Abstract
Background
The purpose of this study was to evaluate the long-term cost-effectiveness (including hospitalizations and cardiometabolic consequences) of atypical antipsychotics among adults with schizophrenia.
Methods
A 5-year Markov cohort cost-effectiveness model, from a US payer perspective, was developed to compare lurasidone, generic risperidone, generic olanzapine, generic ziprasidone, aripiprazole, and quetiapine extended-release. Health states included in the model were patients: on an initial atypical antipsychotic; switched to a second atypical antipsychotic; and on clozapine after failing a second atypical antipsychotic. Incremental cost-effectiveness ratios (ICERs) assessed incremental cost/hospitalization avoided. Effectiveness inputs included discontinuations, hospitalizations, weight change, and cholesterol change from comparative clinical trials for lurasidone and for aripiprazole, and the Clinical Antipsychotic Trials of Intervention Effectiveness for other comparators. Atypical antipsychotic-specific relative risk of diabetes obtained from a retrospective analysis was used to predict cardiometabolic events per Framingham body mass index risk equation. Mental health costs (relapsing versus nonrelapsing patients) and medical costs associated with cardiometabolic consequences (cardiovascular events and diabetes management) were obtained from published sources. Atypical antipsychotic costs were estimated from Red Book® prices at dose(s) reported in clinical data sources used in the model (weighted average dose of lurasidone and average dose for all other comparators). Costs and outcomes were discounted at 3%, and model robustness was tested using one-way and probabilistic sensitivity analyses.
Results
Ziprasidone, olanzapine, quetiapine extended-release, and aripiprazole were dominated by other comparators and removed from the comparative analysis. ICER for lurasidone versus risperidone was $25,884/relapse-related hospitalization avoided. At a $50,000 willingness-to-pay threshold, lurasidone has an 86.5% probability of being cost-effective, followed by a 7.2% probability for olanzapine, and 6.3% for risperidone. One-way sensitivity analysis showed the model is sensitive to lurasidone and generic risperidone hospitalization rates.
Conclusion
Generic risperidone is the least costly atypical antipsychotic. Lurasidone is more costly and more effective than risperidone and is cost-effective at willingness-to-pay thresholds of greater than $25,844 per hospitalization avoided. The favorable cost-effectiveness of lurasidone is driven by its clinical benefits (eg, efficacy in preventing hospitalizations in patients with schizophrenia) and its minimal cardiometabolic adverse effect profile.
Introduction
The diagnosed prevalence of schizophrenia in the US is only 0.51%,Citation1 yet the disease imposes a significant burden on patients, caregivers, and society, resulting in an estimated total annual excess cost of $62.7 billion in 2002 in the US.Citation2 Schizophrenia is also one of the most challenging diseases to treat due to its variable presentation, the heterogeneity of clinical response to treatment, poor adherence, and low rates of persistence with treatment.Citation3,Citation4
Poor adherence to antipsychotic treatment has been shown to increase the risk of relapse and subsequent hospitalization and to increase related resource utilization and costs.Citation3,Citation5,Citation6 According to 2008 data from the Healthcare Cost and Utilization Project, there were 356,000 hospital stays for schizophrenia and other psychotic disorders in the US, comprising 19% of all mental health and substance abuse-related hospitalizations. Patients admitted to hospital for schizophrenia have the highest average total cost per stay ($7,500), with an average duration of 11.1 days.Citation7 Patients who had experienced a relapse of psychotic symptoms within the previous 6 months incurred four times higher costs than schizophrenia patients without a recent relapse (P < 0.01).Citation8
While atypical antipsychotics (AAPs) are relatively well tolerated, they are often associated with metabolic side effects. These adverse effects may include weight gain, hyperglycemia, insulin resistance, and lipid abnormalities. The American Diabetes Association Consensus on Antipsychotic Drugs and Obesity and Diabetes recognizes that certain atypical antipsychotic agents are also associated with increased risk of developing metabolic syndrome, new-onset diabetes, and cardiovascular disease.Citation9 It has been reported that patients taking AAPs have approximately two times the risk of metabolic syndrome and diabetes compared with the general population.Citation10,Citation11 In addition, patients on AAPs have been found to be 9% more likely to develop diabetes than those taking conventional antipsychotics.Citation12,Citation13 Metabolic side effects of atypical antipsychotics, especially weight gain, may contribute to premature treatment discontinuation and poor adherence,Citation4,Citation14 which can lead to symptom worsening, relapse, and greater health care resource utilization.Citation15,Citation16
There has been continuing unmet clinical and economic need for new AAPs that not only effectively reduce the occurrence of acute relapses but also have a neutral or minimal impact on metabolic parameters. Such agents may have the potential to reduce the costs of care by reducing the incidence of new-onset diabetes or cardiovascular disease and/or improving treatment compliance and reducing acute exacerbations and subsequent hospitalizations. In clinical studies, lurasidone (Latuda®, Sunovion Pharmaceuticals, Marlborough, MA, USA), an AAP approved by the US Food and Drug Administration in October 2010, has demonstrated lower annual rates of relapses and relapse-related hospitalizations compared with quetiapine extended-release. In addition, lurasidone also has been reported to have a more favorable cardiometabolic profile compared with other major AAPs in both clinical trials and in the real-world practice setting, thus potentially offering a cost-effective alternative therapy for patients with schizophrenia.Citation17 Therefore, the objective of this health economic model was to assess the cost-effectiveness of lurasidone compared with other available generic and branded atypical antipsychotics in the treatment of schizophrenia from a US payer perspective, including direct medical costs; direct nonmedical costs and indirect costs, such as lost productivity, were not included in the model.
