Abstract
Background
February 2013 US treatment guidelines recommend the once-daily tablet of efavirenz/emtricitabine/tenofovir (Atripla®) as a preferred regimen and the once-daily tablet of elvitegravir/cobicistat/emtricitabine/tenofovir (Stribild™) as an alternative regimen for first-line treatment of human immunodeficiency virus (HIV). This study assessed the clinical and economic trade-offs involved in using Atripla compared with Stribild as first-line antiretroviral therapy in HIV-infected US adults.
Methods
A Markov cohort model was developed to project lifetime health-related outcomes, costs, quality-adjusted life years (QALYs), and cost-effectiveness of Stribild compared with Atripla as first-line antiretroviral therapy in HIV-1-infected US patients. Patients progressed in 12-week cycles through second-line, third-line, and nonsuppressive therapies, acquired immune deficiency syndrome, and death. Baseline characteristics and first-line virologic suppression, change in CD4 count, and adverse effects (lipid, central nervous system, rash, renal) were based on 48-week clinical trial results. These results demonstrated equivalent virologic suppression between the two regimens. Point estimates for virologic suppression (favoring Stribild) were used in the base case, and equivalency was used in the scenario analysis. Published sources and expert opinion were used to estimate costs, utilities, risk of acquired immune deficiency syndrome, mortality, subsequent-line CD4 count, clinical efficacy, and adverse events. Costs were reported in 2012 US dollars. Sensitivity analyses were conducted to assess robustness of results.
Results
Compared with patients initiating Atripla, patients initiating Stribild were estimated to have higher lifetime costs. Stribild added 0.041 QALYs over a lifetime at an additional cost of $6,886, producing an incremental cost-effectiveness ratio of $166,287/QALY gained. Results were most sensitive to first-line response rates, product costs, and likelihood of renal adverse events. When equivalent efficacy was assumed, Atripla dominated Stribild with lower costs and greater QALYs.
Conclusion
At a societal willingness to pay of $100,000/QALY, Stribild was not cost-effective in the base case compared with Atripla for first-line HIV treatment.
Introduction
February 2013 guidelines from the US Department of Health and Human Services include four preferred initial combination antiretroviral treatment regimens for antiretroviral-naïve human immunodeficiency virus type 1 (HIV-1)-infected patients who are not pregnant. These regimens incorporate as their “backbone” the nucleoside reverse transcriptase inhibitors (NRTIs) tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC). The regimens include one that is nonnucleoside reverse transcriptase inhibitor (NNRTI)-based two that are protease inhibitor-based and one that is integrase strand transfer inhibitor-based.Citation1
One of these preferred regimens, Atripla® (efavirenz/emtricitabine/tenofovir; Bristol-Myers Squibb, Princeton, NJ, USA; Gilead Sciences Inc, Foster City, CA, USA) is a once-daily single tablet combining the NNRTI regimen efavirenz with the NRTI backbone (TDF/FTC). Among the list of alternative regimens is Stribild™ (elvitegravir/cobicistat/emtricitabine/tenofovir; Gilead Sciences Inc), a recently introduced once-daily single-tablet integrase inhibitor composed of elvitegravir and cobicistat with the NRTI backbone (TDF/FTC). In a 48-week Phase III clinical trial of adults aged 18+ years, Atripla and Stribild were found to have similar clinical efficacyCitation2 Adverse event rates for the two regimens were similar, except that patients receiving Atripla experienced more central nervous system reactions and rash, whereas those receiving Stribild experienced more renal events.
To better understand the potential trade-offs involved in choosing between these two agents, this analysis used decision-analytic modeling to evaluate the cost-effectiveness of Atripla compared with Stribild in first-line treatment for antiretroviral-naïve HIV-infected US adults.
