?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Assessing the cost-effectiveness of treatments in rheumatoid arthritis (RA) is of growing importance due to the chronic nature of the disease, rising treatment costs, and budget-constrained health care systems. This analysis assesses the cost-effectiveness of modified-release (MR) prednisone compared with immediate-release (IR) prednisone for the treatment of morning stiffness due to RA.
Methods
A health state transition model was used to categorize RA patients into four health states, defined by duration of morning stiffness. The model applied a 1-year time horizon and adopted a UK National Health Service (NHS) perspective. Health benefits were measured in quality-adjusted life years (QALYs) and the final output was the incremental cost-effectiveness ratio (ICER). Efficacy data were derived from the CAPRA-1 (Circadian Administration of Prednisone in Rheumatoid Arthritis) study, drug costs from the British National Formulary (BNF), and utility data from a direct elicitation time-trade-off (TTO) study in the general population. Sensitivity analyses were conducted.
Results
Mean treatment costs per patient were higher for MR-prednisone (£649.70) than for IR-prednisone (£46.54) for the duration of the model. However, the model generated an incremental QALY of 0.044 in favor of MR-prednisone which resulted in an ICER of £13,577. Deterministic sensitivity analyses did not lead to significant changes in the ICER. Probabilistic sensitivity analysis reported that MR-prednisone had an 84% probability of being cost-effective at a willingness-to-pay threshold of £30,000 per QALY. The model only considers drug costs and there was a lack of comparative long-term data for IR-prednisone. Furthermore, utility benefits were not captured in the clinical setting.
Conclusion
This analysis demonstrates that, based on the CAPRA-1 trial and directly elicited public preference values, MR-prednisone is a cost-effective treatment option when compared with IR-prednisone for RA patients with morning stiffness over one year, according to commonly applied UK thresholds (£20,000–£30,000 per QALY). Further research into the costs of morning stiffness in RA is required.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease with prevalence rates varying from 0.45% in southern Europe up to 0.66% in northern/middle Europe and the US, which increase to approximately 2% in those aged $60 years.Citation1 RA patients present with progressive damage to the synovial-lined joints, leading to symptoms such as joint swelling, tenderness, stiffness, and severe impairment of movement.Citation2
RA patients have reduced health-related quality of life (HRQoL), including impaired physical, psychologic, and social functioning.Citation3 Synovial symptoms of RA, such as joint stiffness and functional disability, are particularly severe in the morning.Citation2,Citation4,Citation5 This morning stiffness has been shown to contribute to the worsening of HRQoL. A survey in 11 European countries found that 82% of RA patients stated that their morning symptoms had a significant impact on their HRQoL.Citation6 This is noteworthy, considering that morning stiffness is prevalent in 41% and 79% of patients with controlled and uncontrolled RA, respectively.Citation7 Further, 73% of patients in paid employment reported that impairment in morning function affected their working life, including time off work and reduced career progression.Citation6
The economic consequences of RA and morning stiffness, including loss of earnings and out-of-pocket costs, are substantial for the individual.Citation8 RA is also a considerable burden to the state in terms of health care spending, welfare payments, and decreased productivity.Citation8 RA is treated chronically by a number of therapies, including disease-modifying antirheumatic drugs, glucocorticoids, nonsteroidal anti-inflammatory drugs (NSAIDS), and biologics,Citation3 which relieve symptoms and modify the disease process.Citation9 Whilst the introduction of biological therapies has provided a notable advance in the treatment of RA, it has also significantly increased the direct costs.Citation9 Given this and the prevalence of RA, the treatment and management of RA represents a significant economic burden to health care systems. In the UK in 2009, estimates suggest that National Health Service (NHS) health care costs attributable to RA amounted to approximately £560 million.Citation10
Glucocorticoids such as prednisone inhibit the circadian release of proinflammatory cytokines and hence reduce the duration of morning stiffness symptoms.Citation11 Immediate-release (IR) prednisone is taken upon waking,Citation11 which is too late to impact upon morning symptoms. Preventing the timely rise of proinflammatory cytokines through appropriate timing of drug administration or use of modified-release (MR) preparations may result in an improvement in morning stiffness duration.Citation12 MR-prednisone (Lodotra®; Horizon Pharma AG, Reinach, Switzerland) is indicated for the “treatment of moderate to severe, active rheumatoid arthritis in adults, particularly when accompanied by morning stiffness”. Lodotra tablets have an MR formulation which is designed to deliver prednisone at the most physiologically efficient time in order to relieve morning symptoms in RA.Citation13 No adverse impact of MR-prednisone on the hypothalamic-pituitary-adrenal axis was detected.Citation14
