Abstract
Purpose
Pulmonary arterial hypertension (PAH) is a rare and progressive pulmonary vascular disease that can result in right heart failure and death. Oral prostacyclins play an important role in the management of intermediate-low risk PAH. This targeted literature review (TLR) aimed to identify and compare evidence supporting use of oral prostacyclin pathway agents (PPAs: selexipag and oral treprostinil) in intermediate-low risk PAH.
Methods
A targeted literature review was conducted. Literature databases (MEDLINE, Embase, and Cochrane reviews) were searched for studies describing clinical practice and treatment outcomes for oral treprostinil and selexipag globally, published in English (2012 to 2022). Electronic searches were supplemented by manual-searches of targeted conferences (2020 to 2022), and reference lists of identified publications were reviewed. One reviewer assessed studies for eligibility.
Results
In total, 95 publications met inclusion criteria: 47 full-text articles (selexipag n = 22; oral treprostinil n = 16; selexipag and oral treprostinil n = 9) and 48 conference materials. Selexipag and oral treprostinil target the prostacyclin pathway differently; their label-supporting trials had different primary endpoints (disease progression and hospitalization vs exercise capacity and disease progression), differing baseline therapy (0, 1 or 2 vs 0 or 1 baseline treatments), titration duration and dosing (personalized dose capped at 1600 ug twice daily (BID) vs increasing doses over time with no maximum dose), respectively. While both oral PPAs have demonstrated reduced risk of disease progression, only selexipag showed reduction in hospitalization rates. Oral PPAs have been shown to reduce healthcare costs in real-world clinical practice. This difference is reflected in labeled indications.
Conclusion
Given differences in trial- and real-world outcomes, number of prior therapies, and dosing, personalizing the choice of oral PPA is critical to maximizing the benefit for individual patients.
Plain Language Summary
PAH is a condition that causes heart failure. It is important to take medicines to slow down this process. For people with early disease, there are some medicines that can be taken as a tablet rather than as an injection to slow down disease progression. The differences between two of the tablet options – selexipag and oral treprostinil, are unclear. We reviewed publications describing how, when and why these medicines are used and how well they work, to improve our understanding of the value of these medicines to people with PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a rare disease resulting from progressive pulmonary vascular remodeling and luminal narrowing.Citation1 The typical symptoms of PAH (shortness of breath with exercise, fluid retention, lower extremity edema, and presyncope/syncope) are related to progressive decline in right heart function.Citation2 Changes in cardiopulmonary function often occur prior to symptom presentation in PAH.Citation3 The initial symptoms are often misdiagnosed as other cardiovascular and pulmonary conditions such as asthma or congestive heart failure, resulting in an average 2.8-year delay between symptom onset and diagnosis/treatment.Citation4 PAH is associated with high levels of morbidity and hospitalization burden.Citation5 Treatment is required to prevent right heart failure and death.Citation6 Treatment decisions are based on risk categorization, with initial double combination treatment targeting the endothelin and nitric oxide pathways recommended for low- or intermediate-risk patients;Citation6 however, only a small minority of patients maintain low-risk status following double combination treatment at 3-month follow-up.Citation7 The 2022 Guidelines of the European Society of Cardiology (ESC) and European Respiratory Society (ERS) describe the addition of a prostacyclin pathway agent, selexipag for patients on PDE5i and/or endothelin receptor antagonist (ERA) and oral treprostinil for patients on monotherapy (phosphodiesterase 5 inhibitor [PDE5i] or ERA) who are at risk of progression.Citation6
The prostacyclin metabolic pathway is dysregulated in patients with PAH, and PPAs play a key role in managing this condition; PPAs can be intravenous, subcutaneous, inhaled, or oral.Citation6 Treprostinil, a prostacyclin analog, and selexipag, a selective prostaglandin I2 receptor agonist, are the only two oral PPAs approved by the Food and Drug Administration (FDA) and represent a patient–friendly dosage alternative.Citation8,Citation9 Oral treprostinil is not approved for use in PAH by the European Medicines Agency (EMA), whereas selexipag has been approved by the EMA. Earlier utilization of PPAs may improve long-term outcomes for PAH patients, delaying disease progression. Oral PPAs may improve uptake of PPAs by creating an easily accessible dosage option; however, information describing their use is diffuse. A review of the literature is needed to consolidate the evidence supporting use of the oral PPAs selexipag and treprostinil in clinical practice, to inform clinical and formulary decisions.