Materials and methods
We developed a Markov cohort model that projected from the payer perspective, the cost-effectiveness of using Atripla versus Stribild as first-line treatment in an antiretroviral-naïve HIV-infected US adult population ( and ). A Markov model is constructed of mutually exclusive and exhaustive health states, and patients are simulated through the health states at fixed intervals known as model cycles. Once the model has simulated all patients until death, outcomes (ie, costs and survival) are summed for the entire cohort.
Figure 1 Model schematic for health states of an antiretroviral therapy-naïve HIV-1-infected adult US population.
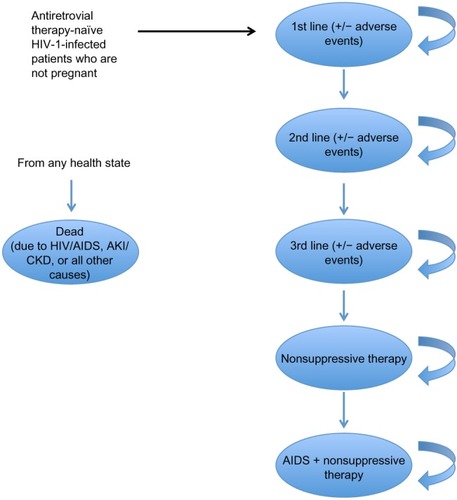
Figure 2 Model schematic for adverse eventsb in an antiretroviral therapy-naïve HIV-1-infected adult US population.
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; HIV, human immunodeficiency virus.
Patients entered the model with baseline age, gender, race, and CD4 count estimated from the Phase III clinical trial population.Citation2 Trial participants were adults aged at least 18 years with:
plasma HIV-1 RNA concentrations ≥5,000 copies per mL
estimated glomerular filtration rate of at least 70 mL per minute
aspartate and alanine aminotransferase concentrations of no more than five times the upper limit of normal
total bilirubin of no more than 25.65 μmol/L or a normal direct bilirubin, absolute neutrophil count of at least 1,000 cells per μL
at least 50,000 platelets per μL
hemoglobin concentration of at least 85 g/L
a negative serum pregnancy test.
Patients with new acquired immune deficiency syndrome (AIDS)-defining disorders or serious infections within 30 days of screening were excluded. In total, 348 patients were included in the safety population for the Stribild arm of the trial, and 352 patients were included in the Atripla arm.
The Markov model incorporated 12-week cycles to accommodate the timing of HIV treatment cycles and the shorter nature of treatments for adverse events. During each 12-week model cycle, patients could respond to and continue their current treatment with or without elevated lipids, they could discontinue treatment due to adverse events or virologic failure, or they could die due to HIV infection, AIDS, acute kidney injury, chronic kidney disease, or other causes. Patients who discontinued initial treatment progressed through second-line and third-line treatments and nonsuppressive therapy, whereas those who experienced elevated lipid levels continued to receive their current HIV treatment and received lipid-lowering therapy (40 mg of atorvastatin once daily) until 6 months after progression to the next line of HIV therapy. Second-line treatment was defined as a market basket of either atazanavir or darunavir, each with ritonavir and two NRTIs. Third-line treatment and nonsuppressive therapy were defined as: 1) darunavir with ritonavir, etravirine, and two NRTIs; or 2) maraviroc, raltegravir, and two NRTIs. For both lines, patients were equally divided between the treatment options.
For each model strategy (ie, Atripla or Stribild), we projected total costs, life years, quality-adjusted life years (QALYs), virologic response, AIDS events, and adverse events (rash, renal abnormalities, central nervous system symptoms, elevated lipids). QALYs allow for inclusion of the impact of treatments on health-related quality of life. Incremental costs, life years, and QALYs of Stribild compared with Atripla were calculated to estimate incremental cost-effectiveness ratios (ICERs), a measure of the value of an intervention. Costs were reported in 2012 US dollars, and both costs and clinical outcomes were estimated using a 3% annual discount rate to incorporate the diminishing value of cost and clinical outcomes in future years. The model was developed using Tree Age Pro 2012 (Tree Age Software Inc, Williamstown, MA, USA), a commonly used modeling software which allows for comparison across treatment strategies.
