Abstract
Background
In patients with type 2 diabetes mellitus, basal-bolus strategies can improve treatment by offering dosing flexibility, and improved satisfaction, adherence, and clinical outcomes. The purpose of this study was to compare real-world outcomes between US patients initiating analog insulin therapy with insulin glargine and those initiating with a premixed analog insulin (PMX).
Methods
This was a retrospective study of data from patients (≥18 years) with type 2 diabetes mellitus in the IMPACT® database who initiated insulin treatment with insulin glargine (GLA) or a PMX. Clinical and economic outcomes were measured over one year, including persistence and adherence, consumption of insulin, glycemic outcomes, incident hypoglycemia, and health care resource utilization and cost.
Results
Data from 2,502 patients were included in the analyses (n = 834 for PMX, n = 1,668 for GLA). Compared with PMX, persistence was higher and consumption of insulin was lower for GLA (both P < 0.0001). Adherence, glycemic outcomes, and hypoglycemia-related events were similar between groups, as were health care utilization and total health care costs. Diabetes-related drug and supply costs were lower for GLA than for PMX (P < 0.0001 and P = 0.046, respectively).
Conclusion
In US patients with type 2 diabetes mellitus, initiating insulin with once-daily GLA, rather than a PMX, is associated with increased treatment persistence and similar clinical and hypoglycemic outcomes, but lower diabetes pharmacy and supply costs. GLA may be a more flexible option than PMX. However, these results also show suboptimal glycemic control in the real-world setting despite change in treatment regimens and call for optimization in management of patients with type 2 diabetes mellitus.
Introduction
Oral antidiabetic drugs and lifestyle interventions are initially recommended for the management of most patients with type 2 diabetes mellitus (T2DM),Citation1 but since T2DM is a progressive disease, these interventions often fail over time.Citation2,Citation3 Current guidelines for the management of T2DM recommend initiating insulin if noninsulin therapy at maximal tolerated doses does not achieve or maintain glycemic control over 3–6 months.Citation1,Citation4 Typically, patients will initiate analog insulin therapy with a single daily injection of basal analog insulin,Citation1,Citation5 such as a regular human insulin, the intermediate-acting neutral protamine Hagedorn insulin, or the long-acting analog insulin glargine (GLA) or detemir. To target meal-related glucose excursions, prandial insulin (eg, rapid-acting GLA or aspart) may also be added to the regimen. Such a “basal-bolus” therapeutic regimen may be the most appropriate strategy when basal analog insulin alone is no longer sufficient to reach the glycated hemoglobin (A1c) target.Citation5,Citation6 In these cases, rapid-acting insulin can be added to the basal analog insulin, providing physical, psychologic, and treatment satisfaction benefits to patients.Citation7 A simplified, stepwise approach of one or two preprandial injections before the meals of greatest glycemic impact can be used, as well as a traditional three preprandial injections approach.Citation8 Alternatively, patients may initiate insulin treatment with (or switch to) premixed analog insulins (PMX; a fixed combination of intermediate-acting insulin with regular insulin or a rapid-acting insulin).Citation9,Citation10
Although basal-bolus treatment is more complicated for patients than PMX treatment, it allows for more flexibility, especially with irregular mealtimes; however, PMX regimens generally result in larger decreases in A1c levels. Of note, dose flexibility has been shown to be an important attribute of injectable treatments for T2DM from the patient perspective,Citation11 which can contribute to increased patient satisfaction with therapy.Citation12 Furthermore, lower treatment satisfaction is associated with poor adherence to medication;Citation13 greater satisfaction with treatment is related to better clinical outcomes.Citation14,Citation15 In addition, GLA and detemir are associated with smaller increases in weight and a reduced incidence of hypoglycemia compared with, for example, neutral protamine Hagedorn insulin.Citation1
A previously published randomized clinical trial showed that GLA-based basal-bolus treatment is as effective as PMX treatment,Citation16 and that GLA-based basal-bolus treatment causes less hypoglycemia than PMX. Patient-reported outcomes are also more positive for GLA-based basal-bolus treatment when compared with PMX treatment.Citation17 However, few studies have compared real-world outcomes following initiation of basal only or basal-bolus therapy versus PMX; such information would assist with treatment decisions and help optimize the management of patients with T2DM.