PAH is a rare disease, and many clinical studies in this indication are conducted in small patient populations. PAH describes a heterogeneous patient population comprising of patients at varying stages of progression and differing etiologies, and these differences are associated with impacts on clinical outcomes. Furthermore, there are differences in clinical trial endpoints between pivotal studies and differences in how morbidity endpoints are defined. There are challenges when performing meaningful indirect treatment comparisons between clinical trials in this indication for the reasons outlined. We performed a targeted literature review (TLR) to identify and compare all of the published data describing use of the oral PPAs treprostinil and selexipag (including the pivotal trials FREEDOM and GRIPHON, respectively) in PAH. The TLR identified clinical studies, prescribing practices, and clinical and economic outcomes for oral treprostinil and selexipag.
Methods
The TLR involved searching literature databases (MEDLINE, Embase, and Cochrane reviews) for articles describing clinical practice and treatment outcomes for oral treprostinil and selexipag in PAH. Aggregate search terms describing PAH, treprostinil, selexipag, reviews, real-world studies, and clinical trials were employed (see for PICOS inclusion and exclusion criteria). The search was global and limited to publications in English from January 1, 2012, to June 23, 2022, including publications from targeted conferences. Targeted conferences (American College of Cardiology, American College of Chest Physicians, American College of Rheumatology, American Thoracic Society, British Society of Rheumatology, European Respiratory Society, European Society of Cardiology, International Society for Heart and Lung Transplantation, Pulmonary Hypertension Association, Pulmonary Vascular Research Institute) from 2020 to 2022 were reviewed. Article title and abstract (screen 1) and fulltexts (screen 2) were screened by a single reviewer, followed by a quality check conducted by a second independent reviewer. Studies in populations less than n = 20 were excluded from this analysis. The literature search was supplemented by a review of reference lists. A single reviewer screened article titles and abstracts (screen 1) and full texts (screen 2), followed by a quality check conducted by a second independent reviewer. Studies in populations of fewer than 20 people were excluded from this analysis.
Table 1 PICOS Inclusion and Exclusion Criteria for the TLR
Results
As illustrated in the PRISMA diagram illustrated in , 992 articles were identified through MEDLINE, Embase, and Cochrane reviews; 7 additional articles were identified through review of conference materials and a further 12 articles were identified through a review of reference lists. One hundred and ninety-three articles progressed to full content screening with 95 publications meeting the inclusion criteria for extraction: 48 conference materials, 47 full-text articles (selexipag n = 22; oral treprostinil n = 16; selexipag and oral treprostinil n = 9). Most studies identified were observational and clinical studies describing dose titration and safety. No studies were identified that described unmet need, treatment patterns, patient or physician preferences, or patient and caregiver experiences.
The findings from this TLR confirmed the diffuse nature of the literature describing outcomes for these oral PPA treatments. Half of the literature identified had been published within conference materials. Many of the studies identified were in small patient numbers and reflected the heterogeneous nature of these patient populations (both stage of disease progression and etiology).
Randomized Controlled Trials (RCTs) and FDA Indications for Selexipag and Oral Treprostinil
Primary endpoints and outcomes of the RCTs of selexipag and oral treprostinil are summarized in . Several studies have suggested that PAH-related morbidity events are prognostic for mortality.Citation10,Citation11 Clinical trials of selexipag (GRIPHON) and oral treprostinil (FREEDOM-EV) have used composite endpoints to measure disease progression as a primary endpoint.Citation11–14
Table 2 Registrational Clinical Trials of Selexipag and Oral Treprostinil