shows the characteristics of the model baseline patient population, which is based on the Phase III clinical trial population.Citation2 Age-specific and gender-specific all-cause mortality and HIV-specific and AIDS-specific mortality were based on national US data,Citation3,Citation4 and mortality associated with acute kidney injury and chronic kidney disease plus dialysis were based on estimates from the published literature.Citation5,Citation6 Virologic response and change in CD4 count were estimated from clinical trial data and published literature. CD4 counts were categorized and plateaued after 3 years.Citation7–Citation9 Although the clinical trialCitation2 showed equivalent virologic suppression between the two regimens, the more conservative point estimates (favoring Stribild) were used as model inputs. Clinical efficacy data are shown in .
Table 1 Model baseline population of antiretroviral therapy-naïve HIV-1-infected adult patients
Table 2 Model clinical efficacy estimates
Patients who progressed (due to virologic failure or a severe adverse event) through three lines of therapy and onto nonsuppressive regimens had a viral load of ≥50 copies/mL and CD4 counts that plateaued at their most recent prior value. Patients who received nonsuppressive therapy could progress to AIDS, at which point their CD4 counts were categorized from 0 to <50 cells/mm3 or 50 to ≤200 cells/mm3 ().Citation10,Citation11
The model included the following adverse events: rash, renal abnormalities, central nervous system symptoms, elevated lipids causing initiation of lipid-lowering therapy, and other adverse events causing treatment discontinuation. Adverse events were defined as those causing treatment discontinuation except for elevated lipids, for which treatment continued with additional costs. Rates of adverse events were based on published literature and expert opinion (), and were included in the model after being converted to 12-week probabilities. Rash, central nervous system symptoms, and other adverse events causing treatment discontinuation occurred only during the first cycle of treatment; renal abnormalities and elevated lipids causing initiation of lipid-lowering therapy occurred at any time. Adverse events except for elevated lipids occurred at the end of week 8 of the 12-week model cycle, at which time patients discontinued treatment and took a 4-week drug holiday before initiating their subsequent line of therapy in the next cycle. Patients who initiated lipid-lowering therapy continued for 24 weeks (two model cycles) after discontinuing the HIV treatment that caused initiation of such therapy.
Table 3 Model adverse eventTable Footnotea,Table Footnoteb rates by treatment
A proportion of patients with renal abnormalities experienced acute kidney injury and were at risk of developing chronic kidney disease and requiring hemodialysis (dialysis) in the cycle following the acute kidney injury event. The likelihood of acute kidney injury was based on patients experiencing renal failure in the clinical trial,Citation2 and the likelihood of chronic kidney disease plus dialysis was based on expert opinion. Patients with acute kidney injury who did not develop chronic kidney disease experienced added costs and decreased utility and proceeded to the next line of therapy. Those with chronic kidney disease also proceeded to the next line of therapy, and for all remaining model cycles remained on dialysis with utility decrements and added costs.
Costs per 12-week cycle are shown in and were inflated to 2012 dollars using the Consumer Price Index.Citation12 The analysis was conducted from the payer perspective, with included costs as follows: product acquisition; baseline monitoring; and treatment for adverse events. Costs of productivity losses were not included.
Table 4 Model costs by treatment, patient monitoring characteristic, and adverse event
Product acquisition costs were based on 30-day wholesale acquisition costs.Citation13 A market basket of commonly used second-line and third-line treatment regimens was selected based on expert opinion, and nonsuppressive and third-line therapy regimens were assumed to be equivalent. Regimen dosing schedules were based on published guidelines.Citation1 To account for adjusted dosing of abacavir/lamivudine (ABC/3TC) in patients with stage V chronic kidney disease, 1% of patients within the NRTI market basket received the reduced ABC/3TC dosage recommended in the February 2013 guidelines.
