Abstract
Background
Erythropoiesis-stimulating agents (ESAs) are the mainstay of anemia therapy. Continuous erythropoietin receptor activator (CERA) is a highly effective, long-acting ESA developed for once-monthly dosing. A multitude of clinical studies has evaluated the safety and efficiency of this treatment option for patients with renal anemia. In times of permanent financial pressure on health care systems, the cost-effectiveness of CERA should be of particular importance for payers and clinicians.
Objective
To critically analyze, from the nephrologists’ point of view, the published literature focusing on the cost-effectiveness of CERA for anemia treatment.
Methods
The detailed literature search covered electronic databases including MEDLINE, PubMed, and Embase, as well as international conference abstract databases.
Results
Peer-reviewed literature analyzing the definite cost-effectiveness of CERA is scarce, and most of the available data originate from conference abstracts. Identified data are restricted to the treatment of anemia due to chronic kidney disease. Although the majority of studies suggest a considerable cost advantage for CERA, the published literature cannot easily be compared. While time and motion studies clearly indicate that a switch to CERA could minimize health care staff time in dialysis units, the results of studies comparing direct costs are more ambivalent, potentially reflecting the differences between health care systems and variability between centers.
Conclusion
Analyzed data are predominantly insufficient; they miss clear evidence and have to thus be interpreted with great caution. In this day and age of financial restraints, results from well-designed, head-to-head studies with clearly defined endpoints have to prove whether CERA therapy can achieve cost savings without compromising anemia management.
Background
Anemia is associated with reduced quality of life, significant morbidity, and increased mortality.Citation1 Partial correction of anemia, the maintenance of stable hemoglobin (Hb) levels, and a reduced frequency of therapeutic interventions are common goals in anemia therapy.Citation2,Citation3 Besides treatment of underlying diseases and compensation of iron, vitamin B12, or folate deficiencies, red blood cell (RBC) transfusions were traditionally used for the correction of anemia.Citation4 The introduction of erythropoiesis-stimulating agents (ESAs) more than two decades ago has often nearly completely replaced donation of RBC transfusions.Citation5 Today, the two major therapeutic areas for ESA treatment are anemia associated with chronic kidney disease (CKD) and anemia due to chemotherapy in cancer patients.Citation6,Citation7 Different ESA types currently available in the European Union (EU) are summarized in .
Table 1 Selection of ESAs, excluding “biosimilars”, which are currently available in the EU
Although ESA therapy can often result in dramatic benefits initially, long-term improvement in outcomes is disappointing, particularly in patients with CKD or end-stage renal disease.Citation9 Furthermore, randomized controlled trials in CKD patients not on dialysis, with or without diabetes, resulted in serious concerns about the safety of ESA therapy.Citation10–Citation13 In addition to more cautious and individualized ESA use, current trends in anemia management mainly focus on simplifying and economizing ESA treatment, as this kind of treatment still generates significant costs.
The pegylated erythropoietin continuous erythropoiesis receptor activator (CERA) (methoxy polyethylene glycol-epoetin beta; Mircera®; Hoffman-La Roche Ltd, Basel, Switzerland) has a unique erythropoietin receptor binding kinetic, allowing for once-a-month dosing.Citation14,Citation15 CERA has been extensively tested in preclinical and clinical studies.Citation16,Citation17 In CKD patients, the safety profile of CERA and its efficacy – as compared to that of other ESAs, including the longer acting ESA analog, darbepoetin alfa (DA) (Aranesp®; Amgen Inc., Thousand Oaks, CA, USA) – was not always convincing.Citation18,Citation19 In advanced non-small-cell lung cancer patients receiving chemotherapy, CERA treatment was prematurely stopped due to high mortality.Citation20
This safety signal had significant market impact, so that CERA was only approved for treatment of renal anemia by the EU in July 2007 and by the United States Food and Drug Administration (FDA) in November 2007.Citation21 Patent disputes between Hoffman-La Roche Ltd, and its rival, Amgen, have prevented CERA importation into, or use in, the US until mid-2014, when patents for Amgen’s Aranesp® expire in most territories.Citation22
The incidence and prevalence of patients with anemia is growing worldwide.Citation23,Citation24 In view of increasing health care costs and escalating financial pressure on public and private payers, it is desirable to improve the cost-effectiveness of ESA therapy while maintaining high standards of care.Citation25 Adoption of a reasonably priced ESA regimen providing predictable and stable Hb responses with minimal administration frequency should be most welcome.
Recent data already argue that the introduction of CERA could offer relevant cost savings compared to a conventional first-generation ESA (epoetin [EPO] alpha or beta) therapy: firstly, the cost-effectiveness of pegylated drugs has been indicated in various clinical settings;Citation26 secondly, the nursing staff costs in particular are directly affected by the dosing frequency of the ESA.Citation27–Citation29 Finally, a landmark study by Schiller et alCitation30 demonstrated that use of a once-monthly ESA to correct anemia in dialysis patients may provide substantial time, resource, and cost savings.
But are these findings the simple solution to this complex question: can the administration of once-monthly CERA result in cost-effectiveness or even cost savings when compared to other available ESAs? To solve this pivotal issue from the clinicians’ perspective, available data reporting the costs associated with CERA treatment for anemia in chronic disease were critically reviewed by a nephrologist.