The FDA-approved indication for selexipag is for treatment of PAH (World Health Organization [WHO] Group 1) to delay disease progression and reduce the risk of hospitalization.Citation18 This reflects findings from GRIPHON, as selexipag significantly reduced the risk of morbidity and mortality events versus placebo by 40%,Citation13 and resulted in a 30% risk reduction for the secondary endpoint, risk of death or hospitalization due to PAH worsening, compared with placebo (hazard ratio [HR]: 0.70; 95% confidence interval [CI]: 0.54, 0.91); 87.4% of the events for this endpoint were PAH-related hospitalizations.Citation13 In GRIPHON, time from diagnosis was ≤6 months in 34.9% of patients and >6 months in 65.1% of patients. The value of earlier initiation of selexipag after diagnosis (≤6 months versus >6 months) has been established in a post hoc analysis of the GRIPHON trial; patients who initiated selexipag earlier experienced a more pronounced effect on the time to first disease progression event than those who initiated later (HR: 0.45; 95% CI: 0.33, 0.63, and HR: 0.74; 95% CI: 0.57, 0.96, respectively; P = 0.0219 for interaction).Citation19
Oral treprostinil is indicated by the FDA for the treatment of PAH (WHO Group 1) to delay disease progression and improve exercise capacity.Citation20 Oral treprostinil was studied in a series of randomized, prospective, placebo-controlled clinical trials (the “FREEDOM” trials). Six-minute walk distance (6MWD) at week 12 was the primary endpoint in the FREEDOM-M trial. It showed a significant benefit compared with baseline values (median Hodges-Lehmann treatment effect of 23.0m [95% CI, 4–41 m; P = 0.0125]), leading to FDA approval of oral treprostinil for use as a monotherapy to improve exercise capacity in PAH.Citation15 Trials of oral treprostinil added to background therapy (FREEDOM-C and FREEDOM-C2) failed to demonstrate a significant improvement in the primary endpoint, 6MWD.Citation16,Citation17 The primary endpoint of FREEDOM-EV was time to first adjudicated clinical worsening event. In FREEDOM-EV, 90 (26%) participants in the oral treprostinil group experienced a clinical worsening event compared with 124 (36%) of placebo participants (HR: 0.74; 95% CI: 0.56, 0.97; P = 0.028). The treatment-attributable difference in clinical worsening was driven by reduced incidence of disease progression in the oral treprostinil group (HR 0.39; 95% CI 0.23, 0.66; P < 0.001).Citation14 Findings from FREEDOM-EV expanded the oral treprostinil FDA label to include delay in disease progression.Citation20
Survival Data
GRIPHON was not powered to determine survival outcomes, and no statistical difference in the number of deaths among patients receiving oral selexipag (17.4%) was observed versus placebo (18.0%).Citation13 GRIPHON mortality data are difficult to interpret because of treatment switching following a non-fatal primary endpoint event, with many patients switching from placebo to oral selexipag.Citation13 Post hoc analysis of the FREEDOM-EV study found that mortality was lower at study closure in patients receiving oral treprostinil versus placebo (11% vs 17.4%, respectively; P = 0.0324).Citation21 However, this finding is difficult to interpret because mortality was similar between oral treprostinil and placebo at the end of randomized treatment (4.9% vs 5.2%, respectively, P = 0.9781) or open-label extension study (8.7% vs 12.2%, respectively, P = 0.43), and death status was unknown for 11% of the original FREEDOM-EV population.Citation21
Survival outcomes in longer term open-label studies for progressive conditions such as PAH can be impacted by variation in subsequent treatments. Long-term follow-up of patients who received selexipag in the placebo-controlled GRIPHON study and the open-label extension study showed that Kaplan–Meier estimates of overall survival at 1, 2, 5, and 7 years were 92%, 85%, 71%, and 63%, respectively.Citation22 Long-term follow-up of patients who received oral treprostinil in the placebo-controlled FREEDOM-EV and open-label extension study showed Kaplan–Meier estimates of overall survival at 1, 3, and 5 years were 96%, 88%, and 79% versus 95%, 80%, and 70% for placebo, respectively.Citation23 It is not possible to directly compare survival data between GRIPHON and FREEDOM-EV due to differences in background therapy (ie, 32.5% of patients in GRIPHON received dual-combination PAH treatment, while all patients in FREEDOM-EV received PAH monotherapy);Citation19,Citation21 and function class with a greater proportion of FC III patients in GRIPHON compared with FREEDOM-EV (52.5% vs 33.9% respectively).