Materials and methods
Model design
A Microsoft Excel®-based Markov cohort model was developed to assess the cost-effectiveness of lurasidone compared with other AAPs available for treating adult patients with schizophrenia. Treatment comparators evaluated in the model included aripiprazole (Abilify®, Bristol-Myers Squibb Company, Princeton, NJ, USA), lurasidone, olanzapine (generic), quetiapine extended-release (Seroquel XR®, AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA), risperidone (generic), and ziprasidone (generic). The cost-effectiveness analysis was conducted over a 5-year time horizon from a third-party payer perspective in the US.
Costs and outcomes associated with AAPs and incorporating treatment switching were modeled using this Markov cohort analysis. Patients in the model start on lurasidone or another AAP (aripiprazole, olanzapine, quetiapine extended-release, risperidone, and ziprasidone). When patients discontinue the first AAP for any cause (adverse event or lack of efficacy), they transition to a “composite” AAP. The composite AAP therapy was incorporated into the model to account for treatment switching within a single state, as patients remain on the composite therapy until they fail due to lack of efficacy and transition to clozapine.Citation18 Patients failing the composite due to adverse events were assumed to remain on the composite and continue to incur the associated costs and outcomes, thereby simulating treatment switching (). The composite therapy was operationalized by averaging the discontinuation rates (transition probabilities), costs, and outcomes of the other comparator AAPs among which the patient might possibly switch (eg, if a patient initiates treatment with lurasidone, then the composite would reflect the average of aripiprazole, olanzapine, quetiapine extended-release, risperidone, and ziprasidone). Patients discontinuing the composite health state due to lack of efficacy were considered to be refractory and were switched to clozapine. Patients switched to clozapine were projected to remain on clozapine for the remainder of the 5-year analysis.
Modeled costs in the analysis include pharmacy, mental health, diabetes management, and cardiovascular event-related costs inflated to 2012 US dollars using the Medical Care Component of the Bureau of Labor Statistics’ Consumer Price Index.Citation19 The model outcome was relapse-related hospitalizations avoided, and the per patient mean value was estimated over the 5-year time horizon. Costs in the model include the cost of hospitalizations; hence, to avoid double-counting, the outcome represents the clinical benefit to patients associated with avoiding relapse and a subsequent hospitalization, and not cost savings.Citation20 A standard discount rate of 3% was used for both costs and outcomes. The model comparators were ranked from least to most costly and then incremental cost-effectiveness ratios (ICERs), representing the difference in cost divided by the difference in outcome between the comparator agents, were calculated after excluding dominated and extendedly dominated options. One-way and probabilistic sensitivity analyses were also conducted to test model robustness.
Model inputs
Patient characteristics
The population for the model included adult patients diagnosed with schizophrenia. Patient characteristics for the base case scenario were specified to reflect the average schizophrenia patient enrolled in the lurasidone clinical trials: male (73% of patients were male), age 38 years, weight 77.3 kg, body mass index (BMI) 26.3, with a mean total cholesterol of 192 mg/dL, high-density lipoprotein of 48 mg/dL, and systolic blood pressure (BP) of 120 mmHg.Citation21,Citation22 In addition, 5.5% of patients were assumed to have diabetes and 67% to be smokers. In the model, patient gender, high-density lipoprotein, systolic BP, and smoking were static and did not change over time. Patient age, total cholesterol, and diabetes were a function of time (age), therapy (diabetes), and time on therapy (total cholesterol). Patient characteristics were used to estimate the risk of cardiovascular events and mortality in the model based on Framingham risk equations.Citation23
Effectiveness parameters
Effectiveness parameters were obtained from adjusted indirect comparisons of various outcome measures from different clinical sources. The annual transition probabilities between the different Markov states were based on rates for total discontinuation, discontinuation due to lack of efficacy (used to determine relapses), and hospitalizations (used to determine relapse-related hospitalizations) from four major data sources ().
Table 1 Discontinuation and hospitalization rates
Discontinuation due to lack of efficacy and hospitalization rates for olanzapine, risperidone, quetiapine, and ziprasidone were obtained directly from the first phase of Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), a prospective double-blind study of 1,493 patients with schizophrenia.Citation4 Given that the first phase of the CATIE trial was an 18-month study, the discontinuation rates over 18 months were adjusted to 12 months, assuming that the rate at which patients discontinued treatment was constant.
