Abstract
Purpose
To compare personalized dosimetry with yttrium-90 (90Y)-loaded glass microspheres (SIRT) vs atezolizumab and bevacizumab (A+B) in hepatocellular carcinoma (HCC) treatment in terms of cost-effectiveness and budget impact from a German statutory health insurance (SHI) perspective.
Patients and Methods
Cost-effectiveness analysis (CEA) and budget impact analysis (BIA) models were developed in MS Excel. The available key studies (IMbrave150 and DOSISPHERE-01) suggest that both strategies are comparable in terms of progression-free survival and overall survival in HCC, but a difference in severe adverse events (SAE) in favor of SIRT was observed. Accordingly, the CEA model investigates the endpoints “cost per SAE avoided” and “cost per quality-adjusted life year (QALY) gained”, whereas the BIA simulates the impact of a stepwise re-allocation of current market share to the option which emerges as more cost-effective from the CEA.
Results
The model suite estimated a mean annual total per-patient costs of € 29,984 for SIRT, compared to € 75,725 for A+B. SIRT was associated with a lower number of SAE and a higher number of QALYs compared to A+B. Switching additionally 25% of the eligible patients (≈500) from systemic therapy to SIRT could generate annual savings of approximately € 22.6 million Euros to the SHI.
Conclusion
SIRT was identified as dominant treatment strategy. SIRT use not only saves SHI expenditure compared to systemic immunotherapy but also yields extra QALYs. This positions SIRT as the dominant and more cost-effective treatment strategy for patients with HCC. The savings to the SHI system, derived from the BIA conducted, become increasingly significant with rising adoption rates of SIRT.
Introduction
Liver cancer is the sixth most common cancer worldwide – ranking fifth among men and ninth among women.Citation1 Its incidence is expected to rise to over 1 million cases by 2025, thus posing a public health challenge. Hepatocellular carcinoma (HCC) is the most common form of liver cancer and accounts for nearly 90% of the cases. The main risk factors of HCC are infection by hepatitis B and hepatitis C viruses; however, non-alcoholic steatohepatitis, known as metabolic syndrome steatohepatitis, associated with metabolic syndrome or diabetes mellitus, is also becoming a more frequent risk factor in the West.Citation2,Citation3 Only 10–20% of the patients are amenable to invasive curative treatment, such as resection or transplant.Citation4 The subset of non-metastasized, non-resectable HCC patients assigned to stages B and partly C (≈25%) on the Barcelona clinic liver cancer (BCLC) scale are eligible for two treatment options, systemic immunotherapy and selective internal radiation therapy (SIRT).Citation4 This is hence the target population of our comparative modelling study: BCLC-B (by definition void of any extrahepatic disease) in its entirety and to include some of the heterogeneity of the BCLC-C stage, a proportion of 25% of the latter. Therapies vary regarding required infrastructure, medical specialization(s), facilities, preparation, conduct, and costs.
Radioembolization is a safe and effective treatment option for both primary and secondary tumors.Citation4 The potential of personalized dosimetry has also been evaluated, resulting in substantially increased response rates and overall survival (OS).Citation4 With systemic immunotherapy and radioembolization yielding comparable efficacy outcomes,Citation5,Citation6 but differing substantially in acquisition cost, a thorough comparative economic analysis is warranted.
The objective of the presented research was to compare the personalized dosimetry with yttrium-90 (90Y)-loaded glass microspheres (TheraSphere®, Boston Scientific, Marlborough, Massachusetts, USA) vs the systemic immunotherapy combination atezolizumab (Tecentriq®, Roche, Basel, Switzerland) and bevacizumab (Avastin®, Roche, Basel, Switzerland) in HCC treatment in terms of cost-effectiveness and budget impact from a German statutory health insurance perspective.
Materials and Methods
A cost-effectiveness analysis (CEA) and a budget impact analysis (BIA) model were developed in MS Excel (Microsoft Excel 365-Version 2307 64 Bit), according to the German guidelines for health economic assessments published by the German national institute for quality and efficiency in health care (IQWiG).Citation7 This model compared personalized dosimetry with yttrium-90 (90Y)-loaded Glass microspheres (SIRT) with Atezolizumab plus Bevacizumab (A+B) in patients with hepatocellular carcinoma in Germany from a statutory health insurance (SHI) perspective.