The Circadian Administration of Prednisone in Rheumatoid Arthritis Study (CAPRA-1) was a Phase III trial involving 288 patients with $45 minutes of morning stiffness due to active RA previously treated with disease-modifying antirheumatic drugs (DMARDS), nonsteroidal anti-inflammatory drugs, and glucocorticoids.Citation13 Patients were randomly assigned to either MR-prednisone (n = 144) or to IR-prednisone (n = 144). The primary endpoint was the relative change in duration of morning stiffness from baseline to the end of the 12-week double-blind phase (calculated from daily patient diaries).Citation13 At the end of the study period, patients in the MR group achieved a mean reduction of 43.9 minutes compared with 22.7 minutes in the IR group.Citation13 In addition to this, a 9-month, open-label extension of the CAPRA-1 trial investigated the long-term efficacy of MR-prednisone.Citation11 The reduction in morning stiffness duration established in the double-blind phase was sustained during follow-up. Further, recently published research from the CAPRA-2 trial reported a significantly greater median relative reduction in duration of morning stiffness at 12 weeks for MR-prednisone compared with placebo (P < 0.004). The response was also achieved rapidly, with a significant difference in response rates for MR-prednisone in comparison with placebo reported as early as week 2.Citation15
The CAPRA-1 trial also collected HRQoL information using the Health Assessment Questionnaire (HAQ) and Short-Form 36 questionnaire (SF-36). The SF-36 data were converted into utility values using the Brazier equation,Citation16 which demonstrated that there was a 0.0132 utility improvement for patients treated with MR-prednisone compared with IR-prednisone;Citation17 this numeric improvement was not statistically significant. This may be due to several reasons. For example, it has been acknowledged that generic instruments can lack sensitivity in chronic diseases,Citation18–Citation21 and in addition, these instruments were not used in the most effective manner in the CAPRA-1 study (HRQoL measures were only captured at week 0 and week 12, and instruments were administered as part of a general visit and not specifically in the morning). It is also important to note that morning stiffness in RA does not necessarily correlate with generic HRQoL scores; Khan et al found that morning stiffness showed only a moderate correlation with Health Assessment Questionnaire (HAQ) scores.Citation7 Due to these limitations, a separate study directly eliciting health state utilities associated with differing durations of morning stiffness in RA has been conducted.Citation22 This UK population-based direct elicitation study demonstrated that a reduction in morning stiffness duration in RA is associated with improved HRQoL.
Economic evidence regarding the use of MR and IR-prednisone in RA patients with morning stiffness is limited. Given the chronic nature and increasing prevalence of RA, the rising costs of treatment, and health care budget constraints, assessing the cost-effectiveness of RA treatment is of growing importance.Citation23 This study aimed to build on the findings of the CAPRA-1 trial and the direct elicitation time-trade-off (TTO) study for health state utilities. We assessed the cost-effectiveness of MR-prednisone compared with IR-prednisone in the treatment of morning stiffness due to RA from a UK health care system perspective.
Materials and methods
Methods
A cost utility model was developed in which health was measured in quality-adjusted life years (QALYs) and costs in British Pounds Sterling (£). The final output of the model was the incremental cost-effectiveness ratio (ICER), which measured the incremental cost and health gain of MR-prednisone compared with IR-prednisone. The model categorized RA patients into a series of four discrete health states defined by duration of morning stiffness symptoms. The distributions of patients were elicited from the pivotal Phase III CAPRA-1 trial comparing MR-prednisone and IR-prednisone.Citation13
Model structure
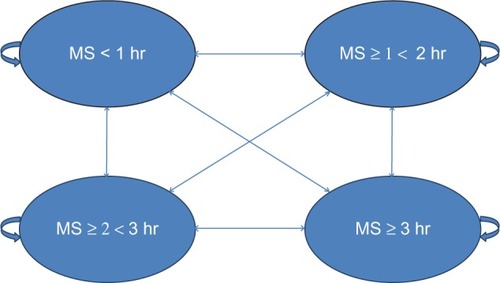
The model applied a 1-year time horizon, meaning that discounting was not necessary. The 1-year time horizon for the model was justified given the 3-month duration of the double-blind CAPRA-1 trial and the 9-month single-arm, open-label extension.Citation13,Citation15 The evaluation adopted the perspective of the UK health care payer, the National Health Service (NHS). No attempt was made to capture costs or benefits which fall outside of the health service. The health state transition model was developed in Microsoft Excel 2007, and an overview of the model structure is provided in .