The purpose of this study was to compare real-world outcomes between US patients with T2DM requiring analog insulin therapy initiating with GLA, with or without a rapid-acting insulin, and those initiating with a PMX.
Materials and methods
Patients
This was a retrospective study of data from IMPACT®, a US, nationally managed care database that comprises about 50 US health care plans and contains medical claims, pharmacy claims, eligibility data, and laboratory results for 107 million patients, of whom 73% had pharmacy benefits and 18% had laboratory results.
Data from adult US patients (≥18 years) diagnosed with T2DM, defined as having at least one inpatient visit or at least two physician visits (≥30 days apart) with a primary or secondary diagnosis of T2DM (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 250.x0 or 250.x2), were identified for inclusion in the analysis. Patients were required to have continuous health plan coverage of both medical and pharmacy benefits for ≥6 months before (baseline period) and at least one year after (follow-up period) initiation of GLA or PMX (the index date), and must have at least one A1c test result at both baseline and at the end of the one-year follow-up period. Patients using only oral antidiabetic drugs or glucagon-like peptide-1 in the baseline period were eligible for inclusion, while patients were excluded if they had used any type of insulin, including a PMX, in the baseline period. Using an intent-to-treat approach, patients were assigned to treatment cohorts according to the type of insulin with which they initiated treatment, ie, GLA or PMX. Within the GLA cohort, patients could initiate rapid-acting insulin any time between 30 days prior to and 360 days after insulin glargine initiation (also known as a basal-bolus/plus treatment regimen) or stay only on GLA, also known as a basal-only treatment regimen.
Outcomes
Clinical and economic outcomes were measured over a one-year follow-up period, including persistence and adherence, consumption of insulin, glycemic outcomes, hypoglycemia event, and health care resource utilization and cost. Treatment persistence was defined as a patient remaining on the study drug during the follow-up period, without discontinuation or switching after initiation.Citation18–Citation22 Study medication was considered discontinued if the prescription was not refilled within the expected time of medication coverage (the 90th percentile of the time, stratified by the metric quantity supplied, between first and second fills among patients with at least one refill). Patients who restarted their initial medication after a period without it during follow-up were considered nonpersistent. Sensitivity analyses were also conducted using 75th and 95th percentiles of the time, so that for patients in the GLA group, the treatment persistence was based on GLA use; since rapid-acting insulin could be added anytime during the follow-up period, treatment persistence with rapid-acting insulin was not measured. For patients in the PMX group, the treatment persistence was based on PMX use; patients could switch between different types of PMX (aspart or lispro) during the follow-up period, but would still be considered as persistent users of PMX. Treatment adherence was measured by both traditional medication possession ratio (MPR) and adjusted MPR; the traditional MPR does not take into account the difference in package sizes between insulin medications, and the adjusted MPR addresses this limitation by using the total number of days of drug supply during the follow-up period divided by the total number of days in the follow-up period, multiplied by the average days between prescription refills divided by average days of drug supply for patients.Citation23 The daily average consumption of insulin was calculated as the total number of units dispensed before the last refill of study drug divided by the total number of days between initiation and last refill during the follow-up period.
With regards to glycemic control outcomes, A1c level was analyzed as the follow-up value, change from baseline, and percentage of patients meeting target A1c (<7.0%). Hypoglycemia was also reported as a health care encounter (outpatient, inpatient, or emergency room visit) with a primary or secondary ICD-9-CM diagnosis code for hypoglycemia (ICD-9 code 250.8, diabetes with other specified manifestations; 251.0, hypoglycemic coma; 251.1, other specified hypoglycemia; or 251.2, hypoglycemia, unspecified).Citation24 The setting of the hypoglycemic event (outpatient, emergency room, or hospital) was used as proxy for severity of the event.
In addition, for economic outcomes, health care resource utilization was determined using outpatient visits, emergency room, visits, inpatient admissions, inpatient length of stay (days), endocrinologist visits, and diabetes-related health care resource utilization using claims with a primary or secondary diagnosis of diabetes (ICD-9-CM code 250.xx). Health care costs were also computed as plan-paid amounts of adjudicated claims, and diabetes-related health care costs included costs from medical claims with a primary or secondary diagnosis of diabetes (ICD-9-CM code 250.xx), antidiabetic medications, and glucose meters and test strips.