Cost-Effectiveness Analysis
The CEA model estimated the cost per serious adverse event (SAE) avoided and the cost per quality-adjusted life year (QALY) gained, comparing SIRT vs A+B from a SHI perspective. The model used a partitioned survival modelling (PSM) approach to distribute patients among the health states “progression-free”, “progression”, and “death”. Partitioned survival models are commonly employed to inform reimbursement decisions for oncology drugs.Citation8
Prospective studies such as IMbrave150 for A+BCitation5 and DOSISPHERE-01 for SIRT,Citation6 suggest that both strategies are potentially comparable in terms of progression-free survival (PFS) and OS in HCC. compares the eligibility criteria and compares the eventual study populations of the two respective pivotal trials; both study populations are deemed to be of similar severity and hence comparable. The Dosisphere-01 trial included patients with large tumors and significant portal vein infiltration, both known to have negative prognostic factors. Irrespective of these small differences in the prognostic factors favoring the IMbrave150 (A+B) population, the model conservatively assumes that both populations are comparable.
Table 1 Comparison of Eligibility Criteria from Participation in the Pivotal Trials
Table 2 Comparison of the Study Populations of the Respective Trials
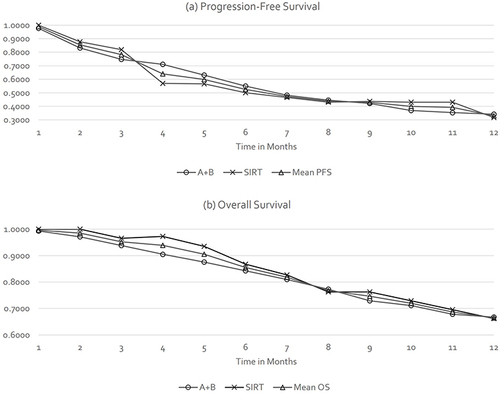
Therefore, the PSM calculations were informed by the combined PFS and OS Kaplan-Meier estimates taken from the IMbrave150 (A+B) and DOSISPHERE (SIRT) studies, as presented in , for the 12-month CEA model time horizon.
Figure 1 Progression-Free Survival (a) and Overall Survival Estimates (b) Used in the CEA Model.
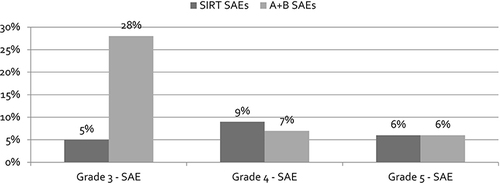
Unlike the efficacy data, a difference in SAEs favoring SIRT was observed in IMbrave150 for A+BCitation5 and DOSISPHERE-01 for SIRT,Citation6 as highlighted in .
Figure 2 Serious Adverse Events Rates Used in the CEA Model.
The CEA model investigated the endpoint of “cost per SAE avoided”. Additionally, the model simulated the impact of SAEs on a patient’s health utility by considering published SAE-related disutilities, categorized by severity grade; which in turn reduced the base health utility. The base utility was calculated using cardinal utilities obtained from published literatureCitation9 and linked to the health states defined in the PSM – “progression-free”, “progression”, and “death”. These utilities were weighted based on their occurrence, as simulated by the model in monthly cycles. All CEA model inputs are presented in .