Discontinuation and hospitalization rates for aripiprazole and lurasidone were based on an indirect comparison with the drugs included in the CATIE trial. Aripiprazole was directly compared with olanzapine in a long-term, open-label study,Citation25 in which the relative risk for all-cause discontinuation for aripiprazole was 1.349, and the relative risk of discontinuation due to lack of efficacy was 1.851. Therefore, the rates of total discontinuation and discontinuation due to lack of efficacy of olanzapine from the CATIE trialCitation4 were multiplied by these relative risks to obtain estimates of the discontinuation rates for aripiprazole. Because no hospitalization rate was available for aripiprazole, estimation of this rate was based on the relative risk of lack of efficacy for aripiprazole in Chrzanowski et alCitation25 and on the hospitalization rate for olanzapine from the CATIE trial.Citation4
In a similar manner, lurasidone was indirectly compared with the other model comparators using a direct comparison with quetiapine extended-release in a double-blind, parallel-group, 12-month comparative study.Citation24 In that study, lurasidone was found to have a relative risk of discontinuation for any cause of 0.787, a relative risk of discontinuation due to lack of efficacy of 0.728, and a relative risk of hospitalization of 0.404 versus quetiapine extended-release. These respective rates for lurasidone were then multiplied by the rates of discontinuation for any cause, rates of discontinuation due to lack of efficacy, and rates of hospitalization for quetiapine immediate-release from the CATIE trial to derive the respective rates for lurasidone. Based on the results of randomized clinical trials comparing quetiapine extended-release 400 mg/day and quetiapine immediate-release 400 mg/day, it was assumed that quetiapine extended-release and quetiapine immediate-release have similar efficacy and safety profiles.Citation26
Finally, the rates for clozapine were based on an 18-month study by McEvoy et alCitation27 and were adjusted to 12-month rates. Standard errors for the transition probabilities were calculated based on the proportions and sample sizes from the published studies.
Cardiometabolic parameters
The model structure also incorporates the costs and outcomes of cardiometabolic consequences of treatment with each AAP. Many AAPs have been associated with increased cardiovascular risk by causing weight gain, an increase in lipid levels, and a higher risk of diabetes.Citation28 As such, cardiometabolic parameters in the model included annual weight change (kg/year), annual cholesterol change (mg/dL/year), and diabetes relative risk ().
Table 2 Cardiometabolic parameters
The model incorporates these risk factors by utilizing data from comparative clinical trialsCitation4,Citation24,Citation25 of the rate of weight gain and lipid increase of AAPs and data from a large retrospective analysis of the risk of diabetes.Citation29 In order to track time on therapy to estimate the amount of weight gain and lipid increase, time-dependent substates (tunnel states) based on the time at which a segment of the cohort initiates a new therapy, were incorporated into the cohort analysis.Citation18 For each annual cycle in the model, the age of the patient cohort increases, baseline weight is adjusted by time on AAP therapy, total cholesterol is adjusted by time on AAP therapy, and diabetes is adjusted by the relative risk based on the AAP. These adjusted risk factors were then applied to the Framingham 10-year cardiovascular risk profile using the Framingham BMI risk equation.Citation23 According to the BMI equation, cardiovascular risk is a function of age, BMI, untreated systolic BP, treated systolic BP, smoking, and diabetes. Finally, the 10-year risks were adjusted to one-year risks to calculate the expected number of annual cardiovascular disease events based on the cohort risk factors.
Mortality rates
Published age-specific and gender-specific mortality tables were used to determine patient mortality over the 5-year time horizon.Citation30 The mortality risk due to cardiovascular events and suicide was estimated separately in the model; therefore, the population mortality rates were adjusted to exclude the increased mortality risks associated with suicide and cardiovascular disease among patients with schizophrenia. Patient suicides were calculated based on the rate per 100,000 patient-years (mean 579, standard error 52) from a published systematic review of suicide ratesCitation31 and were assumed to be identical for all AAPs. Cardiovascular disease mortality was estimated by multiplying the number of patients experiencing a cardiovascular event by the fatal cardiovascular event rate (mean 9.5%, standard error 2.0%).Citation32
Drug costs and resource utilization
Annual drug costs were estimated based on the wholesale acquisition costs of each AAP (generic olanzapine, generic risperidone, generic ziprasidone, aripiprazole, quetiapine extended-release, and clozapine) as reported in the Red Book® as of October 9, 2012Citation33 using the weighted average cost calculated based on the average daily dose from the CATIE trial (). Annual costs for lurasidone were based on patient utilization from a 12-month, multicenter, double-blind, parallel-group study of flexibly dosed lurasidone (40–160 mg/day),Citation24 in which 15% of patients received a dose of 40 mg or 80 mg and 85% of patients received a dose of 120 mg or 160 mg. Because lurasidone 160 mg was not an approved dose and was as effective as the 120 mg dose, for the purposes of the model, it was assumed that all patients receiving lurasidone 160 mg received lurasidone 120 mg.