Statistical analyses
Selection bias is inherent in real-world retrospective studies, because patients prescribed one treatment often differ systematically from patients prescribed a comparison treatment. The analysis was conducted using an intent-to-treat approach. Propensity score matching at a 1:2 ratio was used to match patients in the PMX cohort with those in the GLA cohort to adjust appropriately for a lack of randomization between the cohorts by removing observed differences in baseline demographic and clinical characteristics.Citation25 Each patient is assigned a propensity score, which is a fitted value of the probability of being a member of the overall cohort. To match patients from the two cohorts, a 1:2 nearest neighbor greedy match between glargine and premix initiators was performed using the propensity score for each patient. A patient from one cohort could only be matched to a patient from the other cohort if their propensity scores were very similar (±0.01 units apart); patients who could not be matched were dropped from the analysis. A propensity score weighted generalized linear model was used as the main multivariate analysis method; age, baseline A1c, copay, initial year, region, health plan, diabetes education, baseline comorbidities, diabetes medication, health care utilizations, and costs were controlled in the model.
Among matched patients, baseline characteristics, clinical outcomes, and economic parameters were summarized and compared with P-values provided by Student’s t-test or χ2 test as appropriate. Kaplan–Meier curves were used to examine time to treatment discontinuation between both cohorts and time to add rapid-acting insulin in the GLA cohort. For the health care resource utilization and health care costs analysis, raw data are compared; regression modeling was not used in this study, thus transformation was not needed.
Results
Patient characteristics
Data from a total of 2,502 matched patients were included in the analyses (834 in the PMX cohort and 1,668 in the GLA cohort). All baseline demographic and clinical characteristics, health care utilizations, and health care costs were balanced after propensity score matching (). Overall, 47.6% of patients were women, the mean age was 55.8 years, the mean baseline A1c was 9.6%, and the mean number of oral antidiabetic drugs at baseline was 2.1.
Table 1 Clinical and economic characteristics at baseline
Treatment persistence and adherence
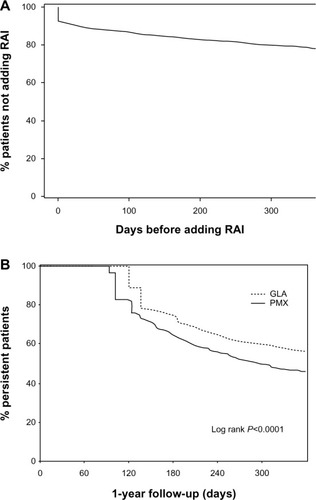
Patients in the GLA cohort were more treatment-persistent with initiated insulin than those in the PMX cohort (55.9% versus 45.4%, P < 0.0001, ). These results remained consistent in the sensitivity analyses using the 75th percentile of the time (75th percentile, 28.1% versus 23.4%, P = 0.0125). On average, patients in the GLA cohort remained on treatment for 26 days longer than those in the PMX cohort (280 days versus 254 days, P < 0.0001), and the Kaplan–Meier survival curve shows that patients in the PMX cohort discontinued treatment more quickly than those in the GLA cohort ().
Figure 1 Treatment persistence (A) and adherence (B) in the PMX and GLA cohorts.
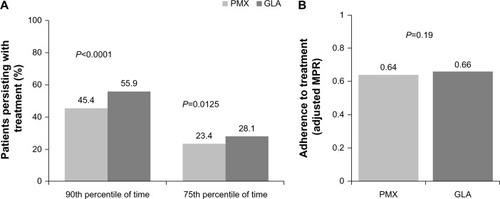
Figure 2 Kaplan–Meier curves for the time to adding RAI (A) and for time to treatment discontinuation (B).
Adherence rates were similar between the two cohorts (adjusted MPR 0.66 versus 0.64, P = 0.19 for GLA and PMX, respectively, ). By the end of one-year follow-up, 8.5% of patients in the GLA cohort had switched to a PMX; however, of patients in the PMX cohort, 10.9% received GLA and 12.5% received a rapid-acting insulin.