Table 3 Overview of Input Values to the CEA and BIA Models
A targeted literature search was performed to identify appropriate utility values for the PSM health states and the disutilities associated with the occurrence of SAEs. The utility values for the health states were informed by a recent publication of EQ-5D-5L utility values from the IMbrave150 study.Citation9 Additionally, the SAE-related disutilities were obtained from two studies that investigated the impact of Grade 3/4 AEs based on the EQ-5D-5L,Citation21,Citation22 whereas the disutilities of Amdahl et al 2016Citation21 were used in the base-case analysis, which reflects a conservative approach as they show a lower impact of SAEs on the quality of life than the disutilities of Howell et al 2022.Citation22
The model included the therapy costs of SIRT and A+B, as well as the costs of AEs related to both therapies. However, it did not include the costs of follow-up therapies and ongoing screening as these were assumed to be comparable for both alternatives. The annual therapy costs of A+B were calculated based on official reimbursement tariffs (from a SHI perspective) sourced from the official German drug compendium.Citation15 These calculations assumed a therapy duration of 6.8 months, aligning with the median PFS. The annual therapy costs of SIRT were determined using the related diagnosis related group (DRG) (H29Z) and additional fee (Zusatzentgelt, ZE) (ZE2021-65) as reimbursement fees.Citation16 This calculation also considered additional costs of the SIRT workup, and for potentially necessary repeated workupsCitation17 as well as potential repeat SIRT procedures,Citation18 utilizing estimates from the published literature.Citation17,Citation18
The costs associated with SAE were calculated using the matrix, presented in , that delineated healthcare resource use (HRU) by AE grade. For grade 3 SAEs the average cost of a hospital stay were estimated, as those are per definition usually leading to a hospitalization. For grade 4 SAEs the average cost of a hospital stay with intensive care unit (ICU) stay were estimated, as those are per definition usually life threatening or disabling adverse events. For grade 5 SAEs 75% of the SAE Grade 4 costs were estimated, 75% of SAE Grade 4 costs, as these are per definition fatal adverse event resulting in death. Here, it was assumed that the patients die on the ICU. This matrix was validated by expert opinion and then contextualized in the German healthcare setting using official German cost references.Citation19,Citation20
The CEA results presented were derived from probabilistic sensitivity analysis (PSA) using a Monte-Carlo simulation approach PSA involving 10,000 iterations.Citation23 Additionally, a one-way sensitivity analysis was performed to assess the impact of varying SAE-related disutilities.
Budget Impact Analysis
The BIA model assessed the implications of shifting current market share towards the more cost-effective treatment option. The analysis was informed by estimates of the current and future treatment mix based on the HCC incidence, the number of patients eligible for SIRT or A+B, and on the current number of SIRT procedures performed for HCC in Germany. The BIA allowed for the simulation of the budget impact resulting from a shift in the current therapy mix in annual steps over a 5-year time horizon. The BIA applies a 5-year time this time horizon is most-frequently requested by healthcare payers.Citation24 This analysis was conducted from a healthcare payer perspective. The input data used in the BIA model are presented in .
Over the BIA model’s 5-year time horizon, it was assumed that the SIRT market share would increase linearly from approximately 25% (year 0, baseline) to approximately 50% (year 5). These assumptions align with insights from clinical experts, considering the constraints of radiation therapy capacities and thus reflect a realistic growth potential.
Results
The model estimated mean annual total per-patient costs of €29,984 for SIRT, compared to €75,725 for A+B, as presented in . As SIRT was associated with a lower number of SAEs and a higher number of QALYs compared to A+B, it was identified as the dominant treatment strategy.
Table 4 Overview of Deterministic CEA Model Results
The incremental cost-effectiveness plane demonstrates the robustness of these results when considering the uncertainty in efficacy and cost inputs. The results, based on 10,000 iterations (“runs”) of the model, consistently positioned in the south-east Quadrant of the coordinate plane. This position signifies lower incremental total costs in conjunction with a higher efficacy/effectiveness in favor of SIRT compared to A+B, as shown in .
Figure 3 Incremental Cost-Effectiveness Planes Comparing A+B vs SIRT from the SHI Perspective.