Mortality outcomes are difficult to determine for oral PPAs due to the absence of comparative data,Citation22–24 and the short duration of clinical trials.Citation13–15
Place in Therapy
Based on 2022 ESC/ERS guidelines, initial triple oral combination therapy with oral PPAs is not recommended but plays a role for patients who present at intermediate-low risk of death while receiving ERA or PDE5i therapy.Citation6 Sequential triple therapy with selexipag is supported by post hoc analysis of patients on dual combination background therapy in GRIPHON.Citation24 TRITON assessed initial triple combination with selexipag versus initial dual combination with selexipag over 26 weeks; although no significant difference in pulmonary vascular resistance was observed, exploratory analyses suggested a possible signal for improved long-term outcomes.Citation25 Data supporting the use of oral treprostinil in triple combination therapy is lacking; additional studies will be required to evaluate the efficacy of adding oral treprostinil to dual combination therapy (see ).Citation16,Citation17
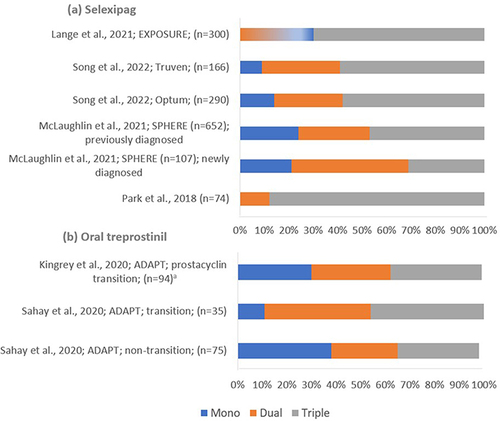
As illustrated in , real-world evidence indicates that, overall, oral selexipag is predominantly used within a triple combination regimen (31.0% to 88.0% of patients receiving selexipag);Citation26,Citation27 newly diagnosed patients more often initiate selexipag treatment with a dual combination regimen with an ERA or a PDE5i.Citation26 As illustrated in , oral treprostinil is equally used as a monotherapy or within a dual/triple-combination regimen with an ERA and/or PDE5i. Studies from the ADAPT registry describe oral treprostinil use within triple combination (33.3% to 45.7% of patients receiving oral treprostinil).Citation28,Citation29 However, neither of the early oral treprostinil trials, FREEDOM-C and FREEDOM-C2, demonstrated an improvement in 6MWD with the addition of oral treprostinil to double oral combination therapy (see ).Citation16,Citation17
Figure 2 Real-world evidence studies describing proportions of patient using selexipag and oral treprostinil within a combination regimen. (a) illustrates the proportions of patients taking selexipag as a monotherapy or within dual- or triple-combination regimens in reported studies; (b) illustrates the proportions of patients taking oral treprostinil as a monotherapy or within dual- or triple-combination regimens in reported studies. aThis value has been calculated based on patient baseline characteristics, assuming the background therapy has been supplemented with one additional therapy rather than replaced.
Dosing and Titration of Oral PPAs
As summarized in , both oral PPAs require initial titration. Individualized dose titration is required for oral PPAs to identify an upper maintenance dose that avoids unmanageable side effects.Citation31
Table 3 Selexipag and Oral Treprostinil Dosing in RCTs and Clinical Practice
Selexipag Titration and Dosing
GRIPHON describes the titration regimen for selexipag of 200 µg BID increased in increments of 200 µg BID weekly until side-effects cannot be managed by adequate treatment or to the maximal dose of 1600 µg BID. Across all doses, selexipag demonstrated a reduction in the risk of morbidity and mortality, supporting individual-dose titration. For patients experiencing a prostacyclin-associated side effect that is unmanageable, the dose is reduced by 200 µg.Citation13
Real-world evidence (SPHERE registry) suggests that most patients (87.8%) receiving selexipag titrate more slowly than the label recommended 200 µg BID weekly, with over one-fifth of patients (21.6%) titrating at 200 µg BID every two weeks.Citation32 The majority of studies that reported selexipag titration described completing titration over 7–9 weeks;Citation33,Citation39,Citation40 SPHERE registry data reported a median time from selexipag initiation to an individualized maintenance dose of 8.1 weeks,Citation32,Citation40–42 and a median maintenance dose of 1200 µg BID (n = 496).Citation40 SPHERE registry data indicate that the average maintenance dose continued to increase and began to stabilize at 6 months. At 18 months, 77.6% of patients enrolled in SPHERE completed the study; of the 22.4% who discontinued early, 11.4% discontinued due to adverse events (AEs) related to PAH progression and 11.2% discontinued due to AEs not related to progression (7.2% attributable to selexipag).Citation32
Oral Treprostinil Titration and Dosing
Across the FREEDOM trials, oral treprostinil titration and dosing evolved with variation in target doses (). These variations arose from changes in the available tablet strength, adoption of three times daily (TID) versus BID dosing, and expert panel recommendations.Citation15–17,Citation20 The FREEDOM-EV trial describes a higher dose than earlier trials, with daily up-titration in 0.125 mg increments for the first 4 weeks, then 0.25 mg increments daily to a maximum of 12 mg (TID dosing with food), achieving an upper median dose of 3.56 mg TID by week 24.Citation23
The minimum effective dose for oral treprostinil is not clear from the literature; however, the literature indicates a dose-dependent treatment effect.
An open-label study of patients participating in FREEDOM trials followed 37 patients and reported mean oral treprostinil doses of 4.3 ± 2.3 to 11.7 ± 5.8 mg/24 hours between 3 and 24 months. Oral treprostinil dose was inversely associated with a change in pulmonary vascular resistance (r = −0.42, P < 0.05), with a greater change among patients in the highest dosing quartile.Citation43
Analysis of oral treprostinil dose and response in 1619 patients with PAH indicated that higher doses of oral treprostinil are required to achieve significantly longer times for first PAH-related and all-cause hospitalization; a trend towards improvements in 6MWD was observed with higher doses.Citation44
The target dose across FREEDOM studies varied, as reflected in real-world studies (). A study using specialty pharmacy service shipment records indicated that in the overall TID dosing group (n = 1200), the median total daily dose (TDD) varied from 5.3 mg to 10.8 mg between 3 and 18 months. In the BID dosing group (n = 400), median TDD increased from 2.5 mg to 5.5 mg between 3 and 18 months.Citation45 More prevalent use of TID has improved tolerability, leading to higher TDDs.