Methods
Search strategy
In July 2013, electronic databases including MEDLINE® (US National Library of Medicine, Bethesda, MD, USA), PubMed (National Center for Biotechnology Information, US National Library of Medicine), and Embase (Elsevier Inc., Philadelphia, PA, USA) were queried for published literature on the definite or estimated costs and cost-effectiveness of CERA for anemia treatment. Additional studies were identified by searching bibliographies of related publications and using the Google internet search engine (Google, Mountain View, CA, USA). Final updated searches were undertaken in January 2014. Iterative searches of conference abstract databases (including the International Society of Nephrology [Brussels, Belgium], the American Society of Nephrology [Washington, DC, USA], the European Renal Association–European Dialysis and Transplantation Association [Parma, Italy], the American Society of Hematology [Washington, DC, USA], the European Hematology Association [the Hague, the Netherlands], and the International Society For Pharmacoeconomics and Outcomes Research [Lawrenceville, NJ, USA]) were conducted to find relevant abstracts presented between 2008 and 2013.
The applied search terms were: “Mircera”, “methoxy polyethylene glycol epoetin-beta”, “continuous erythropoiesis receptor activator”, or “CERA”; and “anemia”, “anaemia”, “haemoglobin”, or “hemoglobin” and “cost”, “costs”, or “cost-effectiveness”.
Study selection
Inclusion criteria were: 1) language of publication restricted to English; 2) studies or trials relating to costs and the cost-effectiveness of CERA treatment in anemia related to CKD, cancer, or chronic heart failure; 3) studies published in peer-reviewed journals or abstracts presented at conferences of international societies; and 4) studies involving adult patients.
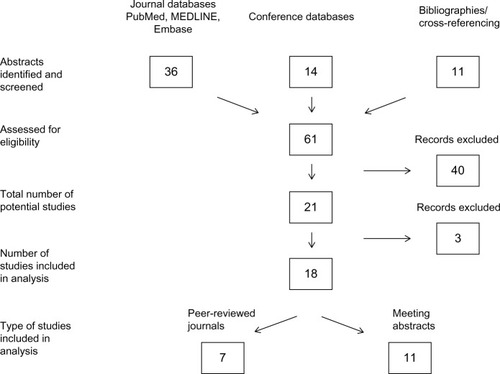
The exclusion criteria for this study were: 1) non-English language publications; 2) studies concentrating solely on outcomes or quality of life without any description of costs or time savings associated with CERA treatment; and 3) editorials and scholarly reviews. A flowchart of the study selection procedure is depicted in .
From the initial search of the three selected journal databases, 36 potentially relevant abstracts were found. A further eleven abstracts were included in this initial search from cross-referencing. Searches of the conference abstract databases produced 14 additional abstracts. A total of 61 selected abstracts were reassessed and further analyzed by the author. Forty of the 61 abstracts were immediately excluded, as they did not fulfill the proposed inclusion criteria, or they were duplicates. The remaining 21 abstracts were analyzed for relevance, with three of these excluded as they did not directly discuss CERA-associated costs or associated time savings. Eighteen papers or abstracts were finally chosen for discussion in this review.
Results
The literature query focusing on the costs and cost-effectiveness of CERA treatment for anemia showed a relative paucity of research published to date. With respect to the different potential indications, relevant studies were restricted to patient cohorts with renal anemia.
The 18 selected studies can be divided into seven papers that were published in peer-reviewed journals and eleven meeting abstracts presented at conferences of international societies. As a matter of principle, the literature can be divided into one of the following groups: studies demonstrating an increase of costs; or studies arguing for a cost reduction associated with CERA treatment. A further subcategorization of the latter selected studies was made: studies demonstrating definite cost reduction; studies suggesting potential cost reduction; and studies suggesting time reduction leading to a subsequent reduction of costs.
Studies demonstrating increasing costs after a switch to CERA therapy are summarized in . These included one cost-effectiveness analysis (CEA), one cost-minimization analysis (CMA), and four single-center studies with predominantly small patient numbers and limited study duration of 6 months or 12 months, respectively. Of note, four of the six studies analyzed hospital-based centers. Silva et alCitation31 used a CEA and Markov model to evaluate the impact of a hypothetical switch to CERA in Brazilian dialysis patients and found conventional EPO to be more cost effective for the public health system. The results were confirmed by a sensitivity analysis. Unfortunately, more detailed information regarding the treatment’s cost-effectiveness (for example, median costs/patient/month for EPO versus CERA) was not available from the abstract. A CMA conducted in two Spanish tertiary hospitals compared EPO, DA, and CERA in CKD patients on and not on dialysis and found the highest median costs/patient/month for CERA treatment.Citation32
Table 2 Studies demonstrating increased medication costs after a switch to CERA therapy
Overall, median ESA costs/patient under CERA treatment in studies demonstrating an increase in costs ranged between €115/month and €290/month. Exceptionally high average costs/patient/month of €290.1±€69.0 were demonstrated in a prospective analysis including hemodialysis (HD) patients.Citation33 At month 6 after switching from subcutaneous EPO to subcutaneous CERA, the monthly cost/patient further increased to €361.6±€169.3. Only a slight but not significant increase in costs after switching from DA to CERA was obvious in a retrospective study that included 15 patients treated with peritoneal dialysis.Citation34
Two studies reported a more subtle result related to different comparator ESAs or total treatment costs, respectively. In a mixed cohort of 190 predialysis patients, Padullés-Zamora et alCitation35 found a significant increase in costs after switching from EPO beta to CERA, whereas a switch from DA to CERA was cost effective. Of particular note, when comparing the observed costs with the expected costs based on the theoretical dosage, the authors found additional possible savings. Lower doses than those recommended in the drug leaflet allowed for sufficient Hb control during the first 3 months postswitch.