During one-year follow-up in the GLA cohort, 21.7% (n = 363) of patients added a rapid-acting insulin. At baseline, these patients had significantly higher levels of illness than those who stayed on GLA only (baseline Charlson comorbidity index, 0.85 versus 0.66, P = 0.01; hospitalization, 21.7% versus 10.8%, P < 0.001). The average time for adding rapid-acting insulin was 95.54 ± 111.64 days (median 42 days, ). The percentage of patients in the GLA cohort using a rapid-acting insulin was similar for each quarter of the follow-up period, with 13.0%, 11.9%, 12.8%, and 13.0% filling a prescription for a rapid-acting insulin in quarters one, two, three, and four, respectively.
Clinical outcomes
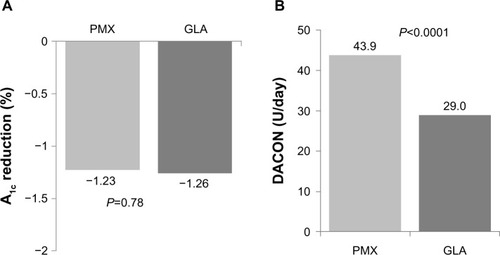
At the end of one-year follow-up, the reduction from baseline A1c level was similar between the GLA and PMX cohorts (−1.26 versus −1.23%, P = 0.784), and only 25% patients achieved the goal of glycemic control (A1c <7.0%) at the end of follow-up (GLA 25.2% versus PMX 24.7%, P = 0.769, ). However, there was a significant difference in the insulin doses between the groups, with the daily average consumption for patients in the GLA cohort being 29.0 U/day (30 U/day of rapid-acting insulin if added) and 43.9 U/day for patients in the PMX cohort (P < 0.0001, ).
Figure 3 A1c reduction (A) and DACON (B) at the end of one-year follow-up.
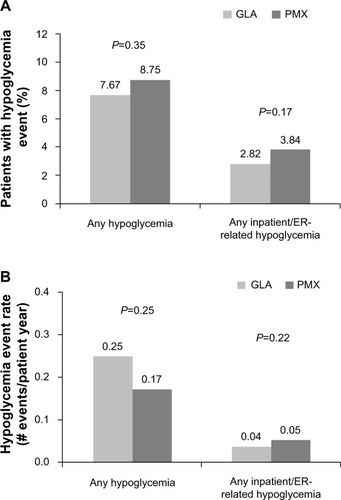
Hypoglycemia-related outcomes were also similar between the GLA and PMX cohorts. The respective hypoglycemia outcomes for the GLA and PMX cohorts were: 7.67% versus 8.75% for prevalence of any hypoglycemia (P = 0.3492); 2.82% versus 3.84% for prevalence of inpatient/emergency room-related hypoglycemia (P = 0.1693); 2.54 versus 0.75 events per patient year for incidence of any hypoglycemia (P = 0.2472); and 0.24 versus 0.32 events per patient year for incidence of inpatient/emergency room-related hypoglycemia (P = 0.2190, and ).
Health care utilization and cost outcomes
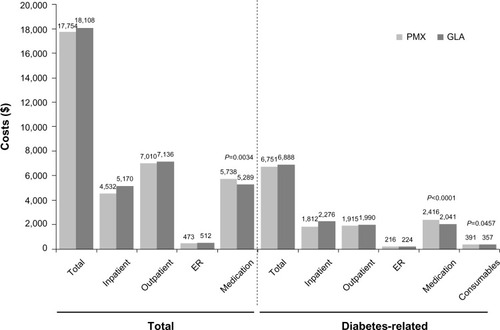
At one year of follow-up, health care utilization outcomes were similar for the GLA and PMX cohorts, except for the number of endocrinology visits, which was higher for PMX than for GLA (1.97 versus 1.68, P = 0.01). Although mean total health care costs were similar between the GLA and PMX cohorts over one year ($18,108 versus $17,754, P = 0.735), diabetes drug costs were significantly lower in the GLA than in the PMX cohorts ($2,041 versus $2,416, P < 0.0001, ). In addition, diabetes supply costs were lower in the GLA than in the PMX cohorts at one year ($357 versus $391, P = 0.046, ).