Safety
Twenty-two studies were identified reporting safety findings for selexipag and 8 studies for oral treprostinil.
The most commonly reported AEs for oral selexipag in GRIPHON were headache (65%), diarrhea (42%), nausea (34%), pain in jaw (26%), and vomiting (18%) (Table 2).Citation13,Citation15 In FREEDOM-M, FREEDOM-C, and FREEDOM-C2 OLE, the most frequently reported AEs were headache (71%), diarrhea (55%), nausea (46%), flushing (35%), vomiting (21%), and pain in jaw (25%).Citation17 Findings in FREEDOM-EV were similar.Citation21
EXPOSURE (observational study) found that selexipag maintenance treatment at the individualized dose was well tolerated in clinical practice.Citation46 A registry study of oral treprostinil found that the rate of AEs decreased over time, with a large reduction in reported rates of prostacyclin-related AEs by month 6.Citation30
Effects of Oral PPAs on Healthcare Utilization and Costs (Oral Selexipag versus Oral Treprostinil)
PAH is characterized by frequent hospitalizations and high medical costs.Citation5 Eight studies were identified that describe economic outcomes for selexipag, including two comparing outcomes with oral treprostinil. No head-to-head clinical trials have compared the impact of these two oral PPAs on hospitalization.
A retrospective claims analysis of 222 people in the US compared outcomes in patients receiving oral PPAs; compared with oral treprostinil (n = 99), selexipag (n = 123) was associated with a 46% lower risk of all-cause hospitalization (P = 0.02) and a 47% lower risk of pulmonary hypertension (PH)-related hospitalization (P = 0.03).Citation47
In a retrospective cohort study using the IBM MarketScan database, Dean et al studied patients aged ≥18 years receiving selexipag (n = 126) or oral treprostinil (n = 130). At month 6, the mean number of inpatient visits was similar between treatment groups (adjusted all-cause inpatient visits: 1.3 ± 0.6 [oral treprostinil] vs 1.9 ± 0.8 [selexipag] (P = 0.2);Citation48 and adjusted PAH-related inpatient visits: 1.2 ± 0.6 vs 1.2 ± 0.6, respectively; P = 1.0). After adjusting for covariates, treatment with selexipag was associated with 51.4% higher total all-cause healthcare costs versus oral treprostinil, predominantly attributable to all-cause pharmacy costs (68.2% higher in patients receiving selexipag compared with those receiving oral treprostinil).Citation48,Citation49
A retrospective cohort study of 1,310 patients with PAH (using the Optum database) reported all-cause hospitalization costs of US$39,983 for oral treprostinil versus US$20,635 for selexipag. Total PAH-related medical costs were 40% lower (US$4,03,987) for patients receiving selexipag versus oral treprostinil (P = 0.006).Citation50
A retrospective claims analysis of 583 patients in the US reported that after adjustment for baseline characteristics, selexipag had the lower risk for hospitalization when compared to inhaled iloprost and parenteral treprostinil (relative rate ratio [95% CI], 0.40 [0.22, 0.75], and 0.26 [0.17, 0.39]) and outpatient visits (0.66 [0.56, 0.78] and 0.76 [0.66, 0.88], respectively).Citation34
Discussion
Although oral treprostinil and selexipag both target the prostacyclin pathway, these medications are not clinically equivalent and have different treatment effects on specific health outcomes (eg, hospitalization) and further differences in safety profiles, titration, and maintenance management. Efficacy is also variable depending on the number of concomitant PAH therapies (one vs two) and dose level (individualized dose vs need to maximize dose). Population health decision-makers should strive to ensure that all PAH therapies are made available for patients given the risk of severe negative outcomes, including hospitalization and death in patients with poorly controlled disease, enabling expert clinicians to individualize therapy, including the choice of PPAs based on their experience, available evidence, and guidelines. To optimize outcomes with oral PPAs, careful management of initial side-effects is essential to complete titration and initiate maintenance treatment successfully. Patient tolerability is likely to improve, as side-effects are reported to decrease over time.Citation44
The cost for selexipag is fixed and predictable as it does not vary by dose. Oral treprostinil cost is subject to variation by dose, with increasing costs associated with higher maintenance doses, and so it is difficult to predict and model annual costs incurred with this treatment. Treatment costs should not be viewed in isolation for oral PPAs, as this is a rare fatal disease associated with significant burden on patients and the healthcare system. Oral PPAs deliver many economic benefits, including reduced healthcare costs, reduced hospitalization rates (selexipag), and delayed disease progression, offsetting costs associated with use of these medications.