Despite the higher medication costs associated with a switch from conventional EPO to CERA, the total treatment costs per patient and year were lower in a small cohort of 17 dialysis patients.Citation36 This effect was due to fewer transfusions and hospitalizations in the CERA-treated patients. Reproducibility of these retrospective observational data is difficult, as data were obtained from a single center, and comparatively very high costs were reported for EPO in this center.
In studies demonstrating increasing costs associated with CERA therapy, median CERA doses revealed a broad variation, with average values ranging from 75–200 μg/month.Citation33–Citation36
Five studiesCitation37–Citation41 demonstrating definite cost reduction with CERA compared to conventional ESA therapy are summarized in . Again, these are predominantly retrospective single-center studies including small patient numbers. Data were available from meeting abstracts alone, and to date, none of these studies was published in a peer-reviewed journal.Citation37–Citation41 One cannot exclude that two of the studies analyzed an identical cohort of dialysis outpatients at different time points.Citation39,Citation40 There is considerable variation in cost reduction, which ranges between 14%–45%,Citation37–Citation41 with the lowest cost reductions reported in the single prospective multicenter study.Citation38 Compared to the studies that reported cost increases, the average CERA doses were slightly higher and varied between 92–228 μg/month; however, further assessment of the cost impact related to dosing errors was not noted in any of the cited studies.Citation37–Citation41
Table 3 Studies demonstrating definite cost-reduction after a switch to CERA therapy
In all studies comparing different ESA regimens, the targeted Hb value, as well as the baseline and endpoint values for Hb and iron status (ie, transferrin saturation and ferritin), are critical. The available data for Hb levels are outlined in . The Hb targets were significantly varied in the studies demonstrating higher costs after switching to CERA, and these targets were not defined in the majority of studies demonstrating definite cost reduction. Furthermore, a lack of standardization of iron parameters was obvious (data not shown).
Table 4 Reported hemoglobin levels in studies analyzing the cost-effectiveness of CERA
A literature search of the conference abstract databases revealed four other studies that predicted the probability of cost savings after the introduction of CERA, or following the switch from a regimen with short-acting EPO to CERA ().Citation42–Citation45 All these data were presented at European International Society for Pharmacoeconomics and Outcomes Research conferences, but they are still not available as regular articles in peer-reviewed journals. These studies include two CEAs that presented decision trees simulating treatment costs for the Mexican and Ukrainian public health care systems, respectively, and one CMA from Poland.Citation42–Citation44 Gonzalez et alCitation42 estimated a slight reduction of treatment costs after switching to CERA from EPO alpha, with probabilities of 0.60 for cost savings and 0.99 for cost efficiency. Moreover, the hospital stay of treated patients due to Hb variations was reduced by 37%.Citation42 For the Ukrainian dialysis population, estimated cost savings were 5%–35%, depending on the route of administration.Citation43 Kawalec et alCitation44 performed a CMA from the perspective of the public payer for predialysis patients and found a cost savings of €262.4/patient over a 2-year horizon compared to treatment with DA. Finally, assuming that CERA achieves a market share of 40%, Walsh et alCitation45 calculated a possible ESA budget reduction by 15% in five EU countries based on a United Kingdom budget impact model.
Table 5 Studies predicting cost reduction with CERA treatment
Three observational multicenter time and motion (TAM) studies predicted an average annual time savings of >80% assuming 100% CERA use for the treatment of anemia in dialysis patients ().Citation46–Citation48 Of note, a significant overlap in the available data cannot be excluded, as results presented by Klatko and FelisiakCitation48 from three Polish HD centers seem to be included in a study from De Cock et al,Citation47 who incorporated a total of 20 centers from five EU countries. In both studies, time (but not direct costs) was investigated.
Table 6 Studies predicting a significant gain in time with CERA treatment in dialysis centers
Saueressig et alCitation46 calculated that with the adoption of once-monthly CERA, health care staff members’ time for “observed” activities (including the preparation, distribution, injection, and ordering of ESAs) alone could be reduced by 70%–84%, saving 24–35 working days per year for a center of 100 patients. When nonobserved activities are considered as well, once-monthly CERA could offer potential annual time savings of 43 days in an average German center, and 37 days in an average UK center. Translated into monetary units, this could lead to an estimated reduction in annual costs of 58% for the German center and 35% for the UK center. Similar time saving ratings were confirmed by De Cock et al,Citation47 prognosticating a 67%–95% time reduction, depending on center size and the initial distribution of conventional ESAs. In addition, a comparison between DA and CERA indicated that there was still a substantial reduction in estimated annual time savings, ranging between 40% in France and 58% in Italy.Citation47
Discussion
ESAs are effective in the management of anemia, but they substantially add to the overall treatment costs. Simplifying and economizing ESA treatment is, therefore, of significant importance for both payers and clinicians. The administration of CERA once a month could have an advantage of cost and time reduction. Thus, the currently published literature focusing on the cost-effectiveness of CERA in anemia treatment was critically analyzed.