In each cycle, patients underwent baseline monitoring consisting of a physician visit and laboratory workup. Patients with virologic failure underwent more intensive monitoring, and those who started a new line of therapy had an additional office visit to ensure medication compliance.
For each instance of rash, central nervous system symptoms, or renal abnormalities without acute kidney injury, patients had two office visits, the first to diagnose the adverse event and the second to determine when the patient was ready to begin the next line of treatment. Costs were applied during the cycle in which events occurred. Patients who required lipid-lowering therapy received one 40 mg atorvastatin tablet per day and incurred additional patient monitoring costs. Patients with renal abnormalities with acute kidney injury incurred a one-time acute kidney injury treatment costCitation14 and the cost of two additional patient monitoring visits. Patients who progressed to chronic kidney disease with dialysis incurred costs in each remaining model cycle for inpatient and outpatient services, emergency department visits, and all other services.Citation15
Utility weights for patients with HIV and AIDS were estimated from the literatureCitation16 and varied by CD4 count (). The impact of adverse events was applied as a utility decrement to the baseline HIV or AIDS utility weight during the cycle in which the adverse event occurred (which, for chronic kidney disease with dialysis, includes every subsequent model cycle). Because the specific adverse events grouped as “others causing treatment discontinuation” varied between treatments, no disutility was applied for these events.
Table 5 Model utility estimates by CD4 count and adverse event
In the base case, we evaluated the cost-effectiveness of the preferred and alternative first-line strategies in antiretroviral-naïve HIV-infected US adults. ICERs were calculated as the ratio of incremental costs to incremental QALYs and reported over a lifetime period. One-way and probabilistic sensitivity analyses were conducted to evaluate the impact of parameter uncertainty on model outcomes. In one-way sensitivity analyses, each parameter was varied individually at ±10% of the base case value. In the probabilistic sensitivity analyses, all parameters were varied simultaneously for 5,000 model iterations.
Results
Base case
When Stribild was compared with Atripla in the base case, lifetime costs were higher by $6,886, life expectancy was higher by 0.0188 years, and quality-adjusted life expectancy was higher by 0.0414 QALY. This resulted in an ICER of $166,287/QALY (). Without quality adjustment, this ICER increased to $365,750/LY.
Table 6 Summary results for Atripla versus StribildTable Footnotea
At 3 years, quality-adjusted survival was greater among patients receiving Atripla than among those receiving Stribild. Over a lifetime, Atripla patients experienced a greater number of adverse events than did Stribild patients. However, patients receiving Stribild experienced acute kidney injury or chronic kidney disease with dialysis, whereas those receiving Atripla did not.
For both strategies, HIV medications accounted for a large proportion of the total costs, totaling $635,764 per patient for Atripla and $637,296 per patient for Stribild. The costs for patients who were receiving first-line medication were higher for patients receiving Stribild ($53,628) than for those receiving Atripla ($28,486), but for both strategies most costs were accrued among patients receiving nonsupportive therapy or those with AIDS.
Sensitivity analyses
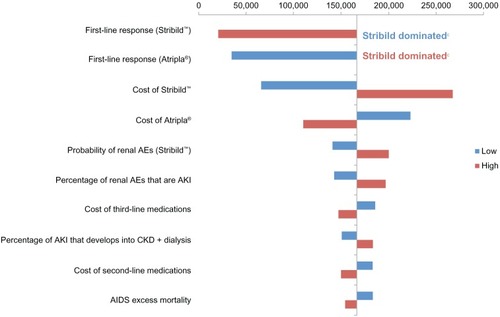
The tornado diagram () shows the impact of varying all parameters ±10% when comparing Stribild with Atripla. Results were most sensitive to first-line response rates of Stribild and Atripla, followed by the cost of Stribild. A 10% decrease in Stribild response or a 10% increase in Atripla response resulted in Atripla’s costing less and being more effective than Stribild. A 10% increase in Stribild response or a 10% decrease in Atripla response caused the ICER to drop to just over $20,000 and $34,000/QALY, respectively. Varying Stribild costs resulted in ICERs ranging from $65,487 to $267,088/QALY, and when Atripla costs were varied, this range was $109,898 to $222,676/QALY. Subsequent treatment (ie, second-line and third-line and nonsuppressive therapy) costs had less impact on ICERs than first-line costs, whereas the costs and rates of adverse events other than renal failure had the least impact on results.