Discussion
In this real-world study, patients with T2DM who initiated insulin treatment with once-daily GLA-based regimens were more persistent with their therapy than those who initiated with a PMX, while showing a similar A1c reduction. Previous studies have found that, when compared with basal insulin alone, premixed insulin formulations lower A1c levels to a greater extent but at the same time result in slightly more hypoglycemic eventsCitation9,Citation26,Citation27 and more weight gain.Citation9,Citation26–Citation28 Similarly, others have reported fewer hypoglycemic events with GLA in a basal-bolus regimen than with PMX.Citation16,Citation29–Citation31 In this study, there were no statistically significant differences in the rates of hypoglycemia between the GLA and PMX cohorts. The data also indicate that the glycemic control achieved by patients in this study, in general, was not good, with average A1c levels of 8.3% at the end of follow-up and a responder rate of approximately 25% only. Better outcomes for both GLA and insulin detemir are usually reported from clinical trials.Citation32 This suggests that, in the real-world setting, patients might not be titrating appropriately, whether on GLA or PMX, and may explain the unexpected similarity in rates of hypoglycemia.
The American Diabetes Association and the European Association for the Study of Diabetes (ADA/EASD) guidelines recommend that when initiating insulin therapy, either basal insulin (with or without rapid-acting insulin) or premixed insulin, both the patient’s A1c levels and willingness to take more than one injection per day should be considered.Citation4 The ADA/EASD guidelines acknowledge the inability to titrate the shorter-acting separately from the longer-acting component of premixed insulins as a disadvantage of these formulations. Nonetheless, the inflexible but relatively simple insulin regimen may be appropriate for those patients who have a regular lifestyle and eating habits.Citation4
In this study, most patients in the GLA cohort stayed on the GLA-only regimen during the one-year follow-up, and thus better maintained a once-daily treatment regimen compared with the twice-daily regimen required with a PMX. This is important, since patients prefer, or are more satisfied with, analog insulin therapy that requires fewer injections,Citation33,Citation34,Citation35 and insulin omission has been related to the need for more daily injections.Citation36 The use of GLA plus a rapid-acting insulin has been reported to result in better patient satisfaction than PMX.Citation17 Furthermore, a basal-insulin-based therapeutic strategy allows more flexibility than use of a PMX. In this study, patients in the GLA cohort could start with once-daily GLA injections, and then add additional injections of a rapid-acting insulin as necessary. In fact, the data show that those patients in the GLA cohort who added rapid-acting insulin were already sicker at baseline. In contrast, those who were treated with PMX had to follow a daily regimen of two injections. This might explain the high rate of switching to a GLA-based treatment regimen in the PMX group.
Despite better treatment persistence and lower diabetes drug and supply costs, the GLA group had total health care costs similar to those in the PMX group. This could be contributed to the fact that the GLA group itself was a heterogeneous group, consisting of patients being treated with either basal-only and basal-bolus/plus regimens, the latter of whom were sicker at baseline and could therefore have incurred a higher follow-up cost. A recent Canadian study reported that self-measured blood glucose testing among insulin users constitutes a significant proportion of diabetes-related pharmacy costs. The annual number of self-measured blood glucose tests was lower among premixed insulin users compared with users of basal insulin with or without bolus insulin. Premix users had lower annual pharmacy costs than basal and basal-bolus insulin users. The proportional costs for insulin therapy as a part of total pharmacy costs were highest for basal-bolus users (54%) and lowest for basal-only users (43%). Similarly, the annual costs associated with self-measured blood glucose testing accounted for a smaller proportion of total pharmacy costs among basal insulin users (38%) compared with basal-bolus and premix users (both about 41%).Citation37
This study has a number of limitations. Firstly, the length of follow-up was only one year and may not have been long enough to detect the long-term clinical and economic benefits of the better treatment persistence associated with the GLA cohort. Secondly, it is a retrospective observational analysis of claims data which may be subject to selection bias and confounding. The presence of a claim for a filled prescription does not indicate whether the medication was actually consumed, or that it was taken as prescribed, and the presence of a diagnosis code on a medical claim may be incorrectly coded or included as rule-out criteria rather than actual disease. In addition, patients switching study drug during the follow-up period in each cohort could result in bias in the adjusted results. Further, dosing information was estimated based on filled pharmacy claims which could introduce a bias to the results based on the frequency and dose of treatment differences. The number of rapid-acting insulin injections is also not known in the real-world setting of this study. Finally, the analyses were based on data from a managed care population and patients with missing baseline or outcomes data were excluded from the study, and may not be representative of other populations or generalizable to all patients with T2DM.