From the clinician’s point of view, although multiple studies have supposedly demonstrated the clinical safety and efficacy of CERA, peer-reviewed literature analyzing the definite cost-effectiveness of CERA is scarce. Most of the available data originate from conference abstracts and are therefore surrounded by a considerable degree of uncertainty and are open to biases of unknown magnitude and direction. Possible caveats in the interpretation of the reviewed literature are summarized in . In addition, it is noteworthy that the reviewed literature was analyzed and interpreted by a clinician and not a pharmacoeconomist.
Table 7 Caveats for the interpretation of studies assessing the cost-effectiveness of CERA
The identified literature was completely restricted to the treatment of anemia due to CKD. Although the majority of studies suggested a considerable cost advantage for CERA, the published literature cannot easily be compared. While TAM studies clearly indicate that a switch to CERA could minimize ESA treatment time and its subsequent costs, the results of studies comparing direct medication costs are more ambivalent, potentially reflecting significant differences between health care systems and centers. In addition, the selected literature presents a mix of cost estimates in European and non-European currencies, and despite attempts to translate the currency into Euros, the comparability between different currencies and different public health systems is not possible. In general, whether the studies have relied on published prices rather than actual market prices remains unclear.
For the switch from short-acting to long-acting ESAs, a conversion guideline is mandatory.Citation49–Citation51 Most clinicians act in accordance with the dose conversion referral provided by the manufacturer to reduce potentially harmful Hb variability. Due to the deficiency in the available data, it remains unclear whether applied dose conversion ratios were comparable between the cited studies.
Furthermore, the CERA doses required to maintain stable Hb values over longer treatment periods seem to vary significantly in different anemic cohorts.Citation39,Citation41,Citation44,Citation45 For example, in stable HD patients, a couple of studies have suggested decreasing CERA doses over time, whereas others argue for increasing dosages.Citation52,Citation53 A study conducted with 52 Japanese HD patients showed that CERA doses decreased during a 28-week study.Citation52 In contrast, the randomized comparative PATRONUS (comPArator sTudy of CERA and darbepOetin alfa in patieNts Undergoing dialySis) trialCitation53 conducted with 490 HD patients demonstrated a dose increase of 6.8% after switching from once-weekly DA to once-monthly CERA.
For unstable or critically ill patients in particular, estimations of the CERA dose requirements and their associated costs are currently unpredictable. Albero Molina et alCitation33 reported a further increase in the average costs associated with CERA in HD patients at month 6. This is potentially due to the fact that treated patients were more critically ill, as reflected by a distinct drop-out rate during follow-up. Of the 30 patients who began the study, 13 were withdrawn during the 6-month study because of “death, transplantation or a process that might interfere with the Hb level”.Citation33
Time savings that can be converted into cost reductions is an important reason for a clinician to switch to a long-acting ESA with reduced dosing frequency.Citation30 However, it is still unclear whether there really is a cost advantage for switching between two long-acting ESAs. Outside of interventional clinical studies, only limited information is published on switching ESA treatment from DA to CERA in renal anemia. In the AFFIRM (Aranesp® Efficiency Relative to Mircera®) study,Citation54 HD patients were switched from DA to CERA. The number of RBC transfusions increased approximately threefold from the preswitch to the postswitch period. In addition, compared to DA, the authors discovered a lower Hb response to CERA and inferior iron utilization, as estimated by hepcidin levels, using dosages recommended by the company. Unfortunately, health care resource utilization and cost data were not collected in this study, preventing a comparison of these variables between the preswitch and postswitch periods.
Of note, the current literature query found cost-effectiveness after switching from DA to CERA in the majority of the studies analyzed.Citation35,Citation38,Citation41,Citation43–Citation45 A total of six studies reported cost savings, another study by De CockCitation47 demonstrated substantial time savings, whereas only two authorsCitation32,Citation34 reported slightly higher costs for CERA compared to DA (median costs/patient/month of €147.5 and €105±€26 versus €134.4 and €115±€17, respectively).Citation32,Citation34,Citation35,Citation38,Citation41,Citation43–Citation45,Citation47 However, it remains unclear whether general conclusions can be drawn from these observations, as many patients may also be successfully managed with a once-monthly dose of DA.Citation55,Citation56
Recent randomized trials comparing target Hb levels >13 g/dL with target levels of 10–12 g/dL resulted in serious concerns about the safety of ESA therapy in CKD patients not on dialysis.Citation10–Citation13 Consequently, the US FDA now recommends using the lowest possible ESA doses with gradual increases in order to avoid the need for transfusions, but without exceeding Hb concentrations of 12 g/dL.Citation57 Although Hb targets were predominantly not defined,Citation31,Citation32,Citation36,Citation37–Citation40 or given that they showed a distinct variation in the analyzed studies (see ),Citation33–Citation35,Citation41 the average baseline and endpoint Hb values reached above the proposed levels of 10–12 g/dL. A detailed declaration regarding the number of RBC transfusions administered in the preswitch and postswitch periods, as well as specifications regarding vitamin B12 or folate supplementation, are missing.