Prior authorizations, step-edits, and quantity limits are common for oral PPAs due to the high treatment costs. Utilization management strategies that are too restrictive may delay initiation of therapy, resulting in poorer outcomes. Indeed, prior authorizations for life-saving medications may be a misplaced strategy when the delay can lead to worsening outcomes or incremental costs, or both. Prompt PPA therapy initiation has been associated with improved or stabilized clinical status for this rapidly progressing disease with a high mortality rate.Citation19
ESC/ERS guidelines recommend risk assessment every 3 to 6 months, which serves as an early signal for treatment escalation with an oral PPA. Population health decision-makers may implement risk assessments to detect patients at risk of worsening and treatment escalation.
Limitations
Studies conducted on fewer than 20 patients were not included in the evidence synthesis. The heterogeneity of clinical trial design (endpoint definition, patient population – etiology, stage of disease progression) are limitations when comparing outcomes across the clinical studies describing in this literature review. The findings of the literature review reflect publications up to June 2022 and do not include subsequent publications.
Conclusions
The 2022 ESC/ERS guidelines describe the addition of selexipag for patients receiving PDE5i and/or ERA and oral treprostinil for patients receiving monotherapy (PDE5i or ERA) who are at risk of progression. This TLR provides population health decision-makers with important insights to evaluate the distinct profiles of oral PPA treatment options and to inform formulary and coverage decisions for the treatment of patients, with PAH ensuring access to critical PAH therapies.
Disclosure
Dr Charles Burger reports personal fees from Janssen, personal fees from INSMED, personal fees from Merck, and non-financial support from United Therapeutics, during the conduct of the study. Yuen Tsang is a former employee and owns stock in Johnson & Johnson. Dr Marie Chivers, Nikki Atkins and Ms Riya Vekaria report employment with Avalere Health which was in receipt of payment from Janssen for this element of the study. Dr Gurinderpal Doad and Dr Sumeet Panjabi are employees and stockholders of Johnson & Johnson, the company that markets oral selexipag. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Hassoun PM, Taichman DB. Pulmonary Arterial Hypertension. N Engl J Med. 2021;385(25):2361–2376. doi:10.1056/NEJMra2000348
- Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: the clinical syndrome. Circ Res. 2014;115(1):115–130. doi:10.1161/CIRCRESAHA.115.301146
- Austin ED, Kawut SM, Gladwin MT, Abman SH. Pulmonary hypertension: NHLBI workshop on the primary prevention of chronic lung diseases. Ann Am Thorac Soc. 2014;11(Suppl 3):S178–85. doi:10.1513/AnnalsATS.201312-443LD
- Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. doi:10.1378/chest.09-1140
- Burger CD, Ghandour M, Padmanabhan Menon D, Helmi H, Benza RL. Early intervention in the management of pulmonary arterial hypertension: clinical and economic outcomes. Clinicoecon Outcomes Res. 2017;9:731–739. doi:10.2147/CEOR.S119117
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. doi:10.1093/eurheartj/ehac237
- McLaughlin VV, Channick R, De Marco T, et al. Results of an expert consensus survey on the treatment of Pulmonary Arterial Hypertension with oral prostacyclin pathway agents. Chest. 2020;157(4):955–965. doi:10.1016/j.chest.2019.10.043
- American College of Cardiology. FDA approves selexipag for PAH patients. Available from: https://www.acc.org/latest-in-cardiology/articles/2015/12/22/11/39/fda-approves-selexipag-for-pah-patients/. Accessed May 17, 2024.