Table 3 Annual treatment costs and resource utilization
Resource utilization costs were obtained from the published literature (). The annual costs of psychiatric care were obtained from a published prospective, observational, noninterventional study of schizophrenia in the US comparing 310 patients with and 1,247 patients without a relapse.Citation34 Relapse was defined as any of the following: psychiatric hospitalization, use of emergency services, use of a crisis bed, or a suicide attempt, and was determined by systematic abstraction of data from patients’ medical records. Drug costs were estimated using average wholesale price minus 15%, and psychiatric hospitalizations were based on per diem costs adjusted across sites using their relative value units. Cost components included costs of medications (antipsychotics; other psychotropics, such as mood stabilizers, anticholinergics, antidepressants, antianxiety drugs; and sleep agents), psychiatric hospitalizations, day treatment, emergency services, psychosocial group therapy, medication management, individual therapy, and assertive community treatment/case management. Costs for patients with a relapse and a psychiatric hospitalization versus those with a relapse and no psychiatric hospitalization were differentiated by subtracting the hospitalization costs from the former group.
The costs of diabetes management were obtained from a studyCitation35 published by the American Diabetes Association that estimated the annual attributable costs of diabetes based on data from multiple sources, including the Medical Expenditure Panel Survey. This estimate included costs associated with hospital inpatient care, outpatient and physician office visits, emergency visits, nursing facility stays, home health visits, visits with other health professionals, and prescription drug and medical supply use. The rate of diabetes was multiplied by the annual diabetes cost to estimate the total costs of diabetes.
The annual costs of a cardiovascular event were estimated based on the one-month attributable costs from a large administrative claims analysis in the USCitation32 using an incidence-based cost of illness that included costs for myocardial infarction, cardiac arrest, congestive heart failure, angina pectoris, transient ischemic attack, hemorrhagic stroke, ischemic stroke, peripheral vascular disease, coronary artery bypass graft surgery, and coronary angioplasty. The number of cardiovascular events was multiplied by the cost per event to estimate the total cost of cardiovascular disease events for the cohort.
Sensitivity analyses
The robustness of the model results were tested using a oneway deterministic sensitivity analysis and a probabilistic sensitivity analysis. The one-way deterministic sensitivity analysis was conducted to quantify the impact of uncertainty around the mean value of individual model parameters. For the low and high values, the one-way sensitivity analysis used the 95% confidence interval based on the mean and standard error for all model parameters.
In addition, sensitivity analyses using other scenarios listed below were conducted to evaluate the potential impact on the cost-effectiveness results:
Using the Framingham lipid risk equation in place of the Framingham BMI risk equation
Changing the discount rate from 3% to a range of 0%–5%
Running the analysis with pharmacy costs only and removing cardiometabolic costs.
A probabilistic sensitivity analysis was conducted to quantify the impact of uncertainty of all model parameters by simultaneously sampling from the 95% confidence interval for each parameter distribution. Beta distributions were used for probabilities and percentages, log normal distributions were used for relative risks, and normal distributions were used for the remainder of parameters.
Results
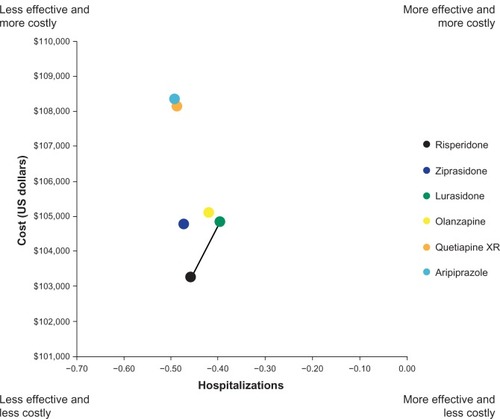
Over the 5-year time horizon of the model, generic ris-peridone patients had the lowest total discounted health care costs, followed by generic ziprasidone, lurasidone, generic olanzapine, and other branded products, quetiapine extended-release and aripiprazole (). Lurasidone was associated with the lowest number of relapse-related hospitalizations (0.40), followed by olanzapine (0.42). Aripiprazole had the highest number of relapse-related hospitalizations (0.49). Full disaggregated model results are shown in .
Table 4 Discounted clinical outcomes and costs for atypical antipsychotics
In the incremental cost-effectiveness analysis, aripiprazole, quetiapine extended-release, and ziprasidone were dominated by risperidone (ie, more costly and less effective) whereas aripiprazole, quetiapine extended-release, and olanzapine were dominated by lurasidone and were therefore removed from the incremental analysis (). The ICER for lurasidone versus risperidone was $25,884 per relapse-related hospitalization avoided.
Figure 2 ICER per relapse-related hospitalization avoided.