The health states were developed with input from three key opinion leaders in the RA field, three RA patients with morning stiffness, and clinical trial data from CAPRA-1. Four health states were identified, each with a 1-hour difference in duration of morning stiffness.Citation22 The four health states applied in the model were based on a morning stiffness duration of less than 1 hour, 1–2 hours, 2–3 hours, and $3 hours (). The health states were deemed to be a clinically meaningful change for patients and key opinion leaders (KOLs), and the duration was also expected to lead to a tangible change in health status.Citation22
The distributions of patients in such health states were determined from the pivotal Phase III CAPRA-1 trial for MR-pred-nisone, the primary objective of which was to assess whether MR-prednisone is superior to IR-prednisone in reducing the duration of morning stiffness following 3 months of treatment.Citation11 This randomized, multicenter, double-blind trial included 288 subjects with a documented history of RA, who met the American College of Rheumatology criteria for RA, including symptoms of morning stiffness (average daily duration of 45 minutes during the last 7 days of the screening process), joint pain, tender and swollen joints, and an inflammatory state with elevated erythrocyte sedimentation rate or C-reactive protein. provides a full breakdown of patient demographics. The development of these health states is described further in a direct preference elicitation study designed to identify utility values attributable to each health state.Citation22
Table 1 Patient demographics for CAPRA-1
Costs
For costs, j represents treatment, where j = 1 refers to MR-prednisone and j = 2 refers to IR-prednisone; Cj is the total cost of drug for treatment j; Y is the expected duration for each treatment (months); and Dj is the monthly average cost per treatment arm (see ). Hence,
Table 4 Drug cost and quantities per day/month (UK 2012)Citation29
Therefore, the incremental cost between the treatment arms is:
Quality-adjusted life years
Qj is the total QALY for treatment; K represents health states 1–4; t is time; Pjtk is the distribution for treatment j across health state k, at time t (see ), and Uk is the health state utility values (see ). For a particular treatment, the total QALY is:
Table 2 Patient distribution across health states for baseline and months 1–3 (unadjusted baseline values and adjusted baseline to month 3 as used in analysis)
Table 3 Utility values for health states based on different durations of morning stiffness in RA
Therefore, the ICER is:
Efficacy inputs
Treatment efficacy data were derived from the CAPRA-1 trial. Analyses were conducted using the original trial data to obtain patient distributions for the different health states.Citation24 Monthly distributions of patients across the health states were obtained from this analysis at baseline, and at 4, 8, and 12 weeks (see ). These outcomes were used to populate the model at baseline, month 1, month 2, and month 3, respectively.
An average distribution of patients across both treatment arms was used at baseline to ensure that any differences in treatment efficacy were not attributable to the baseline patient distributions. For example, patient distribution for those with less than 1 hour morning stiffness (K = 1) at baseline for MR-prednisone was 0.104 compared with 0.085 in the IR-prednisone arm; this was adjusted to a mean distribution of 0.095 for both arms. This adjustment was made by calculating the difference in distribution at baseline and applying these differences to the distributions for each arm at each time point of the model. This analysis did not capture age-related mortality of patients; it was deemed reasonable to assume that mortality is equivalent between the treatment arms.
The model applied a 1-year time horizon; after month 3, patients were assumed to remain in the same health state for the remainder of the analysis to reflect the availability of double-blind clinical data. However, longitudinal open-label data suggest that the efficacy of MR-prednisone continues to increase over time.Citation11 Therefore, it may be reasonable to assume that the modeling method chosen in the base case is conservative with regard to the results for MR-prednisone.
Utility input data
Utility input data were derived from a previously published direct elicitation study.Citation22 Four health states were developed based on evidence derived from peer reviewed literature,Citation7,Citation25,Citation26 clinical trial data,Citation13 and patient diaries.Citation27 Each health state was developed with the Euro-QoL 5DCitation28 as the contextual framework. The health states were refined through input from two expert rheumatologists, a consultant rheumatology nurse, and three RA patients with morning stiffness. The four health states were then tested using a TTO approach which was conducted with 109 members of the general public sampled from seven regions in the UK in 2011.Citation22 These published utility scores, based on the four health states represented in this model, are presented in .