- de Lartigue J. Oral treprostinil for the treatment of pulmonary arterial hypertension. Drugs Today. 2014;50(8):557–565. doi:10.1358/dot.2014.50.8.2207312
- Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL Registry analysis. Chest. 2013;144(5):1521–1529. doi:10.1378/chest.12-3023
- McLaughlin VV, Hoeper MM, Channick RN, et al. Pulmonary arterial hypertension-related morbidity is prognostic for mortality. J Am Coll Cardiol. 2018;71(7):752–763. doi:10.1016/j.jacc.2017.12.010
- Burger CD, Long PK, Shah MR, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest. 2014;146(5):1263–1273. doi:10.1378/chest.14-0193
- Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–2533. doi:10.1056/NEJMoa1503184
- White RJ, Jerjes-Sanchez C, Bohns Meyer GM, et al. Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind placebo-controlled clinical trial. Am J Respir Crit Care Med. 2020;201(6):707–717. doi:10.1164/rccm.201908-1640OC
- Jing ZC, Parikh K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127(5):624–633. doi:10.1161/CIRCULATIONAHA.112.124388
- Tapson VF, Torres F, Kermeen F, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142(6):1383–1390. doi:10.1378/chest.11-2212
- Tapson VF, Jing ZC, Xu KF, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. 2013;144(3):952–958. doi:10.1378/chest.12-2875
- FDA. FDA label UPTRAVI. 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214275s000lbl.pdf. Accessed May 25, 2023.
- Gaine S, Sitbon O, Channick RN, et al. Relationship between time from diagnosis and morbidity/mortality in pulmonary arterial hypertension: results from the Phase III GRIPHON study. Chest. 2021;160(1):277–286. doi:10.1016/j.chest.2021.01.066
- FDA. FDA label ORENITRAM; 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203496s011lbl.pdf. Accessed May 25, 2023.
- White RJ, Pepke-Zaba J, Balasubramanian VP, et al. Oral treprostinil treatment is associated with improved survival in pulmonary arterial hypertension participants in FREEDOM-EV and the FREEDOM-EV open-label extension study. Pulmon Vasc Dis. 2022;2022:1.
- Galie N, Gaine S, Channick R, et al. Long-term survival, safety and tolerability with selexipag in patients with pulmonary arterial hypertension: results from GRIPHON and its open-label extension. Adv Ther. 2022;39(1):796–810. doi:10.1007/s12325-021-01898-1
- White R, Pepke-Zaba JP, Balasubramanian V, et al. Oral treprostinil treatment is associated with improved survival in pulmonary arterial hypertension participants in FREEDOM-EV and the FREEDOM-EV open-label extension study. Chest. 2022;162(4):A2301–A2303. doi:10.1016/j.chest.2022.08.1909
- Coghlan JG, Galié N, Gaine S, et al. Long -term survival and safety with selexipag in patients with pulmonary arterial hypertension: results from the GRIPHON study and its open-label extension (id S122). BMJ J. 2018;2:1.
- Chin KM, Sitbon O, Doelberg M, et al. Three- versus two-drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol. 2021;78(14):1393–1403. doi:10.1016/j.jacc.2021.07.057
- McLaughlin V, Chin K, Farber H, et al. Clinical characteristics of patients with pulmonary arterial hypertension and treatment patterns: a real-world analysis from SPHERE (Selexipag: the Users Drug Registry). Chest. 2021;160(4):A2292–A2295. doi:10.1016/j.chest.2021.07.2001
- Park BD, Chin KM, Bartolome S Change in objective parameters after long-term selexipag therapy for pulmonary arterial hypertension: a single-center observational study. presented at: American Thoracic Society International Conference 2018; 2018.
- Sahay S, Ravichandran A, Parikh K, et al. Real-World transitions from parenteral, inhaled, and oral prostacyclin-class therapies to oral treprostinil: interim date from the ADAPT registry. presented at: American Thoracic Society International Conference Abstract; 2020.
- Kingrey J, Swisher J, Ravichandran A, et al. Interim data from the ADAPT registry: real-world tolerability and management of adverse events in patients receiving oral treprostinil. Chest. 2020;158(4):A2169–A2170. doi:10.1016/j.chest.2020.08.1864
- Lange TJ, Soderberg S, Biedermann P, et al. Selexipag titration and dosing patterns in patients with pulmonary arterial hypertension (PAH) in a real-world clinical setting: insights from the EXPOSURE study. presented at: American Thoracic Society International Conference 2021; 2021.
- Corporation UT. The Orenitram Titration kit. Available from: https://www.orenitram.com/kit. Accessed April 22, 2023.