Sensitivity analyses
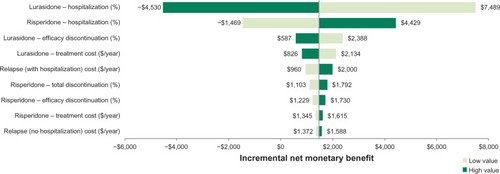
shows the results of the one-way sensitivity analysis comparing lurasidone and risperidone for the most impactful parameters at an assumed willingness-to-pay threshold of $50,000 per hospitalization avoided. At this threshold, lurasidone is the preferred therapeutic option, with an incremental net monetary benefit of $1,480 compared with risperidone. As shown in the tornado diagram, the model is sensitive to the following two parameters: lurasidone hospitalization rate (incremental net monetary benefit range, −$4,530 to $7,489) and risperidone hospitalization rate (−$1,469 to $4,429). The model results were insensitive to the other model parameters over the tested ranges.
Figure 3 One-way sensitivity analysis results (tornado diagram).
When costs of relapses and hospitalization are included and cardiometabolic costs are excluded from the analysis, the model results in an ICER of $26,109 for lurasidone versus risperidone.
Results of the probabilistic sensitivity analysis at a willingness-to-pay threshold of $50,000 per hospitalization avoided indicated that lurasidone is the most cost-effective AAP, followed by olanzapine, risperidone, ziprasidone, quetiapine extended-release, and aripiprazole (). At a willingness-to-pay threshold of $50,000, lurasidone has an 86.5% probability of being cost-effective, followed by a 7.2% probability for olanzapine, and 6.3% for risperidone (). At willingness-to-pay thresholds below approximately $26,000 per relapse avoided, risperidone has the highest probability of being cost-effective
Figure 4 Cost-effectiveness acceptability curve.*
Abbreviations: WTP, willingness to pay; XR, extended-release.
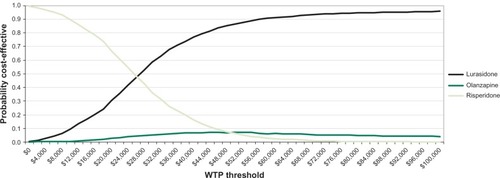
Table 5 Probabilistic sensitivity analysis results
Discussion
A similar study comparing lurasidone with aripiprazole for second-line use in patients with schizophrenia found lurasidone to be cost-effective.Citation36 This is the first cost-effectiveness analysis comparing lurasidone with other AAPs for firstline use in patients with schizophrenia. The results of this cost-effectiveness analysis are generally comparable with the cost-effectiveness analyses of other AAPs that were recently published. A microsimulation model evaluating the cost-effectiveness of the AAPs olanzapine, risperidone, quetiapine, ziprasidone, and aripiprazole from a third-party payer perspective in the US over a one-year period showed that olanzapine had the lowest mean direct costs, followed by generic risperidone. Olanzapine was the dominant therapy for costs per quality-adjusted life-year (QALY).Citation37 The total costs of adverse events, including extrapyramidal symptoms, weight gain, diabetes, and lipid abnormalities, were greatest for olanzapine, but the cost-effectiveness was driven by reduced inpatient hospitalizations. Another study comparing the cost-effectiveness of ziprasidone with that of olanzapine, risperidone, and quetiapine based on data from the CATIE trial from the Canadian health care perspective over a 5-year period found that ziprasidone was less costly and more effective than olanzapine and quetiapine;Citation38 however, the model included olanzapine at branded price. Similar to the results of this analysis, the model by McIntyre et alCitation38 found that olanzapine had the highest costs related to cardiovascular and type 2 diabetes. Two other cost-effectiveness analyses comparing olanzapine and aripiprazoleCitation39 and risperidone and olanzapineCitation40 yielded disparate results: the analysis by Ascher-Svanum et al,Citation39 based on patient-level data from a randomized controlled trial, showed that olanzapine dominated risperidone. The analysis by Cooper et al,Citation40 based on real-world insurance claims data in Canada, showed that use of risperidone was more cost-effective than olanzapine. These cost-effectiveness analyses, however, did not evaluate lurasidone as it was not available on the US market until early 2011.
The current therapeutic standard of care to manage symptoms and prevent clinical relapses associated with schizophrenia are AAPs,Citation41,Citation42 which are generally perceived to have an improved side effect profile over typical antipsychotics.Citation43 However, prior to approval of lurasidone, results from two large non-industry-sponsored studies (CATIE and CUtLASS [Cost Utility of the Latest Antipsychotics in Schizophrenia Study]) demonstrated that some of the atypical agents, particularly clozapine and olanzapine, may be associated with metabolic side effects, including serious weight gain, diabetes, and hyperlipidemia.Citation4,Citation44,Citation45 In a review article published in 2006, among the agents that were available on the market, the relative tendency to cause weight gain was as follows: clozapine > olanzapine > risperidone = quetiapine > ziprasidone = aripiprazole.Citation46 Clozapine and olanzapine are also associated with an increased risk of diabetes and dyslipidemia.Citation45,Citation47 Based upon a recent review of labels for all AAPs, including lurasidone, the relative tendency to cause weight gain is hypothesized to be as follows: olanzapine = quetiapine > risperidone = iloperidone > ziprasidone > aripiprazole > quetiapine extended-release > paliperidone = asenapine > lurasidone.Citation48
A recent literature review of lurasidone clinical trials concluded that lurasidone has a minimal impact on body weight, with no clinically relevant associated changes observed in blood glucose, cholesterol, triglycerides, or the electrocardiographic QT interval.Citation17 In clinical trials, glucose, lipid parameters, and weight with lurasidone were generally similar to placebo. Pooled data from short-term, placebo-controlled studies comparing lurasidone 40 mg, 80 mg, 120 mg, and 160 mg with placebo found a mean change from baseline in serum glucose of 2.6, −0.4, 2.5, 2.5, and 0.0 mg/dL, respectively; mean change from baseline in total cholesterol of −5.7, −6.2, −3.8, −6.9, and −5.8 mg/dL, respectively; mean change from baseline in triglycerides of −5.1, −13.0, −3.1, −10.6, and −13.4 mg/dL, respectively; and mean change from baseline weight of 0.22, 0.54, 0.68, 0.60, and −0.02 kg, respectively.Citation49 Because of the more favorable cardiometabolic profile with lurasidone, the impact of cardiovascular and metabolic-related side effects may be lower, resulting in lower costs for payers.