Cost inputs
The economic evaluation considered the drug costs of MR-prednisone and IR-prednisone. presents the estimates of the daily costs of treatment from the British National Formulary 63.Citation29 Prednisone does not appear in the British National Formulary (BNF) 63, so drug costs for prednisolone were used because prednisone is the prodrug of prednisolone. The costs of both interventions were based on a daily dose of 7 mg as per the mean dose in the trial (6.38 mg/day in CAPRA-1; 6.8 mg/day during the open-label extension). The average dose is therefore a conservative estimate and also accounts for wastage (because the smallest available tablet is 1 mg). MR-prednisone and IR-prednisone are self-administered, so there are no additional resource implications for the NHS that directly relate to drug administration.
Costs associated with adverse events were not included in the cost calculations. Data from the CAPRA-1 trial show that the most frequently reported drug-related adverse events were similar in both treatment groups.Citation30 No single drug-related treatment-emergent adverse event (TEAE) occurred in more than 6% of patients, and there was never more than a 3% difference in drug-related TEAE between treatment arms.Citation30 Therefore, it is reasonable to assume that patients treated with either drug will display similar treatment costs and resource use due to adverse events. Other health care resources incurred by treating RA patients, such as hospital outpatient visits, were not included in this analysis. It is reasonable to assume that RA patients with longer durations of morning stiffness may require additional health care professional attention. However, these potential health care resources are excluded due to an absence of robust evidence.
Sensitivity analysis
Deterministic sensitivity analyses
Deterministic one-way sensitivity analyses were carried out on the utility values. One-way sensitivity analyses for the utility data assessed the impact on the ICER by varying model inputs using the lower and upper bounds of each of the health states of the reported confidence intervals (). The impact of applying a longer time horizon (discount factor of 3.5% used, as per UK National Institute of Health and Clinical Excellence [NICE] guidance),Citation21 to reflect the chronic nature of RA, and a shorter time horizon to reflect the availability of clinical evidence, were also analyzed. After month 3, a conservative assumption of “no further improvement” is applied, as per the base case analysis.
Scenario analyses
Three alternative scenarios related to the efficacy input data were tested as part of the sensitivity analyses:
Scenario 1: extension of effect to 6 months. Distributions of patients from the CAPRA-1 trial were applied for the first 3 months of treatment; following this, the mean change from 0 to 3 months was applied between months 3 and 6. Patients then remained in the same health state for the remainder of the model (up to month 12).
Scenario 2: use of open-label study data. Distributions of patients from the CAPRA-1 trial were applied for the first 3 months for treatment with MR-prednisone. A 9-month, single-arm, open-label extension of the CAPRA-1 trial investigated the long-term efficacy of MR-prednisone (n = 249, all patients from CAPRA-1).Citation11 Data at 12 months from the open-label study (distributions of patients, adjusted for baseline differences: k = 1, 0.416; k = 2, 0.177; k = 3, 0.142; k = 4, 0.266) were applied for this scenario. Monthly distributions of patients across health states were not available between week 12 and week 52; therefore, the model assumed a constant linear monthly change in patient distributions. This distribution was calculated by examining the difference in distributions across health states at week 52 and week 12 and dividing this by 9 to obtain a monthly change in distribution, assuming a linear change. The proportion of patients treated with IR-prednisone was based on the baseline to 3 month CAPRA-1 trial data and no further response was assumed after 3 months.
Scenario 3: patient distributions using CAPRA-1 trial data. To test whether the adjustment for differences in patients’ baseline distribution had any impact on results, a scenario was undertaken where no adjustment was made. Actual monthly distributions of patients across the health states were applied from the CAPRA-1 trial (see baseline distribution in ) from baseline to month 3. After month 3, a conservative assumption of “no further improvement” is applied, as per the base case.
Probabilistic sensitivity analysis
The robustness of the ICER and uncertainty in the two key input parameters of patient distributions across health states and utility values was also investigated by probabilistic sensitivity analysis. The Dirichlet distribution was applied to patient distributions across the four different health states at baseline, month 1, month 2, month 3, and month 12 for both MR-prednisone and IR-prednisone. Probabilities for the Dirichlet distribution were determined by random draw in Excel (a random number from 0 to 1), and alpha distributions were determined by the number of patients in each health state in the base case.
For the utility data, standard errors and mean utility values were used to calculate the alpha and beta values for the utility scores associated with each health state. The formulas used for calculating alpha and beta distributions are presented below:
SE = standard error; μ = expected value.
Monte Carlo simulations employing 1,000 iterations were performed.