Functional iron deficiency with low circulating iron and normal or increased storage iron translated into low transferrin saturation; moreover, normal or high serum ferritin is commonly seen in CKD patients.Citation58,Citation59 Inadequate iron availability limits the response to ESA.Citation6 Unfortunately, all studies summarized in this review do not include a detailed description of iron administration. This lack of standardization poses a challenge and can lead to confusion when comparing these data. Although there are some promising data for CERA regarding its improvement of iron utilization, further studies have to prove if maximal cost-effectiveness after CERA switch can only be reached with optimal iron substitution.Citation32,Citation60,Citation61
In a health economic evaluation analysis of different health care interventions, a variety of methods can be applied by the investigators.Citation62 These methods to assess costs and effects between (for example) two or more ESA comparators should include a cost–benefit analysis, a cost–utility analysis, CEA, or CMA.Citation63–Citation66 Only six of the 18 selected studies in this review established a CEA or CMA to evaluate the cost-effectiveness of CERA.Citation31,Citation32,Citation41–Citation44 Moreover, for all of the studies that applied CEA or CMA, access was limited to abstracts and not to full-text articles. Therefore, it is currently not possible to estimate if all obligatory requirements for CEAs concordant with published consensus guidelines were fulfilled in these studies. For example, it has been recommended that CEA be conducted from a societal perspective, and that a lifetime horizon be employed, since only these approaches avoid allocation biases that may be introduced by a narrower approach.Citation67–Citation69 In addition, it is not possible to compare the different CEAs, as no universal outcomes were indicated. Finally, some experts in this field believe that CMA is an appropriate method of analysis, but only under rare circumstances.Citation70,Citation71 Taken together, due to methodological ambiguity, conclusions from the reported CEAs and CMAs cannot be easily derived.
TAM studies are defined in the National Library of Medicine’s controlled vocabulary thesaurus as “the observation and analysis of movements in a task with emphasis on the amount of time required to perform the task”.Citation72 TAM studies have proven to be the gold standard method to measure and quantify clinical workflow.Citation73
The collection of observational data for ESA treatment-related activities should allow for a realistic estimation of the average times spent on each activity. Tasks suitable for the TAM studies were the activities related to the preparation, distribution, injection, recordkeeping, and ordering of ESAs.Citation46–Citation48 Of particular note, the respective portion of these activities shows a significant variation in different clinical settings. For example, in ambulatory dialysis units, frequent ESA dosing places a substantial burden on nursing time, whereas self-administration of long-acting ESAs at home is often routine for peritoneal dialysis patients.Citation30,Citation42
In parallel to the landmark study conducted by Schiller et al,Citation30 all three TAM studies included in this review estimated that 100% conversion to once-monthly CERA would reduce nursing ESA administration time by approximately 80% in dialysis units. While costs were not investigated in the reports by De Cock et alCitation47 and Klatko and Felisiak,Citation48 respectively, Saueressig et alCitation46 estimated a resultant cost savings of between 35%–58%.
Here, an important question arises: can time be easily translated into monetary units? All three TAM studies were multicenter-based, which can be a strength but also a weakness.Citation46–Citation48 From the current author’s point of view, these data should be interpreted with caution, as well known limitations of TAM studies are their extreme variability in time for prespecified tasks, and in the results observed between different centers and treatment settings (for example, public hospital versus private practice settings), making the pooling of data with the equal weighting of each center difficult. The inclusion of different regional locations, and even different EU countries with entirely different health care systems, further complicates generalization of these results.
Financial reasons were the driving force behind the development of biosimilar erythropoietins that were introduced after patents of short-acting ESAs had expired.Citation74 EPO biosimilars approved by the European Medicines Agency or the US FDA have been shown to have a comparable efficacy and safety profile to their originators.Citation75 Unfortunately, studies comparing the costs between CERA and biosimilar erythropoietins are still absent in the literature.
Effective long-acting competitors on the ESA market are DA and CERA, with Amgens’ DA acting as a “monopolist” on the US market.Citation21 In this context, a marketing survey assessed nephrologists’ interest in and anticipation of the expected 2014 arrival of CERA on the US market.Citation76 Half of the surveyed nephrologists believed that approximately 40% of their CKD patients are potential CERA candidates, suggesting that CERA could have a significant impact on the US renal anemia market in the coming years.Citation76 Despite these survey results, the fate of CERA on the highly competitive US market is currently unpredictable.
Conclusion
As safety concerns for CERA are still limited, and recent studies have demonstrated similar efficiency compared to DA and conventional short-acting ESAs, the cost-effectiveness of CERA could become the pivotal reason for clinicians to prescribe this remedy. Unfortunately, the current literature provides only little evidence to support such a decision. Therefore, well-designed, head-to-head studies with defined endpoints directly comparing costs in similar patient populations treated with equipotent CERA and comparator doses are now urgently needed.
Acknowledgments
The author thanks Dr Nicole Bick from Roche Pharma AG Grenzach-Wyhlen for helpful assistance in the literature search.
Disclosure
The author reports no conflicts of interest in this work.