The present economic analysis was conducted to estimate the costs and outcomes associated with initial treatment with lurasidone compared with other AAPs in the treatment of schizophrenia. To allow comprehensive evaluation of the costs and outcomes, the potential cardiometabolic adverse events and increased health care costs associated with AAP treatments were also included. The base case results from the cost-effectiveness model found generic risperidone and generic ziprasidone to be the least costly treatments, with varying effects on hospitalization avoidance, as expected. However, lurasidone was found to have the lowest total costs among the branded agents, with best effects on hospitalization avoidance. Lurasidone dominated (was less costly and more effective) olanzapine, quetiapine extended-release, and aripiprazole in terms of relapse-related hospitalizations. Lurasidone was more costly and more effective than generic risperidone, with an ICER of $25,884.
Limitations
The validity of any cost-effectiveness analysis is only as plausible as the inputs and assumptions made within the model. While the authors have attempted to ensure that the model’s assumptions are valid, there are some limitations with the model that need to be recognized. First, the cost-effectiveness model does not account for patient heterogeneity, which is a limitation of all Markov cohort models. In the base case, patients were assumed to reflect the average schizophrenia patient enrolled in the lurasidone clinical trials,Citation21,Citation22 ie, male, aged 38 years, weight 77.3 kg, BMI 26.3, total cholesterol 192 mg/dL, high-density lipoprotein 48 mg/dL, systolic BP 120 mmHg, diabetes rate 5.5%, and smoking rate 67%. To the extent that a specific patient population differs from these characteristics, the model results may change. However, these patient characteristics primarily impact the cardiometabolic risks in the model, which have a minimal impact on the overall costs.
In addition, the model is based on the results of various comparative clinical trials, including CATIE,Citation4 Study 234,Citation24 and a 12-month, open-label study comparing aripiprazole and olanzapine.Citation25 In order to include lurasidone and aripiprazole in the model, these drugs were compared indirectly with the other model comparators from CATIE. Generally, naturalistic studies such as CATIE will show reduced effectiveness compared with clinical trials, which are designed to assess efficacy. However, the effect of this is mitigated to some extent by conducting an indirect treatment comparison, which involves a relative, not an absolute, comparison across treatments. While health care decision-makers increasingly recognize indirect treatment comparisons as an acceptable alternative method of comparison in the absence of real-world parallel-group data, differences in study populations may limit their comparability. CATIE was intended to be a real-world effectiveness trial; therefore, the study population represented a heterogeneous population of patients with chronic schizophrenia and comorbid conditions. Study 234 of lurasidone included patients who were previously treated with lurasidone or placebo for 6 weeks.Citation24 The aripiprazole–olanzapine study included patients with either acute relapsing or chronic, stable schizophrenia.Citation25 However, with the exception of the aripiprazole–olanzapine study, all of these studies involve patients with chronic, potentially stable schizophrenia. While there were some differences in the three different data sources, all these studies included patients who were already on active treatment with antipsychotics when they initiated the study treatment of choice and thus are likely to represent a relatively stable treated population.
This analysis used relapse-related hospitalizations avoided as the outcome measure, as opposed to QALYs, for two reasons. First, the model was developed from a US payer perspective, and it is generally understood that US payers are not interested in QALYs. Second, since none of the studies used to obtain effectiveness data included QALYs as an outcome measure, it was felt that incorporating QALYs would involve additional assumptions about the relationship between patient health states (relapse/no relapse) and QALYS and would not necessarily result in a significant relative difference in the outcomes.
Resource costs were estimated based on published literature and drug costs in the model were estimated based on mean drug utilization and published Red Book costs as of October 9, 2012. Changes in costs since this time may impact model results. The authors have sought to address modest variation in resource and drug costs within the sensitivity analyses; however, to the extent that these costs vary significantly, the model results may be impacted.