Results
Base case results
Over a 12-month period, the mean per patient treatment costs were higher in the MR-prednisone arm compared with the IR-prednisone arm (). However, MR-prednisone generated an increase in QALYs of 0.044 over 12 months, thereby resulting in an ICER of £13,577.
Table 5 Incremental cost-effectiveness results: baseline deterministic findings
Deterministic sensitivity analyses
shows the impact of changes made to the key parameters in the model. Across all of the sensitivity analyses performed, the ICER remained below a cost-effectiveness threshold of £20,000 per QALY.
Table 6 One-way sensitivity analysis results
Scenario analyses
shows the impact of different assumptions related to the efficacy inputs (distribution of patients across health states). The results of the scenario analyses demonstrate that the ICER is relatively insensitive to changes in these assumptions, because the ICER remained below a cost-effectiveness threshold of £20,000 per QALY.
Table 7 Scenario analysis results
Probabilistic sensitivity analysis
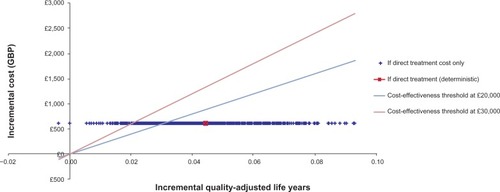
Compared with the base case results, the probabilistic sensitivity analysis resulted in an identical incremental cost of £603.16 (as drug costs were not included in the probabilistic sensitivity analysis), an incremental QALY of 0.044 (95% confidence interval 0.014–0.074) and a similar ICER of £13,617 (95% confidence interval £8,080–£41,326). The cost-effectiveness scatter plot demonstrates the high confi-dence in the input values of the model, which have resulted in a limited spread of results ().
Figure 2 Cost-effectiveness scatter plot for modified-release prednisone.
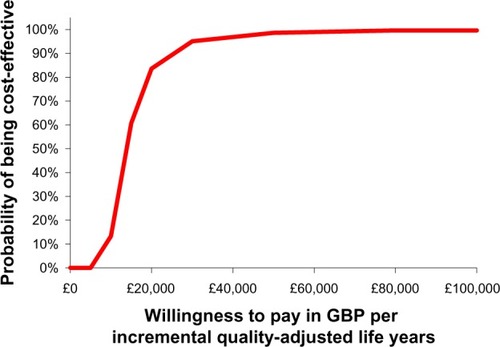
The cost-effectiveness acceptability curve based on the Monte Carlo simulations performed is presented in . The curve shows that, at a willingness-to-pay threshold of £20,000 per QALY gained, the estimated probability that MR-prednisone is cost-effective was 84% and approached 95% at a willingness-to-pay of £30,000 per QALY gained.
Discussion
To the author’s knowledge, this is the first study to investigate the cost-effectiveness of a treatment targeted to RA patients with morning stiffness. For a time horizon of 12 months, the mean treatment costs per patient were higher in the MR-prednisone arm compared with the IR-prednisone arm. However, the model generated an increase in QALYs of 0.044 in favor of MR-prednisone. The ICER was estimated to be £13,577. The cost-effectiveness threshold applied by NICE for decision-making in the UK is normally £20,000–£30,000 per QALY and technologies below this level are deemed cost-effective.Citation21 Our analysis therefore suggests that MR-predni-sone represents a cost-effective use of NHS resources.
Deterministic one-way sensitivity analyses demonstrated that the ICER was relatively insensitive across changes in all of the model parameters. The probabilistic sensitivity analysis also reported that MR-prednisone has an 84% probability of being cost-effective at a willingness-to-pay threshold of £20,000 per QALY, which approaches 95% at a willingness-to-pay of £30,000 per QALY gained. Each of the scenario analyses, including increasing duration of treatment, and changes to efficacy and utility values, results in an ICER which is below £20,000 per QALY. Given the increase in health-related costs and the progressively stretched health care budgets, demonstrating cost-effectiveness is highly important in chronic diseases such as RA.Citation23 The results of this economic model, which demonstrate that MR-prednisone is cost-effective against IR-prednisone, are therefore significant and relevant.
