875
- BachertCVestenbaekUChristensenJCost-effectiveness of grass allergen tablet (GRAZAX) for the prevention of seasonal grass pollen-induced rhinoconjunctivitis – a Northern European perspectiveClin Exp Allergy20073777277917456225
- BertoPPassalacquaGCrimiNEconomic evaluation of sublingual immunotherapy vs symptomatic treatment in adults with pollen-induced respiratory allergy: the Sublingual Immunotherapy Pollen Allergy Italy (SPAI) studyAnn Allergy Asthma Immunol20069761562117165269
- CanonicaGWPoulsenPBVestenbaekUCost-effectiveness of Grazax for prevention of grass pollen-induced rhinoconjunctivitis in Southern EuropeRespir Med20071011885189417611095
- NasserSVestenbaekUBeriot-MathiotACost-effectiveness of specific immunotherapy with Grazax in allergic rhinitis co-existing with asthmaAllergy2008631624162919032235
- BufeAHenmarHGronagerPGrass allergen tablet immunotherapy in children induces IgG4 antibody and IgEblocking responses similar to those seen in adultsPoster presented at the XXVIII EAACI Congress of the European Academy of Allergy and Clinical ImmunologyWarszawa, PolandJune 6–10, 2009
- ALK-Abello LtdGrazax: Summary of product characteristicsHørsholm, DenmarkALK-Abello Ltd2007
- MosbechHOsterballeODoes the effect of immunotherapy last after termination of treatment? Follow-up study in patients with grass pollen rhinitisAllergy1988435235293148282
- British Medical Association and Royal Pharmaceutical Society of Great BritainBritish National Formulary 57London, UKBritish Medical Association and Royal Pharmaceutical Society of Great Britain2009
- CurtisLUnit Costs of Health and Social Care 2008Kent, UKPersonal Social Services Research Unit2008
- Department of HealthNHS Reference Costs 2007/08London, UKDepartment of Health2008
- DasguptaRGuestJFFactors affecting UK primary-care costs of managing patients with asthma over 5 yearsPharmacoeconomics20032135736912627989
- NHS Quality Improvement ScotlandAsthma attack – targeting emergency asthma contacts in children2005 Available from: http://www.healthcareimprovementscotland.org/previous_resources/audit_report/asthma_emergency_contacts.aspx?theme=mobileAccessed October 3, 2013
- DrummondMFSculpherMJTorranceGWMethods for the Economic Evaluation of Health Care Programmes3rd edOxford, UKOxford University Press2005
- PetersenKDKronborgCGyrd-HansenDQuality of life in rhinoconjunctivitis assessed with generic and disease-specific questionnairesAllergy20086328429118269674
- National Prescribing CentreCommon questions about hay feverMeReC Bulletin2004141720
- European Federation of Allergy and Airway Diseases Patients AssociationResults of the patient voice allergy surveyImpact of allergic rhinitis in Europe [Summary Report]2005 Available from: http://www.efanet.org/2005/06/Accessed November 11, 2013
- HanmerJLawrenceWFAndersonJPReport of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scoresMed Decis Making20062639140016855127
- SchermerTRThoonenBPvan den BoomGRandomized controlled economic evaluation of asthma self-management in primary health careAm J Respir Crit Care Med20021661062107212379549
- HM TreasuryAppraisal and Evaluation in Central Government (The Green Book)London, UKHM Treasury2003 Available from: https://www.gov.uk/government/publications/the-green-book-appraisal-and-evaluation-in-central-governentAccessed October 3, 2013
- National Institute for Health and Clinical ExcellenceGuide to the Methods of Technology AppraisalLondon, UKNational Institute for Health and Clinical Excellence2008
- HankinCSCoxLLangDAllergen immunotherapy and health care cost benefits for children with allergic rhinitis: a large-scale, retrospective, matched cohort studyAnn Allergy Asthma Immunol2010104798520143650
- BruggenjurgenBReinholdTBrehlerRCost-effectiveness of specific subcutaneous immunotherapy in patients with allergic rhinitis and allergic asthmaAnn Allergy Asthma Immunol200810131632418814456
- KeidingHJorgensenKPA cost-effectiveness analysis of immunotherapy with SQ allergen extract for patients with seasonal allergic rhinoconjunctivitis in selected European countriesCurr Med Res Opin2007231113112017519078
- PokladnikovaJKrcmovaIVlcekJEconomic evaluation of sublingual vs subcutaneous allergen immunotherapyAnn Allergy Asthma Immunol200810048248918517082
- BuxtonMJSullivanSDAnderssonLFCountry-specific cost-effectiveness of early intervention with budesonide in mild asthmaEur Respir J20042456857415459134
- PaltielADFuhlbriggeALKitchBTCost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy modelJ Allergy Clin Immunol2001108394611447380
- Rutten-van MolkenMPVan DoorslaerEKJansenMCCosts and effects of inhaled corticosteroids and bronchodilators in asthma and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19951519759827697275
- SullivanSDWeissKBLynnHThe cost-effectiveness of an inner-city asthma intervention for childrenJ Allergy Clin Immunol200211057658112373264
- SmithAMRezvaniMBernsteinJAIs response to allergen immunotherapy a good phenotypic marker for differentiating between allergic rhinitis and mixed rhinitis?Allergy Asthma Proc201132495421262098