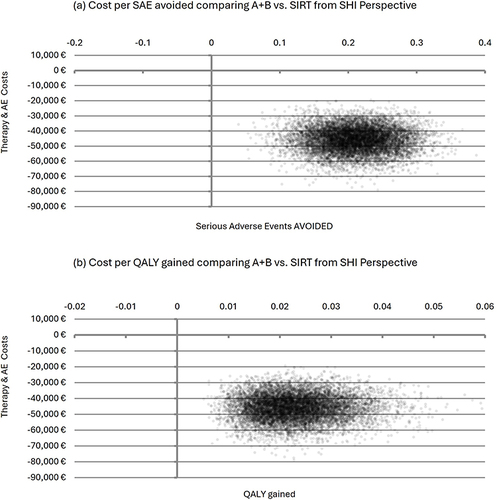
The base case results, as shown in , are based on the application of the lowest published utility reduction in the case of a Grade 3 or Grade 4 SAE (0.1101). This value was drawn from the study by Amdahl et al 2016Citation21 and resulted in additional 0.023 QALYs gained per patient with SIRT. Also, as presented in , even this conservative estimate remained stable when varied in the PSA.
When the higher published utility reductions (0.1800, 0.2044, 0.2300), listed in , were incorporated into the CEA model, the incremental effect in QALYs increased proportionally (0.038, 0.043, 0.048), which pronounces the superior effectiveness of SIRT even more distinctly.
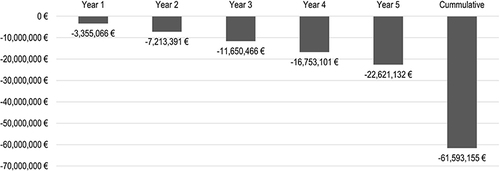
The market share of SIRT among eligible patients with HCC in Germany is about 25% (approximately 500 procedures/year) in Germany.Citation14 It is assumed that the market share of SIRT will increase linearly from approximately 25% at year 0 (baseline) to around 50% at year 5. shows the potential annual savings. Specifically, switching an additionally 25% of the eligible patients from systemic therapy to SIRT could generate annual savings of approximately € 22.6 million to the SHI system and considering the presented linear market share increase results in cumulative savings of € 61.6 million over the 5_year BIA time horizon ().
Discussion
To our knowledge, this is the first economic analysis comparing radioembolization to systemic immunotherapy in dually eligible HCC patients. This analysis comprises both a cost-effectiveness and a budget impact component. The CEA component demonstrates that SIRT not only saves costs compared to systemic immunotherapy but also yields extra QALYs. This positions SIRT as the dominant treatment strategy for patients diagnosed with HCC in terms of cost-effectiveness. In addition, the budget impact component reveals the potential for year-on-year savings to the SHI system. These savings become increasingly significant with the rising adoption rates of SIRT, making it a cost-saving option for healthcare payors.
However, the ultimate treatment decision concerning radioembolization should not be exclusively viewed through an economic lens. Rather, it should be rooted in a collaborative effort involving a multidisciplinary tumor board,Citation4 in accordance with the current clinical guidelines. Wetterwald et al discuss how the new combination therapies – in particular immunotherapy associated with tyrosine kinase inhibitors (TKIs) – hold significant promise in the future treatment of clear cell sarcoma (CCS), so far typically treated by surgical excision with or without radiotherapy.Citation25 Therefore, also in HCC, the therapeutical arsenal may widen further in the future and the need to extend our model may arise.
This study has several strengths. The robust modelling approach employed considered a careful model architecture, such as the use of monthly cycles, the cautious data population (eg not exceeding 50% SIRT uptake), and an adequate sample of 10,000 iterations per treatment arm simulated in the PSA. These constellations consistently yield similar results. This model closely mirrored the real-world clinical practice (eg, SIRT vs A+B), making the results highly applicable to actual patient scenarios. The validation of inputs (eg, eligibility of SIRT, HRU by AE grade, etc.) by clinical experts further strengthens our model. This study adopted a conservative methodology, applying rigorous eligibility criteria and focusing exclusively on SAEs that have the potential to result in additional healthcare expenditure and loss of QALYs. Furthermore, this study accounted for practical constraints that limit the expansion of SIRT, encompassing considerations related to staffing and equipment within the healthcare facilities. Consequently, it is conceivable that the actual cost savings may even exceed our estimations.