References
- CulletonBFMannsBJZhangJTonelliMKlarenbachSHemmelgarnBRImpact of anemia on hospitalization and mortality in older adultsBlood2006107103841384616403909
- WeissGGoodnoughLTAnemia of chronic diseaseN Engl J Med2005352101011102315758012
- GoodnoughLTSchrierSLEvaluation and management of anemia in the elderlyAm J Hematol2014891889624122955
- MurphyMFWallingtonTBKelseyPBritish Committee for Standards in Haematology, Blood Transfusion Task ForceGuidelines for the clinical use of red cell transfusionsBr J Haematol20011131243111328275
- EschbachJWAbdulhadiMHBrowneJKRecombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trialAnn Intern Med19891111299210002688507
- KDIGO clinical practice guidelines for anemia in chronic kidney diseaseKidney Int Suppl201224288291
- RizzoJDBrouwersMHurleyPAmerican Society of Hematology and the American Society of Clinical Oncology Practice Guideline Update CommitteeAmerican Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancerBlood2010116204045405920974674
- rote-liste.de [homepage on the Internet]Frankfurt, GermanyRote Liste® Service GmbH [updated 2014; cited January 23, 2014]. Available from: http://rote-liste.de/Accessed June 13, 2014
- CollinsAJFoleyRNHerzogCExcerpts from the US Renal Data System 2009 Annual Data ReportAm J Kidney Dis2010551 Suppl 1S1S420A6
- BesarabABoltonWKBrowneJKThe effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetinN Engl J Med199833995845909718377
- SinghAKSzczechLTangKLCHOIR InvestigatorsCorrection of anemia with epoetin alfa in chronic kidney diseaseN Engl J Med2006355202085209817108343
- DrüekeTBLocatelliFClyneNCREATE InvestigatorsNormalization of hemoglobin level in patients with chronic kidney disease and anemiaN Engl J Med2006355202071208417108342
- PfefferMABurdmannEAChenCYTREAT InvestigatorsA trial of darbepoetin alfa in type 2 diabetes and chronic kidney diseaseN Engl J Med2009361212019203219880844
- CurranMPMcCormackPLMethoxy polyethylene glycol-epoetin beta: a review of its use in the management of anaemia associated with chronic kidney diseaseDrugs20086881139115618484803
- PanchapakesanUSumualSPollockCNanomedicines in the treatment of anemia in renal disease: focus on CERA (Continuous Erythropoietin Receptor Activator)Int J Nanomedicine200721333817722510
- LevinNWFishbaneSCañedoFVMAXIMA study investigatorsIntravenous methoxy polyethylene glycol-epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non-inferiority trial (MAXIMA)Lancet200737095961415142117950856
- MacdougallICWalkerRProvenzanoRARCTOS Study InvestigatorsCERA corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trialClin J Am Soc Nephrol20083233734718287255
- LocatelliFMannJFAldigierJCCERA safety profile: a pooled analysis in patients with chronic kidney diseaseClin Nephrol20107329410320129016
- DellannaFWinklerREBozkurtFMIRACEL Study GroupDosing strategies for conversion of haemodialysis patients from short-acting erythropoiesis stimulating agents to once-monthly CERA: experience from the MIRACEL studyInt J Clin Pract2011651647221091595
- GasconPPirkerRDel MastroLDurrwellLEffects of CERA (continuous erythropoietin receptor activator) in patients with advanced non-small-cell lung cancer (NSCLC) receiving chemotherapy: results of a phase II studyAnn Oncol201021102029203920335369
- SchmidtRJMethoxy polyethylene glycol-epoetin beta: worth waiting for or a novelty worn off?Expert Opin Pharmacother20091091509151419505218
- Patent docs Biotech & Pharma Patent Law & News Blog [webpage on the Internet] Available from: http://www.patentdocs.org/2009/12/amgen-and-hoffmanlaroche-settle-mircera-litigation.htmlAccessed June 8, 2014
- Meguid El NahasABelloAKChronic kidney disease: the global challengeLancet2005365945633134015664230
- BrayFJemalAGreyNFerlayJFormanDGlobal cancer transitions according to the Human Development Index (2008–2030): a population-based studyLancet Oncol201213879080122658655
- DuhMSWeinerJRWhiteLALefebvrePGreenbergPEManagement of anaemia: a critical and systematic review of the cost effectiveness of erythropoiesis-stimulating agentsPharmacoeconomics20082629912018198931
- BeckerRDembekCWhiteLAGarrisonLPThe cost offsets and cost-effectiveness associated with pegylated drugs: a review of the literatureExpert Rev Pharmacoecon Outcomes Res201212677579323252359
- BurnierMDouchampsJATangheALess frequent dosing of erythropoiesis stimulating agents in patients undergoing dialysis: a European multicentre cost studyJ Med Econ2009122778619450138
- ChurchillDNMacariosDAttardCKallichJGoereeRCosts associated with erythropoiesis-stimulating agent administration to hemodialysis patientsNephron Clin Pract20071064c193c19817596729
- BernardoMCrawfordPHertelJAssessment of time and practice resources required to provide weekly or monthly erythropoiesis-stimulating protein therapy to chronic kidney disease patients in the physician office settingJ Manag Care Pharm200612971472517249904