Conclusion
This real-world study shows that for US patients with T2DM failing oral antidiabetic drugs, initiating insulin with a once-daily GLA-based regimen, instead of a PMX, is associated with increased treatment persistence and similar clinical and hypoglycemic events outcomes, but lower diabetes pharmacy and supply costs. Most patients initiating with GLA stayed on the GLA-only regimen during one-year follow-up; those who added a rapid-acting insulin were already more severely ill at baseline. Thus, GLA may be a more flexible treatment option than PMX and result in better treatment persistence. However, in both cohorts, only one of five patients were able to achieve the goal for glycemic control of A1c < 7.0% by the end of follow-up. This suggested suboptimal titration by the patients and/or their physicians in the real-world setting, and calls for optimal management of patients with T2DM.
Disclosure
OB and LX are employees of STATinMED Research, which received funding to carry out this work from Sanofi US Inc. WW and KT are employees of Sanofi US Inc. The authors received writing/editorial support for preparation of this manuscript from Pim Dekker of Excerpta Medica, which was funded by Sanofi US Inc.
References
- InzucchiSEBergenstalRMBuseJBManagement of hyperglycemia in type 2 diabetes: a patient-centered approachDiabetologia2012551577159622526604
- CobbleMEPetersALClinical practice in type 2 diabetes: after metformin and lifestyle, then what?J Fam Pract200958Suppl 11S7S1419891948
- ShomaliMAdd-on therapies to metformin for type 2 diabetesExpert Opin Pharmacother201112476221142694
- American Diabetes AssociationStandards of medical care in diabetes – 2012Diabetes Care201235 Suppl 1S11S6322187469
- RaccahDOptions for the intensification of insulin therapy when basal insulin is not enough in type 2 diabetes mellitusDiabetes Obes Metab200810768218577159
- OwensDRLuzioSDSert-LangeronCRiddleMCEffects of initiation and titration of a single preprandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof of concept’ studyDiab Obes Metab20111320202027
- NicolucciADel PratoSVespasianiGELEONOR Study GroupOptimizing insulin glargine plus one injection of insulin glulisine in type 2 diabetes in the ELEONOR study: similar effects of tele-care and conventional self-monitoring of blood glucose on patient functional health status and treatment satisfactionDiabetes Care2011342524252621953799
- DavidsonMBRaskinPTanenbergRJVlajnicAHollanderPA step-wise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failureEndocr Pract20111739540321324825
- RaskinPAllenEHollanderPINITIATE Study GroupInitiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogsDiabetes Care20052826026515677776
- RaskinPRHollanderPALewinAGabbayRABodeBGarberAJINITIATE Study GroupBasal insulin or premix analogue therapy in type 2 diabetes patientsEur J Intern Med200718566217223044
- BoyeKSMatzaLSWalterKNVan BruntKPalsgroveACTynanAUtilities and disutilities for attributes of injectable treatments for type 2 diabetesEur J Health Econ20111221923020224930
- PeyrotMRubinRRPolonskyWHBestJHPatient reported outcomes in adults with type 2 diabetes on basal insulin randomized to addition of mealtime pramlintide or rapid-acting insulin analogsCurr Med Res Opin2010261047105420199136
- BidermanANoffEHarrisSBFriedmanNLevyATreatment satisfaction of diabetic patients: what are the contributing factors?Fam Pract20092610210819254969
- BradleyCDiabetes treatment satisfaction questionnaireBradleyCHandbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and PracticeChur, SwitzerlandHarwood Academic Publisher1994
- FinkelMIThe importance of measuring patient satisfactionEmpl Benefits J199722121510165682
- RiddleMCVlajnicAJonesBARosenstockJComparison of 3 intensified insulin regimens added to oral therapy for type 2 diabetes: twice-daily aspart premixed versus glargine plus 1 prandial glulisine or stepwise addition of glulisine to glargineDiabetes201160 Suppl 1A113