This research emphasizes the need for further research into the costs of morning stiffness because this model only considers drug costs and omits other potential costs to the payer and society. These costs have been omitted due to a lack of robust data relating specifically to the health states applied in this evaluation. However, a European study found that drug costs represent only 14% of the costs associated with RA, with other medical costs accounting for 21%, work productivity losses accounting for 32%, informal care for 19%, and nonmedical costs for 14%.Citation1 An observational study, which observed 1,185 RA patients treated with MR-prednisone over a 9-month period, found significant reductions in the number and proportion of patients receiving other therapies for RA, such as disease-modifying antirheumatic drugs. Similar reductions were seen with use of analgesics, nonsteroidal anti-inflammatory drugs, and gastroprotective treatments.Citation31 Additional data from Fautrel suggest that early treatment with glucocorticoids, including MR-prednisone, delays the need for costly biologics,Citation32 and in addition, a recent publication has highlighted the cost savings associated with delaying biologic treatment by use of MR-prednisone.Citation33 These data suggest that use of MR-prednisone may be associated with a reduction in health care costs, which would further reduce the ICER. This is particularly significant given the relatively low incremental drug costs of MR-prednisone. For example, a community nurse visit costs £768 over a 12-month period (assumes 1 hour per month),Citation34 which is more than the incremental cost of MR-prednisone (£603.16).
Another aspect to consider is that the model uses clinical effectiveness data drawn directly from the CAPRA-1 trial which was based in the German and Polish health care settings; however, the economic model is based on the UK NHS perspective. This assumes that the baseline distribution of patients and the treatment effect are similar between Germany, Poland, and the UK. This is a universal limitation of using multinational trial data in country-specific models, and hence this limitation is not exclusive to this model. There is also considerable uncertainty about the long-term efficacy of MR-prednisone due to the limitations of open-label studies, and there is a lack of comparative data for the IR formulation. Despite these limitations, the initial long-term data relating to the efficacy of MR-prednisone are positive, with more than 29% of patients reporting less than 1 hour of morning stiffness after 12 months of treatment with MR-prednisone in the single-arm, open-label study.
The CAPRA-1 trial incorporated health-related quality of life variables, including the SF-36 and HAQ. However, these instruments were not able to capture HRQoL benefits despite clear improvements in the primary endpoint of morning stiffness duration.Citation13 This may be due to several reasons, as noted in the introduction, HRQoL measures were only captured at week 0 and week 12 and instruments were administered during a general visit and not specifically in the morning (ie, outside of the “morning stiffness period”).Citation13 In addition, generic instruments may lack sensitivity in chronic diseases.Citation18–Citation20 This may be due to extended intervals between data collection. For example in CAPRA-1, SF-36 data were collated to cover a recall period of 4 weeks. However, instruments which quantify HRQoL over the previous 24-hour period are much more responsive and the respondent does not need to consider long recall periods.Citation35 Furthermore, the SF-6D, which provides a means of using the SF-36 in economic evaluation, is also known to have a high floor which may limit its use in patients with more severe manifestations of RA.Citation36 Uncertainty over the sensitivity of generic quality of life measures has also been raised in the oncology setting,Citation37 with research suggesting that, at times, the QALY construction methodology can be inadequate because it fails to capture important quality of life issues.
In instances where generic HRQoL instruments do not measure the desired aspects of a disease and there is uncertainty regarding their sensitivity, it is common to use utility values from published sources or to use direct elicitation measures.Citation38 Since there were no previously published utility values relating explicitly to duration of morning stiffness symptoms, a robust utility elicitation exercise was performed.Citation22 NICE recommends use of the TTO method in a representative sample of the UK population, as was the case in this model.Citation21 The published TTO study demonstrated that reduction in morning stiffness duration is associated with improvement in HRQoL for patients with RA.Citation22 Therefore, because MR-prednisone is associated with a reduction in morning stiffness duration, it is reasonable to assume that it improves HRQoL in comparison with IR-prednisone. The utility values cited in this model (from Iqbal et al)Citation22 are consistent with the findings of previous utility studies in RA. Recent research reported that the mean visual analog pain score (VAS) for late-stage RA was 33.52 ± 24.79,Citation39 which is comparable with the score obtained for the anchor state in the Iqbal et al study of 0.34 ± 0.16.Citation22 Further, a study conducted in 345 Irish patients with RA treated with biological therapy found that mean utility values were 0.54 ± 0.09 using the SF-6D and 0.43 ± 0.322 using the EQ-5D.Citation3 These scores are comparable with those elicited by Iqbal et al for the anchor health state (0.45) and health state 1 (0.50).Citation22
The model applied a 1-year time horizon because this reflects the length of the CAPRA-1 trial (3 months) plus the 9-month open-label extension.Citation13,Citation15 However, it is recognized that because RA is a life-long condition, examination of a lifetime time horizon may also be appropriate. Initial results from the long-term observational data show that the efficacy of MR-prednisone continues to increase over time.Citation11 Therefore, it may be reasonable to assume that the ICER presented in this study is a conservative short-term estimate. Furthermore, results from the 5-year sensitivity analysis suggest that MR-prednisone is deemed to be cost-effective over a longer time period.