- SchillerBDossSDE CockEDel AguilaMANissensonARCosts of managing anemia with erythropoiesis-stimulating agents during hemo-dialysis: a time and motion studyHemodial Int200812444144919090867
- SilvaFHCVViannaCMDMSilvaFVCPUK3 Cost-effectiveness of Anemia Treatment in Dialysis Patients in Brazil: ISPOR 3rd Latin America Conference, Mexico City, Mexico, 8–10 September 2011Value in Health2011147A570
- Escudero-VilaplanaVMartínez-NietoCLópez-GómezJMVega-MartínezABellón-CanoJMSanjurjo-SáezMErythropoiesis-stimulating agents in anaemia due to chronic kidney disease: a cost-minimization analysisInt J Clin Pharm201335346346823595914
- Albero MolinaMDLópez-Menchero MartínezRdel Pozo FernándezCÁlvarez FernándezLSánchez RodríguezLEfficiency of monthly subcutaneous administration of methoxy-polyethylene glycol-epoetin beta (Mircera) in stable patients under hemodialysis previously treated with erythropoietinDialisis y Trasplante201334393100 Spanish
- TsaiMHFangYWLeuJGShifting darbepoetin alpha to subcutaneous methoxy polyethylene glycol-epopoetin beta of similar doses and extended dose intervals effectively maintains hemoglobin concentrations in peritoneal dialysis patientsActa Nephrologica201327299103
- Padullés-ZamoraNComas-SugrañesDPineda-Yuste MdelMJódar-MasanésRMartínez-CastelaoAUse of methoxy polyethylene glycol-epoetin beta in stage 3, 4 or 5 non-dialysis chronic kidney diseaseNefrologia201232222122722421952
- OlmosVPignataroJOlmosIDanersMAssessment of Compliance to the Treatment of Anaemia in Patients Receiving a Novel ESA – Methoxy Polyethylene Glycol-Epoetin Beta (MIRCERA®) – Versus Conventional Erythropoietin Available from: http://www.tshp.twmail.net/attachments/106_V17N4-%E5%B0%88%E9%A1%8C.pdfAccessed January 12, 2014
- MüllerAMollMSwitching hemodialysis patients from short-acting ESA to once monthly CERA: a single center experienceWorld Congress of NephrologyApril 8–12, 2011Vancouver, British Columbia Abstract SU484
- FranzSJägerCGauthierTHemoglobin levels and development of ESA dose in hemodialysis patients after conversion to CERA A multi-center observational studySwiss Medical Weekly200913945/46 Suppl 178 Abstract 8S 4.4. Annual Meeting of the Swiss Society of Nephrology; December 2–4; 2009; Interlaken, Switzerland
- CynkeEBenzBFranzSStable hemoglobin values and lower dose requirements after complete conversion from epoetin beta to CERA A single center experienceSwiss Medical Weekly200913945/46 Suppl 178 Abstract 19S 53. Annual Meeting of the Swiss Society of Nephrology; December 2–4; 2009; Interlaken, Switzerland
- FranzSCynkeEDevelopment of dose and costs after conversion to CERA in hemodialysis patientsSwiss Medical Weekly200813847/48 Suppl 167 Abstract 23S 69. 40th Annual Meeting of the Swiss Society of Nephrology; December 3–5; 2008; St Gallen, Switzerland
- Echarri ArrietaEFernandez FerreiroFAlonso ValenteAMartinez GuitinMMartinez CalvoMCost-effectiveness analysis of patients in peritoneal dialysis with methoxy polyethylene glycol-epoetin beta versus darbepoetin alfa in Santiago de Compostela University hospital complex, SpainPoster presented at: OHP020, 17th Congress of the European Association of Hospital PharmacistsMarch 21–23, 2012Milan, Italy
- GonzalezPGomezEVargasJRenal anemia (RA) treatment in Mexican public health care institutions: an evaluation of the costs and consequencesValue in Health2009127A379A380
- BezditkoNIakovlievaLMischchenkoOGerasymovaOKyrychenkoOPUK24 pharmacoeconomic aspects of use of erythropoietin drugs in patients on hemodialysis in UkraineValue in Health2012157A459
- KawalecPKuzmaJSzkultecka-DebekMCost-minimization analysis: Mircera (methoxypolyethylene glycol-epoetin β) vs. Anranesp (darbepoetin α) in patients with chronic kidney disease (CKD) who are not receiving haemodialysis, in Polish settingValue in Health2009127 ISPOR 12th Annual European Congress; October 24–27; 2009; Paris, France
- WalshCMBiltonRRobinsonJEPUK9 real-life dosing of erythropoiesis-stimulating agents (ESAS) in chronic kidney disease (CKD)-associated anemia: budget impactValue in Health2010137A474
- SaueressigUKwanJTDe CockESapèdeCHealthcare resource utilization for anemia management: current practice with erythropoiesis-stimulating agents and the impact of converting to once-monthly CERABlood Purif200826653754618997465
- De CockEDellannaFKhellafKTime savings associated with CERA once monthly: a time-and-motion study in hemodialysis centers in five European countriesJ Med Econ201316564865623402559
- KlatkoWFelisiakJTime and motion study of anaemia management with erythropoiesis stimulating agents in haemodialysis units in PolandJournal of Health Policy and Outcomes Research2013218992
- SharmaAYeeJGandraSRKhanIPetersenJEstimate of maintenance EPO to darbepoetin alfa dose conversion ratio in a hospital-based dialysis patient populationCurr Med Res Opin201026112679268720942616
- RosbergJHBen-HamadiRCremieuxPYFastenauJMPiechCTDose conversion and cost effectiveness of erythropoietic therapies in chemotherapy-related anaemia : a meta-analysisClin Drug Investig20052513348