- PolonskyWHRosenstockJGilmoreASWeiWChaudhsSLRiddleMCPatient reported outcomes using twice-daily insulin aspart premixed versus insulin glargine plus 1 prandial insulin glulisine or stepwise addition of glulisine to glargine in type 2 diabetes uncontrolled with oral agentsDiabetes201160 Suppl 1A616
- DavisSNWeiWGargSClinical impact of initiating insulin glargine therapy with disposable pen versus vial in patients with type 2 diabetes mellitus in a managed care settingEndocr Pract20111784585221550952
- XieLZhouSWeiWGillJPanCBaserODoes pen help? A real-world outcomes study of switching from vial to disposable pen among insulin glargine-treated patients with type 2 diabetes mellitusDiabetes Technol Ther20131523023623336845
- XieLWeiWPanCDuJBaserOA real-world study of patients with type 2 diabetes initiating basal insulins via disposable pensAdv Ther2011281000101122038703
- BaserOWeiWBaserEXieLClinical and economic outcomes in patients with type 2 diabetes initiating insulin glargine disposable pen versus exenatide BIDJ Med Econ20111467368021892858
- LevinPWeiWWangLPanCDouglasDBaserOCombination therapy with insulin glargine and exenatide: real-world outcomes in patients with type 2 diabetesCurr Med Res Opin20122843944622216894
- BaserOBouchardJDeLuzioTHenkHAagrenMAssessment of adherence costs of insulin device (FlexPen®) versus conventional vial/syringeAdv Ther2010279410420352392
- ZhaoYCampbellCRFonsecaVShiLImpact of hypoglycemia associated with antihyperglycemic medications on vascular risks in veterans with type 2 diabetesDiabetes Care2012351126113222432106
- RosenbaumPRRubinDPThe central role of the propensity score in observational studies for causal effectBiometrika1983704155
- IlagLLKerrLMaloneJKTanMHPrandial premixed insulin analogue regimens versus basal insulin analogue regimens in the management of type 2 diabetes: an evidence-based comparisonClin Ther20072912541270
- MaloneJKKerrLFCampaigneBNSachsonRAHolcombeJHLispro Mixture-Glargine Study GroupCombined therapy with insulin lispro mix 75/25 plus metformin or insulin glargine plus metformin: A 16-week, randomized, open-label, crossover study in patients with type 2 diabetes beginning insulin therapyClin Ther2004262034204415823767
- MaloneJKBaiSCampaigneBNReviriegoJAugendre-FerranteBTwice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with type 2 diabetesDiabet Med20052237438115787659
- DaviesMSinnassamyPStormsFGomisRATLANTUS Study GroupInsulin glargine-based therapy improves glycemic control in patients with type 2 diabetes sub-optimally controlled on premixed insulin therapiesDiabetes Res Clin Pract20087936837517980928
- FonsecaVDavidsonJHomePStarting insulin therapy with basal insulin analog or premix insulin analog in T2DM: a pooled analysis of treat-to-target trialsCurr Med Res Opin2010261621162820429817
- FritscheALarbigMOwensDHäringHUGINGER Study GroupComparison between a basal-bolus and a premixed insulin regimen in individuals with type 2 diabetes – results of the GINGER studyDiabetes Obes Metab20101211512320092584
- DaileyGAdmaneKMercierFOwensDRelationship of insulin dose, A1c lowering, and weight in type 2 diabetes: comparing insulin glargine and insulin detemirDiabetes Technol Ther2010121019102721128849
- WolffenbuttelBHvan OuwerkerkBMVeldhuyzenBFGeelhoed-DuijvestijnPHJakobsenGvan DoornLGComparative effects of two different multiple injection regimens on blood glucose control and patient acceptance in type 1 diabetesDiabet Med199076956992147630
- BretzelRGNuberULandgrafWOwensDRBradleyCLinnTOnce-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trialLancet20083711073108418374840
- SwinnenSGDainMPAronsonRA 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugsDiabetes Care2010331176117820200301
- PeyrotMRubinRRKrugerDFTravisLBCorrelates of insulin injection omissionDiabetes Care20103324024520103556
- YeawJLeeWCWoldenMLChristensenTGroleauDCost of self-monitoring of blood glucose in Canada among patients on an insulin regimen for diabetesDiabetes Ther20123722736405