Treatment switching is common among patients with schizophrenia and was incorporated into this Markov cohort analysis through the use of a composite health state, consisting of average discontinuation rates, costs, and outcomes of noncomparator agents. This was done to ensure a consistent, simplified approach to account for several drug switches for tolerability/adverse event reasons across all model comparators. However, it is possible that treatment-switching patterns may potentially differ in the real world, resulting in different costs and outcomes.
Lastly, cardiometabolic risks were assumed to depend on patient characteristics and weight and lipid changes caused by the AAP agents. Weight and lipid changes were assumed to occur in a linear manner based on the amount of time on therapy. These changes were modeled at the cohort level for the average patient. Outcomes and cardiovascular risks for individual patients may vary. As this analysis incorporates only the known risk factors, it may underestimate the cardiovascular risks and costs associated with AAPs.
Conclusion
The cost-effectiveness of lurasidone compares favorably with generic AAPs (risperidone, ziprasidone, and olanzapine) and branded AAPs (quetiapine extended-release and aripiprazole). This may be driven by the clinical benefits of lurasidone, including efficacy in preventing hospitalizations and relapses in patients with schizophrenia, and its minimal cardiometabolic adverse event profile. Notwithstanding some of the limitations of the model, the results of this economic analysis indicate that, depending upon the decision-maker’s willingness-to-pay threshold, lurasidone may be a cost-effective treatment option for patients with schizophrenia over a 5-year period. Given the implications of this model, further investigation through prospective comparative effectiveness studies or claims database analyses is warranted to confirm the cost-effectiveness of lurasidone in real-world settings.
Disclosure
This study was supported by funding from Sunovion Pharmaceuticals Inc. KR, AP, and AL are employees of Sunovion Pharmaceuticals Inc. KOD and KM are employees of Xcenda, a consulting company that provides services to several pharmaceutical companies, including Sunovion Pharmaceuticals Inc.
References
- WuEQShiLBirnbaumHHudsonTKesslerRAnnual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approachPsychol Med200636111535154016907994
- WuEQBirnbaumHGShiLThe economic burden of schizophrenia in the United States in 2002J Clin Psychiatry20056691122112916187769
- Ascher-SvanumHZhuBFariesDEFuriakNMMontgomeryWMedication adherence levels and differential use of mental-health services in the treatment of schizophreniaBMC Res Notes20092619138402
- LiebermanJAStroupTSMcEvoyJPClinical Antipsychotic Trials of InterventionEffectiveness of antipsychotic drugs in patients with chronic schizophreniaN Engl J Med20053531209122316172203
- dosReisSJohnsonESteinwachsDAntipsychotic treatment patterns and hospitalizations among adults with schizophreniaSchizophr Res20081011–330431118255270
- AhnJMcCombsJSJungCClassifying patients by antipsychotic adherence patterns using latent class analysis: characteristics of nonadherent groups in the California Medicaid (Medi-Cal) programValue Health2008111485618237360
- Agency for Healthcare Research and QualityHCUP facts and figures: statistics on hospital-based care in the United States, 2008Rockville, MDAgency for Healthcare Research and Quality2010 Available from: http://www.hcup-us.ahrq.gov/reports.jspAccessed July 6, 2013
- AlmondSKnappMFrancoisCToumiMBrughaTRelapse in schizophrenia: costs, clinical outcomes and quality of lifeBr J Psychiatry200418434635115056580
- American Diabetes AssociationAmerican Psychiatric AssociationAmerican Association of Clinical EndocrinologistsNorth American Association for the Study of ObesityConsensus development conference on antipsychotic drugs and obesity and diabetesDiabetes Care200427259660114747245
- McEvoyJPMeyerJMGoffDCPrevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES IIISchizophr Res2005801193216137860
- BreseeLCMajumdarSRPattenSBJohnsonJAPrevalence of cardiovascular risk factors and disease in people with schizophrenia: a population-based studySchizophr Res20101171758220080392
- MathewsMMuzinaDJAtypical antipsychotics: new drugs, new challengesCleve Clin J Med200774859760617708131
- SernyakMJLeslieDLAlarconRDLosonczyMFRosenheckRAssociation of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophreniaAm J Psychiatry2002159456156611925293
- WeidenPJMackellJAMcDonnellDDObesity as a risk factor for antipsychotic noncomplianceSchizophr Res2004661515714693352
- Liu-SeifertHAdamsDHKinonBJDiscontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugsBMC Med200532116375765
- VelliganDILamYWGlahnDCDefining and assessing adherence to oral antipsychotics: a review of the literatureSchizophr Bull200632472474216707778
- CitromeLLurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychoticInt J Clin Pract201165218921021129135
- RajagopalanKO’DayKMeyerKTime-on-therapy for atypical antipsychotics in a Markov cohort analysisAbstract PMH86 presented at the International Society for Pharmacoeconomics and Outcomes Research Annual International MeetingJune 2–6, 2012Washington, DC