- ShimamatsuKInamasuHA safe and easy introduction of darbepoetin-alpha in patients receiving maintenance hemodialysis and epoetin monotherapy: a “half-and-half ” combination therapyCurr Ther Res Clin Exp2013745824384988
- HiraiTNishizawaYNakazonoHHemoglobin maintenance and dosing strategies using intravenous continuous erythropoietin receptor activator in Japanese hemodialysis patientsTher Apher Dial201317549850324107278
- CarreraFLokCEde FranciscoAPATRONUS InvestigatorsMaintenance treatment of renal anaemia in haemodialysis patients with methoxy polyethylene glycol-epoetin beta versus darbepoetin alfa administered monthly: a randomized comparative trialNephrol Dial Transplant201025124009401720522670
- ChoiPFaroukMManamleyNAddisonJDose conversion ratio in hemodialysis patients switched from darbepoetin alfa to PEG-epoetin beta: AFFIRM studyAdv Ther201330111007101724173670
- JadoulMVanrenterghemYForetMWalkerRGraySJDarbepoetin alfa administered once monthly maintains haemoglobin levels in stable dialysis patientsNephrol Dial Transplant200419489890315031347
- TrachslerJGlückZDickenmannMParameters for successful monthly extended dosing of darbepoetin-alpha in patients undergoing hemodialysisClin Nephrol200971669770219473639
- U.S. Food and Drug Administration [webpage on the Internet]Erythropoiesis-stimulating agents (ESAs) – full versionSilver Spring, MDU.S. Food and Drug Administration2013 Available from: http://www.fda.gov/Drugs/DrugSafety/DrugSafetyPodcasts/ucm077123.htmAccessed January 24, 2014
- EschbachJWCookJDScribnerBHFinchCAIron balance in hemodialysis patientsAnn Intern Med1977876710713931207
- RambodMKovesdyCPKalantar-ZadehKCombined high serum ferritin and low iron saturation in hemodialysis patients: the role of inflammationClin J Am Soc Nephrol2008361691170118922994
- Kakimoto-ShinoMToyaYKujiTFujikawaTUmemuraSChanges in hepcidin and reticulocyte hemoglobin equivalent levels in response to continuous erythropoietin receptor activator administration in hemodialysis patients: a randomized studyTher Apher Dial Epub1242014
- HashimotoTTsugawaYTsunodaMIkeeRSasakiNHashimotoNImportance of intensive iron supplementation for dialysis patients with continuous erythropoietin receptor activatorJ Am Soc Nephrol201223769A770A Abstract SA-PO570. ASN Kidney Week, October 30–November 4, 2012; San Diego, CA, USA22499589
- ChangWYHenryBMMethodologic principles of cost analyses in the nursing, medical, and health services literature, 1990–1996Nurs Res19994829410410190836
- RussellLBGoldMRSiegelJEDanielsNWeinsteinMCThe role of cost-effectiveness analysis in health and medicine. Panel on Cost- Effectiveness in Health and MedicineJAMA199627614117211778827972
- WeinsteinMCSiegelJEGoldMRKamletMSRussellLBRecommendations of the Panel on Cost-effectiveness in Health and MedicineJAMA199627615125312588849754
- SiegelJEWeinsteinMCRussellLBGoldMRRecommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and MedicineJAMA199627616133913418861994
- UdvarhelyiISColditzGARaiAEpsteinAMCost-effectiveness and cost-benefit analyses in the medical literature. Are the methods being used correctly?Ann Intern Med199211632382441530808
- DrummondMFSculpherMJTorranceGO’BrienBStoddartGMethods for the Economic Evaluation of Health Care Programmes2nd edOxford, UKOxford University Press1997
- RobinsonRCost-effectiveness analysisBMJ199330769077937958219957
- LefebvrePVekemanFCremieuxPYComment: the impact of methodological approach on cost findings in comparison of epoetin alfa with darbepoetin alfaAnn Pharmacother2010443595 author reply 595–59620179253
- BriggsAHO’BrienBJThe death of cost-minimization analysis?Health Econ200110217918411252048
- RobinsonRCosts and cost-minimisation analysisBMJ199330769067267288401098
- National Institutes of Health [webpage on the Internet]Fact sheet: Medical Subject Headings (MeSH®)Bethesda, MDU.S. National Library of Medicine2013 Available from: https://www.nlm.nih.gov/pubs/factsheets/mesh.htmlAccessed January 24, 2014
- LopeteguiMYenPYLaiAMEmbiPJPaynePRTime Capture Tool (TimeCaT): development of a comprehensive application to support data capture for Time Motion StudiesAMIA Annu Symp Proc2012201259660523304332
- JelkmannWBiosimilar recombinant human erythropoietins (“epoetins”) and future erythropoiesis-stimulating treatmentsExpert Opin Biol Ther201212558159222471247
- HörlWHDifferentiating factors between erythropoiesis-stimulating agents: an update to selection for anaemia of chronic kidney diseaseDrugs201373211713023338536
- BioTrends Research Group [webpage on the Internet]Should Mircera reach the U.S. market, surveyed nephorologists state that at least 40 percent of CKD-ND and dialysis patients are likely candidatesExton, PABioTrends Research Group2013 Available from: http://www.bio-trends.com/News-and-Events/Press-Releases/Mircera-Nephrolodists-State-Candidates-100113Accessed January 31, 2014