- Kim NH, Hemnes AR, Chakinala MM, et al. Patient and disease characteristics of the first 500 patients with pulmonary arterial hypertension treated with selexipag in real-world settings from SPHERE. J Heart Lung Transplant. 2021;40(4):279–288. doi:10.1016/j.healun.2021.01.006
- Cui X, Lu W, Zhang D, et al. Selexipag-based triple combination therapy improves prognosis in Chinese pulmonary arterial hypertension patients. Front Cardiovasc Med. 2022;9:991586. doi:10.3389/fcvm.2022.991586
- Song C, Kunovszki P, Beaudet A, et al. Comparison of healthcare encounters and drug persistence in patients with pulmonary arterial hypertension receiving oral selexipag, inhaled iloprost, or parenteral treprostinil: a retrospective database analysis. J Health Econ Outcomes Res. 2022;9(1):151–160. PMID: 35800882; PMCID: PMC9178228. doi:10.36469/001c.35246
- McLaughlin V, Kim NH, Hemnes AR et al. Selexipag dosing and titration in the first 500 patients enrolled in SPHERE (SelexiPag: tHe UsErs dRug rEgistry), European Heart Journal 2019;40(Supplement 1):ehz745.0526. doi:10.1093/eurheartj/ehz745.0526
- El-Kersh K, King C, Shen E, Classi P, Balasubramanian V. Contemporary dosing characteristics of oral treprostinil in real-world clinical practice in patients with pulmonary arterial hypertension. Chest. 2020;158(4):A2183–A2184. doi:10.1016/j.chest.2020.08.1873
- Kung. Selexipag: real world data. presented at: CHEST annual meeting. San Antonio; 2012.
- Rahaghi FF, Feldman JP, Allen RP, et al. Recommendations for the use of oral treprostinil in clinical practice: a Delphi consensus project pulmonary circulation. Pulm Circ. 2017;7(1):167–174. doi:10.1086/690109
- Chang S-A, Lee SH, Choi JH, et al. Real-world practice patterns and characteristics of adverse events with selexipag in Korean patients with pulmonary arterial hypertension; 2022.
- Highland KB, Hull M, Pruett J, Elliott C, Tsang Y, Drake W. Baseline history of patients using selexipag for pulmonary arterial hypertension. Ther Adv Respir Dis. 2019;13:1753466619843774. doi:10.1177/1753466619843774
- Chin K, Chakinala M, Hemnes A, et al. Real-world data for selexipag in patients with connective tissue disease-associated pulmonary arterial hypertension: a SPHERE (Selexipag: the Users Drug Registry) Analysis. Chest. 2020;158(4):A2187–A2190. doi:10.1016/j.chest.2020.08.1875
- Farber HW, Chakinala M, Highland KB, et al. Risk assessment at baseline and one year in patients with pulmonary arterial hypertension (PAH): data from the first 250 patients enrolled in SPHERE (Uptravi[Selexipag]: the users drug registry) (id A105). presented at: American Thoracic Society International Conference; 2019.
- Chin KM, Ruggiero R, Bartolome S, et al. Long-term therapy with oral treprostinil in pulmonary arterial hypertension failed to lead to improvement in important physiologic measures: results from a single center. Pulm Circ. 2015;5(3):513–520. doi:10.1086/682224
- Ramani G, Cassady S, Shen E, et al. Novel dose-response analyses of treprostinil in pulmonary arterial hypertension and its effects on six-minute walk distance and hospitalizations. Pulm Circ. 2020;10(3):2045894020923956. doi:10.1177/2045894020923956
- Balasubramanian VP, Messick CR, Broderick M, Nelsen AC. Dosing characteristics of oral treprostinil in real-world clinical practice. Pulm Circ. 2018;8(2):2045894018770654. doi:10.1177/2045894018770654
- Lange TJ, Escribano P, Biedermann P, et al. Clinical characteristics and treatment patterns in patients with Pulmonary Arterial Hypertension (PAH) initiating selexipag in the EXPOSURE observational study. presented at: American Thoracic Society International Conference 2020; 2020.
- McConnell JW, Tsang Y, Pruett J, Drake W. Comparative effectiveness of oral prostacyclin pathway drugs on hospitalization in patients with pulmonary hypertension in the United States: a retrospective database analysis. Pulm Circ. 2020;10(4):2045894020911831. doi:10.1177/2045894020911831
- Dean BB, Saundankar V, Stafkey-Mailey D, et al. Medication adherence and healthcare costs among patients with pulmonary arterial hypertension treated with oral prostacyclins: a retrospective cohort study. Drugs Real World Outcomes. 2020;7(3):229–239. doi:10.1007/s40801-020-00183-x
- Dean BB, Saundankar V, Stafkey-Mailey D, et al. Correction to: medication adherence and healthcare costs among patients with pulmonary arterial hypertension treated with oral prostacyclins: a retrospective cohort study. Drugs Real World Outcomes. 2020;7(3):241–242. doi:10.1007/s40801-020-00198-4
- Papademetriou E, Liu X, Beaudet A, Tsang Y, Potluri R, Panjabi S. Comparative evaluation of costs and healthcare resource utilization of oral selexipag versus inhaled treprostinil or oral treprostinil in patients with pulmonary arterial hypertension. J Med Econ. 2023;1–16. doi:10.1080/13696998.2023.2204769