The model currently only considers the “quality” aspect of the QALY outcome measure because survival is not captured within this economic evaluation. One other potential limitation is the exclusion of age-related mortality, that may overestimate the incremental QALY gain associated with MR-prednisone; however the impact of this is limited because it would affect both treatment groups.
Overall, this study suggests that, using a direct elicitation approach to generation of utility data, MR-prednisone in comparison with IR-prednisone is a cost-effective use of NHS resources in the treatment of RA patients with morning stiffness over a 12-month time horizon. Further, given that morning stiffness is known to be an important factor in reduced productivity and early retirement, the inclusion of drug costs only provides a potentially conservative analysis. Sensitivity analyses demonstrate that changes to key parameters do not lead to significant changes in the ICER, given that, in all sensitivity analyses conducted, ICERs remain below the acceptable cost-effective threshold applied by NICE.
Author contributions
The model was developed and validated by LH, WD, II, IK, and MO.
Acknowledgments
The authors acknowledge the assistance of three RA experts in generating the utility values used in the model (full results published by Iqbal et al).Citation22 These experts were Professor Peter Taylor (a consultant in RA, who received honoraria from Mundipharma International Limited for the utility study), Professor Bhaskar Dasgupta (a consultant in polymyalgia rheumatica, who also received honoraria from Mundipharma International Limited for the utility study), and Dawn Homer, a rheumatology nurse consultant.
Disclosure
This research was funded by Mundipharma International Limited. The authors acknowledge the editorial assistance of Adelphi Values (supported by Mundipharma International Limited). II and IK are former employees of Mundipharma International Limited (both were employed by Mundipharma International Limited at the time of writing). WD is a current employee of Mundipharma International limited. Authors report no other conflicts of interest in this work.
References
- LundkvistJKastangFKobeltGThe burden of rhematoid arthritis and access to treatments: health burden and costsEur J Health Econ2008824960
- GrassiWDeARLamannaGCerviniCThe clinical features of rheumatoid arthritisEur J Radiol19982711824
- AdamsRWalshCVealeDBresnihanBFitzGeraldOBarryMUnderstanding the relationship between the EQ-5D, SF-6D, HAQ and disease activity in inflammatory arthritisPharmacoeconomics201028647748720465316
- CutoloMHow should morning function in rheumatoid arthritis be assessed? Bibliographic study of current assessmentScand J Rheumatol Suppl2011125172221529306
- SierakowskiSCutoloMMorning symptoms in rheumatoid arthritis: a defining characteristic and marker of active diseaseScand J Rheumatol Suppl20111251521529303
- da SilvaJAPhillipsSButtgereitFImpact of impaired morning function on the lives and well-being of patients with rheumatoid arthritisScand J Rheumatol Suppl201112561121529304
- KhanNAYaziciYCalvo-AlenJReevaluation of the role of duration of morning stiffness in the assessment of rheumatoid arthritis activityJ Rheumatol200936112435244219833759
- American Autoimmune Related Disease AssociationThe cost burden of autoimmune disease: the lastest front in the war on spendingNational Institute of Health Progress in Autoimmune Disease Research Report2011 Available from: http://www.aarda.org/pdf/cbad.pdfAccessed July 27, 2013
- National Institute for and Clinical ExcellenceRheumatoid arthritis: The management of rheumatoid arthritis in adultsNICE Clinical Guidelines2009 Available from: http://publications.nice.org.uk/rheumatoid-arthritis-cg79Accessed July 27, 2013
- National Audit OfficeServices for people with rhematoid arthritisReport by the comptroller and auditor general 823, Session HC8232009 Available from: http://www.nao.org.uk/wp-content/uploads/2009/07/0809823.pdfAccessed July 27, 2013
- ButtgereitFDoeringGSchaefflerATargeting pathophysiological rhythms: prednisone chronotherapy shows sustained efficacy in rheumatoid arthritisAnn Rheum Dis20106971275128020542963
- ArvidsonNGGudbjornssonBLarssonAHallgrenRThe timing of glucocorticoid administration in rheumatoid arthritisAnn Rheum Dis199756127319059137