Figure 3 One-way sensitivity analyses.a,b
Abbreviations: AE, adverse event; AIDS, acquired immune deficiency syndrome; AKI, acute kidney injury; CKD, chronic kidney disease; LY, life year; QALY, quality-adjusted life year.
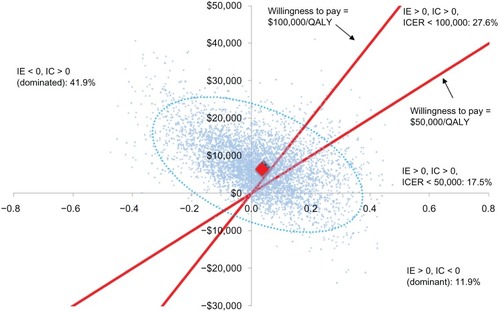
When all 49 parameters were varied simultaneously in probabilistic sensitivity analysis (), results suggested that at a societal willingness to pay of $50,000/QALY, Stribild would be considered cost-effective 17.5% of the time. At a societal willingness to pay of $100,000/QALY, Stribild would be cost-effective 27.6% of the time. Of the 5,000 model simulations, Stribild was dominated in 41.9%, whereas Atripla was dominated in 11.9%. Across all 5,000 iterations, the average incremental costs and QALY between the strategies was $7,897 and 0.0256. This resulted in an ICER of $308,296.
Figure 4 Probabilistic sensitivity analyses.a
Abbreviations: IC, incremental cost; ICER, incremental cost-effectiveness ratio; IE, incremental effectiveness; QALY, quality-adjusted life year.
Scenario analyses were conducted to examine the impact of discounting and assumptions regarding chronic kidney disease risk and treatment efficacy. Without discounting, the ICER increased to $207,273. When the risk of chronic kidney disease with dialysis among Stribild patients was assumed to be half that of base case, the ICER decreased to $96,557/QALY. When Stribild and Atripla were assumed to have equivalent efficacy to reflect the noninferiority clinical trial findings, Atripla dominated Stribild (cost less than Stribild and provided greater life expectancy and quality-adjusted life expectancy), regardless of chronic kidney disease risk or discounting assumptions.
Discussion
In antiretroviral-naïve HIV-1-infected adult patients who were not pregnant, Stribild was not cost-effective compared with Atripla given a commonly used societal willingness to pay threshold of $100,000.Citation17–Citation19 Although model results predicted that Stribild would provide a slight benefit in quality-adjusted life expectancy, the increased costs of Stribild led to an ICER of $166,287. This suggests that using Stribild in this patient population is not an efficient use of economic resources compared with using Atripla.
The results of this model must be considered in light of its limitations. Efficacy inputs from the pivotal Phase III clinical trialCitation2 were based on point estimates rather than the trial’s conclusion of Atripla’s noninferior efficacy. When assuming equivalent efficacy in the scenario analysis, Atripla dominated Stribild, suggesting that our base case results may have overestimated Stribild’s benefit. The risk of death due to chronic kidney disease with dialysis was based on a population of HIV-infected patients with chronic kidney disease, among whom only a portion had end-stage renal disease. Our results may thus overestimate the benefit (and underestimate the ICER) of Stribild compared with Atripla. In addition, although the model only considered severe adverse events causing treatment discontinuation, our utility estimates were derived from patients who also had less severe events. In this way, our model may have underestimated the quality of life decrement associated with adverse events; because the extent of bias could vary between events and therefore regimens, it is unclear how this may have impacted model ICERs.