- Bureau of Labor StatisticsConsumer Price Index, All Urban Consumers Available from: http://data.bls.gov/cgi-bin/surveymost?cuAccessed July 6, 2013
- MullinsCDDouble counting and the reporting of cost per event avoidedClin Ther200628460260316750471
- NasrallahHASilvaRPhillipsDLurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled studyJ Psychiatr Res201347567067723421963
- MeltzerHYCucchiaroJSilvaRLurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo-and olanzapine-controlled studyAm J Psychiatry2011168995796721676992
- D’AgostinoRBSrVasanRSPencinaMJGeneral cardiovascular risk profile for use in primary care: the Framingham Heart StudyCirculation2008117674375318212285
- LoebelACucchiaroJXuJSarmaKPikalovAKaneJMEffectiveness of lurasidone vs quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority studySchizophr Res201314719510223583011
- ChrzanowskiWKMarcusRNTorbeynsANyilasMMcQuadeRDEffectiveness of long-term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52-week, open-label comparison with olanzapinePsychopharmacology2006189225926617058105
- KahnRSSchulzSCPalazovVDStudy 132 InvestigatorsEfficacy and tolerability of once-daily extended release quetiapine fumarate in acute schizophrenia: a randomized, double-blind, placebo-controlled studyJ Clin Psychiatry200768683284217592906
- McEvoyJPLiebermanJAStroupTSCATIE InvestigatorsEffectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatmentAm J Psychiatry2006163460061016585434
- McDermottSMoranRPlattTIsaacTWoodHDasariSHeart disease, schizophrenia, and affective psychoses: epidemiology of risk in primary careCommunity Ment Health J200541674775516328587
- LeslieDLRosenheckRAIncidence of newly diagnosed diabetes attributable to atypical antipsychotic medicationsAm J Psychiatry200416191709171115337666
- AriasEUnited States life tables, 2007National vital statistics reportsHyattsville, MDNational Center for Health Statistics2011 Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_09.pdfAccessed July 6, 2013
- HorKTaylorMReview: suicide and schizophrenia: a systematic review of rates and risk factorsJ Psychopharmacol201024Suppl 4819020923923
- O’SullivanAKRubinJNyamboseJKuznikACohenDJThompsonDCost estimation of cardiovascular disease events in the USPharmacoeconomics201129869370421585226
- RedBook OnlineMicromedex 2.0, Thomson ReutersAccessed October 9, 2012
- Ascher-SvanumHZhuBFariesDEThe cost of relapse and the predictors of relapse in the treatment of schizophreniaBMC Psychiatry2010102 Available from: http://www.micromedexsolutions.com/home/dispatch20059765
- American Diabetes AssociationEconomic costs of diabetes in the US in 2007Diabetes Care200831359661518308683
- RajagopalanKHassanMO’DayKMeyerKGrossmanFCost-effectiveness of lurasidone vs aripiprazole among patients with schizophrenia who have previously failed on an atypical antipsychotic: an indirect comparison of outcomes from clinical trial dataJ Med Econ201316795196123701260
- FuriakNMAscher-SvanumHKleinRWCost-effectiveness model comparing olanzapine and other oral atypical antipsychotics in the treatment of schizophrenia in the United StatesCost Eff Resour Alloc20097419351408
- McIntyreRSCraginLSorensenSNaciHBakerTRoussyJPComparison of the metabolic and economic consequences of long-term treatment of schizophrenia using ziprasidone, olanzapine, quetiapine and risperidone in Canada: a cost-effectiveness analysisJ Eval Clin Pract201016474475520545800
- Ascher-SvanumHStenslandMDPengXCost-effectiveness of olanzapine vs aripiprazole in the treatment of schizophreniaCurr Med Res Opin201127111512221110749
- CooperDMoisanJAbdousBGrégoireJPA population-based cost-effectiveness analysis of olanzapine and risperidone among ambulatory patients with schizophreniaCan J Clin Pharmacol2008153e385e39718953083
- LehmanAFLiebermanJADixonLBAmerican Psychiatric AssociationSteering Committee on Practice GuidelinesPractice guideline for the treatment of patients with schizophrenia, second editionAm J Psychiatry2004161Suppl 215615000267
- KreyenbuhlJBuchananRWDickersonFBDixonLBSchizophrenia Patient Outcomes Research Team (PORT)The schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009Schizophr Bull20103619410319955388
- NaberDLambertMThe CATIE and CUtLASS studies in schizophrenia: results and implications for cliniciansCNS Drugs200923864965919594194
- BuchananRWKreyenbuhlJKellyDLSchizophrenia Patient Outcomes Research Team (PORT)The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statementsSchizophr Bull2010361719319955390
- JonesPBBarnesTRDaviesLRandomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1)Arch Gen Psychiatry200663101079108717015810
- LlorenteMDUrrutiaVDiabetes, psychiatric disorders, and the metabolic effects of antipsychotic medicationsClin Diabetes20062411824
- PatelKHHlavinkaPFSchizophrenia: optimal therapy with second-generation antipsychotic agentsPharmacy Today200713117184
- CitromeLNasrallahHAOn-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does notExpert Opin Pharmacother201213111599161322017361
- Latuda (lurasidone HCl) [package insert]Marlborough, MASunovion Pharmaceuticals Inc2010