- ButtgereitFDoeringGSchaefflerAEfficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trialLancet2008371960820521418207016
- AltenRChronotherapy with modified-release prednisone in patients with rheumatoid arthritisExpert Rev Clin Immunol20128212313322288450
- ButtgereitFMehtaDKirwanJLow-dose prednisone chrono-therapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2)Ann Rheum Dis201372220421022562974
- BrazierJRobertsJDeverillMThe estimation of a preference-based measure of health from the SF-36J Health Econ200221227129211939242
- Mundipharma InternationalData on file: IMS SF-6D results2011
- AletahaDStrandVSmolenJSWardMMTreatment-related improvement in physical function varies with duration of rheumatoid arthritis: a pooled analysis of clinical trial resultsAnn Rheum Dis200867223824317644550
- PapaioannouDBrazierJParryGHow valid and responsive are generic health status measures, such as EQ-5D and SF-36, in schizophrenia? A systematic reviewValue Health201114690792021914513
- EspallarguesMCzoski-MurrayCJBansbackNJThe impact of age-related macular degeneration on health status utility valuesInvest Ophthalmol Vis Sci200546114016402316249475
- National Insititute for Health and Clinical ExcellenceGuide to the methods of technology appraisal2013 Available from: http://www.nice.org.uk/media/D45/1E/GuideToMethodsTechnologyAppraisal2013.pdfAccessed July 27, 2013
- IqbalIDasguptaBTaylorPHeronLPillingCElicitation of health state utilities associated with differing durations of morning stiffness in rheumatoid arthritisJ Med Econ20121561192200022804691
- BenucciMSaviolaGManfrediMSarzi-PuttiniPAtzeniFCost effectiveness analysis of disease-modifying antirheumatic drugs in rheumatoid arthritis. A systematic review literatureInt J Rheumatol2011201184549622162693
- Mundipharma InternationalData on file: transition probabilities for CAPRA-12011
- MurphyKSpenceSMcIntoshCConnor-GorberSHealth state descriptions for canadians: musculoskeletal diseasesStatistics Canada200682138
- YaziciYPincusTKautiainenHSokkaTMorning stiffness in patients with early rheumatoid arthritis is associated more strongly with functional disability than with joint swelling and erythrocyte sedimentation rateJ Rheumatol20043191723172615338490
- National Rheumatoid Arthritis SocietyLiving with RA: case studies Available from: http://www.nras.org.uk/about_rheumatoid_arthritis/living_with_rheumatoid_arthritis/default.aspxAccessed July 27, 2013
- EuroQol Grouphttp://www.euroqol.org/about-eq-5d/eq-5d-nomenclature.htmlAccessed 15 August, 2013
- British National Formulary No 63BNF Prednisone2012 Available from: http://www.medicinescomplete.com/mc/bnf/current/213856.htmAccessed July 27, 2013
- Mundipharma InternationalData on file: Drug related TEAE2006
- PfeifferBKrenzerSDockhornRImpact of modified-release prednisone on functional ability in patients with RARheumatol Int20133361447145423179262
- FautrelBEconomic benefits of optimizing anchor therapy for rheumatoid arthritisRheumatology (Oxford)20125142126
- BoersMButtgereitFA simple model that suggests possible cost savings when modified-release prednisone 5 mg/day is added to current treatment in patients with active rheumatoid arthritisRheumatology (Oxford)20135281435143723584366
- CurtisLPersonal and Social Services Research UnitUnit costs of health and social care Available from: http://www.pssru.ac.uk/Accessed July 27, 2012
- WareJESF-36 Health Survey Update2012 Available from: http://www.sf-36.org/tools/sf36.shtmlAccessed July 27, 2013
- HarrisonMJDaviesLMBansbackNJIngramMAnisAHSymmonsDPThe validity and responsiveness of generic utility measures in rheumatoid arthritis: a reviewJ Rheumatol200835459260218278841
- GarauMShahKKMasonARWangQTowseADrummondMFUsing QALYs in cancer: a review of the methodological limitationsPharmacoeconomics201129867368521599035
- ToshJCLongworthLJGeorgeEUtility values in National Institute for Health and Clinical Excellence (NICE) Technology AppraisalsValue Health201114110210921211492
- MittendorfTDietzBSterzRCifaldiMAKupperHvon der SchulenburgJMPersonal and economic burden of late-stage rheumatoid arthritis among patients treated with adalimumab: an evaluation from a patient’s perspectiveRheumatology (Oxford)200847218819318174232