Because costs and QALYs are likely to accumulate at different rates for each strategy, model-predicted ICERs could differ for time horizons of less than a lifetime. Although such outcomes were not evaluated in this analysis, the lifetime results presented here are based on following patients through all lines of therapy and provide a complete depiction of the clinical and economic trade-offs between strategies. Finally, second-line and third-line therapies were defined as a market basket of products independent of initial treatment, which may oversimplify actual treatment patterns.
By reducing viral load with less renal toxicity and having a lower unit cost, Atripla was predicted in this model to lower rates of acute kidney injury and chronic kidney disease events and decrease total spending compared with Stribild. Using a US cost-effectiveness threshold of $100,000/QALY, the cost of using Stribild is not an effective expenditure of resources compared with using Atripla in antiretroviral-naïve HIV-infected patients. As health care costs increase and more effort is put into controlling these costs, this knowledge may be useful to physicians, policymakers, and payers alike in their efforts at making clinically appropriate yet cost-conscious decisions.
Disclosure
Drs Correll and Juday are employees of Bristol-Myers Squibb. Ms Anene, Drs Bentley and Broder, and Mr Ortendahl are employees of Partnership for Health Analytic Research, LLC, which was paid by Bristol-Myers Squibb to conduct the research described in this manuscript.
References
- Panel on Antiretroviral Guidelines for Adults and AdolescentsGuidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescentsDepartment of Health and Human Services Updated February 12, 2013. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Appendix B, Table 7, and Table 8Accessed March 29, 2013
- SaxPEDeJesusEMillsAGS-US-236-0102 Study TeamCo-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeksLancet201237998352439244822748591
- National Center for Health StatisticsTable 2. Lifetable for males, 2007–2008Atlanta, GACenters for Disease Control and Prevention2012 Available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/NVSR/59_09/Table02.xlsAccessed March 29, 2013
- Centers for Disease Control and PreventionHIV Surveillance Report, volume 22, 2010 Available from: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/Accessed January 11, 2013
- WyattCMAronsRRKlotmanPEKlotmanMEAcute renal failure in hospitalized patients with HIV: risk factors and impact on in-hospital mortalityAIDS200620456156516470120
- ChoiAIRodriguezRABacchettiPBertenthalDVolberdingPAO’HareAMThe impact of HIV on chronic kidney disease outcomesKidney Int200772111380138717805235
- ByakwagaHMurrayJMPetoumenosKEvolution of CD4+ T cell count in HIV-1-infected adults receiving antiretroviral therapy with sustained long-term virological suppressionAIDS Res Hum Retroviruses200925675677619500017
- LeMoing VThiébautRChêneGANRS CO8 (APROCO/COPILOTE) Study GroupLong-term evolution of CD4 count in patients with a plasma HIV RNA persistently <500 copies/mL during treatment with antiretroviral drugsHIV Med20078315616317461859
- MooreRDKerulyJCCD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppressionClin Infect Dis200744344144617205456
- GulickRMLalezariJGoodrichJMOTIVATE Study TeamsMaraviroc for previously treated patients with R5 HIV-1 infectionN Engl J Med2008359141429124118832244
- BakerJVPengGRapkinJTerry Beirn Community Programs for Clinical Research on AIDS (CPCRA)CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infectionAIDS200822784184818427202
- Bureau of Labor StatisticsConsumer Price Index Inflation CalculatorUnited States Department of Labor Available from: http://www.bls.gov/data/inflation_calculator.htmAccessed March 29, 2013