The main limitations of our study stem from the availability of source data. While the unit cost data are robust and validated, the HRU-by-severity (of the AE under study) matrix required additional expert judgement. This was necessary as data at this level of granularity was not available from published sources. However, expert opinions were aligned and confident, which suggests that uncertainty regarding the HRU matrix is likely limited. The linkage of SAEs to utility decrements was straightforward and unlikely to introduce bias.
It should be noted that fixed costs related to SIRT, namely those associated with the required infrastructure (such as facilities and equipment), were not considered in this model. However, this choice aligns with the perspective adopted in this study, specifically the perspective of German SHI, acting as third-party payor that funds the treatment, ie the variable costs. In the German hospital landscape characterized by its dual-financing stream, variable costs (treatments) are covered by the sickness funds whereas fixed costs (buildings, durable equipment) are covered by the federal state hosting the hospital. In this context, the infrastructure can be regarded as a “given”, or constant factor. A more granular picture of cost profiles could possibly be obtained by including liver functional reserve as an outcome predictor, by chosen treatment option and risk factors, as discussed by D’Avola et al in a review concerned with optimizing survival and quality of life in non-surgical treatment of HCC.Citation26 This was beyond the scope of our work, but it would be an interesting case for future investigation.
Future studies evaluating the provider and hospital perspectives jointly may be warranted.
Conclusion
In this comparative assessment of systemic immunotherapy and personalized radioembolization for HCC, SIRT emerges as dominant treatment strategy. The evidence from the CEA and BIM indicates SIRT as a cost-effective treatment strategy, potentially leading to the potential of substantial cost-savings from the perspective of German SHI. Consequently, this study offers valuable support for clinical decision-making, augmenting the decision-making process with a sound economic foundation. As our analysis took a conservative approach (with subsequent under rather than overestimation of savings potential), its implications serve as a call to policymakers and decision-makers to re-align their strategies, ensuring that HCC patients receive optimal care while simultaneously achieving financial efficiencies within the healthcare system.
Disclosure
The authors report no conflicts of interest in this work. This study was funded by Boston Scientific Medizintechnik GmbH. Dr Bjoern Schwander, Mrs Katharina Klesper, and Prof. Dr. York Zoellner report personal fees from Boston Scientific Medizintechnik GmbH, during the conduct of the study.
This paper was presented at the ISPOR Europe 2022 (Vienna, Austria) as a poster presentation with interim findings. The poster and abstract were published in “Poster Abstracts” in Value in Health, Volume 25, Issue 12S (December 2022): https://doi.org/10.1016/j.jval.2022.09.806.
References
- World Cancer Research Fund International. Liver cancer statistics; 2022. Available from: https://www.wcrf.org/cancer-trends/liver-cancer-statistics/. Accessed September 15, 2023.
- Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–1556. doi:10.1016/j.jhep.2023.06.003
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nature Reviews Disease Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
- Weber M, Lam M, Chiesa C, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2022;49(5):1682–1699. doi:10.1007/s00259-021-05600-z
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
- Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label Phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(1):17–29. doi:10.1016/S2468-1253(20)30290-9
- German national institute for quality and efficiency in health care (IQWiG). General Methods for the Assessment of the Relation of Benefits to Costs (Version 6.1); 2022. Available from: https://www.iqwig.de/methoden/general-methods_version-6-1.pdf. Accessed June 15, 2023.
- Woods BS, Sideris E, Palmer S, Latimer N, Soares M. Partitioned survival and state transition models for healthcare decision making in oncology: where are we now? Value Health. 2020;23(12):1613–1621. doi:10.1016/j.jval.2020.08.2094
- Gaugain L, Cawston H, Dubois de Gennes C, et al. Cost-utility analysis of atezolizumab with bevacizumab in untreated unresectable or advanced hepatocellular carcinoma in France. PLoS One. 2023;18(1):e0280442. doi:10.1371/journal.pone.0280442
- Destatis Statistisches Bundesamt. Bevölkerungsstand: amtliche Einwohnerzahl Deutschlands; 2022. Available from: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Bevoelkerungsstand/_inhalt.html. Accessed August 19, 2023.