- PriceRxWolters Kluwer Health 2012 Available from: https://pricerx.medispan.com/NDCNumber.aspxAccessed September 7, 2012
- CandrilliSBellTIrishWMorrisEGoldmanSCairoMSA comparison of inpatient length of stay and costs among patients with hematologic malignancies (excluding Hodgkin disease) associated with and without acute renal failureClin Lymphoma Myeloma200881445118501087
- BergerAEdelsbergJIngleseGWBhattacharyyaSKOsterGCost comparison of peritoneal dialysis versus hemodialysis in end-stage renal diseaseAm J Manag Care200915850951819670954
- SimpsonKNLuoMPChumneyESunEBrunSAshrafTCost-effectiveness of lopinavir/ritonavir versus nelfinavir as the first-line highly active antiretroviral therapy regimen for HIV infectionHIV Clin Trials20045529430415562370
- GrosseSDTeutschSMHaddixACLessons from cost-effectiveness research for United States public health policyAnnu Rev Public Health20072836539117222080
- KingJTJrTsevatJLaveJRRobertsMSWillingness to pay for a quality-adjusted life year: implications for societal health care resource allocationMed Decis Making200525666767716282217
- EichlerHGKongSXGerthWCMavrosPJönssonBUse of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge?Value Health20047551852815367247
- Gilead Sciences IncElvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate single-tablet regimen (EVG/COBI/FTC/TDF, QUAD STR) for treatment of HIV-1 infection in adultsAntiviral Drugs Advisory Committee Meeting Briefing DocumentMay 11, 2012 Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM303397.pdfAccessed June 27, 2013
- RockstrohJKLennoxJLDejesusESTARTMRK InvestigatorsLong-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRKClin Infect Dis201153880781621921224
- BánhegyiDKatlamaCda CunhaCAWeek 96 efficacy, virology and safety of darunavir/r versus lopinavir/r in treatment-experienced patients in TITANCurr HIV Res201210217118122339125
- JohnsonMGrinsztejnBRodriguezC96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failuresAIDS200620571171816514301
- AndersonDDeMasiRDeLaitschLSurlesTCoateBWeek 96 outcomes of patients with less treatment experience versus more treatment experience receiving etravirine in the DUET trialsCurr HIV Res201210325626122497697
- HodderSJayaweeraDMrusJRyanRWitekJGrace Study GroupEfficacy and safety outcomes among treatment-experienced women and men treated with etravirine in gender, race and clinical experienceAIDS Res Hum Retroviruses201228654455122206504
- ClotetBBellosNMolinaJMPOWER 1 and 2 study groupsEfficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trialsLancet200736995681169117817416261
- JohnsonMGrinsztejnBRodriguezCAtazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failuresAIDS200519768569415821394
- GirardPMCampbellTGrinsztejnBPooled week 96 results of the phase III DUET-1 and DUET-2 trials of etravirine: further analysis of adverse events and laboratory abnormalities of special interestHIV Med201213742743522413938
- Stribild [Prescribing information]Foster City, CAGilead Sciences Inc2012
- AntoniouTSmithGSuDImmunologic effectiveness of maraviroc- and raltegravir-containing regimens (R+M+) versus raltegravir-based regimens that do not include maraviroc (R+M−)J Int Assoc Physicians AIDS Care (Chic)201211319219722247337
- SteigbigelRTCooperDAKumarPNBENCHMRK Study TeamsRaltegravir with optimized background therapy for resistant HIV-1 infectionN Engl J Med2008359433935418650512
- MAG Mutual Healthcare SolutionsPhysicians’ Fee and Coding Guide 2012Atlanta, GAMAG Mutual Healthcare Solutions2011
- RoskellNSShearerAGazzardBThe impact of patient, disease, and treatment-related factors on quality of life for HIV patients on HAARTAbstract MOPE0656 presented at the XVI International AIDS ConferenceAugust 13–18, 2006Toronto, Ontario, Canada
- SullivanPWGhushchyanVPreference-based EQ-5D index scores for chronic conditions in the United StatesMed Decis Making200626441042016855129