- Bundesgesundheitsministerium. Gesetzliche Krankenversicherung; Mitglieder, mitversicherte Angehörige und Krankenstand; 2023. Available from: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Statistiken/GKV/Mitglieder_Versicherte/KM1_Januar_bis_Mai_2023_bf.pdf. Accessed August 19, 2023.
- Clouth J, Liepa AM, Moeser G, et al. Hepatocellular carcinoma after prior sorafenib treatment: incidence, healthcare utilisation and costs from German statutory health insurance claims data. Health Econ Rev. 2018;8(1):18. doi:10.1186/s13561-018-0199-1
- Weinmann A, Koch S, Sprinzl M, et al. Survival analysis of proposed BCLC-B subgroups in hepatocellular carcinoma patients. Liver Int. 2015;35(2):591–600. doi:10.1111/liv.12696
- Institut für das Entgeltsystem im Krankenhaus. InEK DatenBrowser; 2019. Available from: https://datenbrowser.inek.org/. Accessed August 19, 2023.
- Lauer-Taxe - Standardwerk der Arzneimittelinformationen. Arzneimittelkosten Atezolizumab und Bevacizumab; 2023. Available from: https://portal.cgmlauer.cgm.com/LF/Seiten/Verwaltung/Kundencenter/1.aspx. Accessed June 15, 2023.
- Institut für das Entgeltsystem im Krankenhaus. Fallpauschalen-Katalog; 2023. Available from: https://www.g-drg.de/ag-drg-system-2023/fallpauschalen-katalog/fallpauschalen-katalog-20232. Accessed August 19, 2023.
- Pollock RF, Colaone F, Guardiola L, Shergill S, Brennan VK. A cost analysis of SIR-Spheres yttrium-90 resin microspheres versus tyrosine kinase inhibitors in the treatment of unresectable hepatocellular carcinoma in France, Italy, Spain and the UK. J Med Econ. 2020;23(6):593–602. doi:10.1080/13696998.2020.1731213
- Marqueen KE, Kim E, Ang C, Mazumdar M, Buckstein M, Ferket BS. Cost-effectiveness analysis of selective internal radiotherapy with yttrium-90 versus sorafenib in locally advanced hepatocellular carcinoma. JCO Oncol Pract. 2021;17(2):e266–e277. doi:10.1200/OP.20.00443
- Destatis Statistisches Bundesamt. Kosten der stationären Krankenhausversorgung; 2023. Available from: https://www.destatis.de/DE/Presse/Pressemitteilungen/2021/04/PD21_194_231.html. Accessed August 19, 2023.
- Kaier K, Heister T, Wolff J, Wolkewitz M. Mechanical ventilation and the daily cost of ICU care. BMC Health Serv Res. 2020;20(1):267. doi:10.1186/s12913-020-05133-5
- Amdahl J, Diaz J, Park J, Nakhaipour HR, Delea TE. Cost-effectiveness of pazopanib compared with sunitinib in metastatic renal cell carcinoma in Canada. Curr Oncol. 2016;23(4):e340–354. doi:10.3747/co.23.2244
- Howell TA, Matza LS, Jun MP, Garcia J, Powers A, Maloney DG. Health State utilities for adverse events associated with chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Pharmacoecon Open. 2022;6(3):367–376. doi:10.1007/s41669-021-00316-0
- Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5(2):157–177. doi:10.1177/0272989X8500500205
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14. doi:10.1016/j.jval.2013.08.2291
- Wetterwald L, Riggi N, Kyriazoglou A, Dei Tos G, Dei Tos A, Digklia A. Clear cell sarcoma: state-of-The art and perspectives. Expert Rev Anticancer Ther. 2023;23(3):235–242. doi:10.1080/14737140.2023.2183846
- D’Avola D, Granito A, Torre-Aláez M, Piscaglia F. The importance of liver functional reserve in the non-surgical treatment of hepatocellular carcinoma. J Hepatol. 2022;76(5):1185–1198. doi:10.1016/j.jhep.2021.11.013