Abstract
Objectives
The aim of this study was to determine budget impact of conversion from cyclosporine (CsA) to sirolimus (SRL) in renal transplant therapy (RTT) from the perspective of insurance organizations in Iran.
Methods
An Excel-based model was developed to determine cost of RTT, comparing current CsA based therapy to an mTOR inhibitor-based therapy regimen. Total cost included both cost of immunosuppressive agents and relative adverse events. The inputs were derived from database of Ministry of Health and insurance organizations, hospital and pharmacy based registries, and available literature that were varied through a one-way sensitivity analysis. According to the model, there were almost 17,000 patients receiving RTT in Iran, out of which about 2,200 patients underwent the operation within the study year. The model was constructed based on the results of a local RCT, in which test and control groups received CsA, SRL, and steroids over the first 3 months posttransplantation and, from the fourth month on, CsA, mycophenolate mofetil (MMF), and steroids were used in the CsA group and SRL, MMF, and steroids were administered in the SRL group, respectively.
Results
The estimated cost of RTT with CsA was US$4,850,000 versus US$4,300,000 receiving SRL. These costs corresponded to the cost saving of almost US$550,000 for the payers.
Conclusion
To evaluate the financial consequence of adding mTOR inhibitors to the insurers’ formulary, in the present study, a budget impact analysis was conducted on sirolimus. Fewer cases of costly adverse events along with lower required doses of MMF related to SRL based therapies were major reasons for this saving budgetary impact.
Introduction
Renal transplantation has been considered a cost-effective alternative to other renal replacement therapies such as hemodialysis or peritoneal dialysis for patients suffering from end-stage renal disease (ESRD).Citation1
Immunosuppressive drugs are major components of renal transplant therapy (RTT), which improve graft and patient survival.Citation1 mTOR inhibitors are quite potent new immunosuppressive agents which modulate immune response in a way quite different from agents such as tacrolimus.Citation2 Sirolimus (SRL) (Rapamune®; Pfizer, Inc., New York, NY, USA) is an mTOR inhibitor obtained first US Food and Drug Administration (FDA) approval for kidney transplantation in 1999 having considered the successful Phase III clinical trial resultsCitation3 and it provides effective maintenance therapy by decreasing common adverse events related to cyclosporine (CsA) such as nephrotoxicity, gingival hypertrophy, and hirsutism.Citation4
In 2006, a comprehensive meta-analysis study on advising mTOR inhibitors as a primary immunosuppression therapy was conducted by Webster et al.Citation5 After considering different adverse events such as cardiovascular accident risks, cytomegalovirus (CMV) infection, and bone marrow suppression, they concluded that the benefit–harm trade-off of using mTOR inhibitors depended on patient groups.Citation5 According to Büchler et al, an SRL based regimen with mycophenolate mofetil (CellCept®) was as effective as CsA based regimen in terms of graft and patient survival and maintaining low rate of acute rejection (AR).Citation6 In 2011, Han et al reported considerable improvement in the long-term renal graft survival in Chinese patients through a 4-year period conversion from CsA into SRL.Citation1 In general, a calcineurin inhibitor (CNI)-free regimen using SRL-MMF could achieve excellent renal function along with fewer AR episodes while experiencing a high rate of adverse events and drug discontinuation.Citation6
In 2012, Nafar et al published a randomized controlled trial (RCT) in Iran, comparing immunosuppression effects of SRL versus CNI (CsA) based therapies among Iranian patients. One hundred patients from Shahid Labbafinejad Teaching Hospital were randomly selected and enrolled in the trial; they were then followed-up for 4 years (2004–2007) in this trial.Citation7 In the present study, the above mentioned locally performed RCT is the reference clinical trial used to obtain health outcomes, probabilities, and resource utilization.
Materials and methods
The current study was performed in accordance with the report of International Society for Pharmacoeconomics and Outcomes Research (ISPOR) task force on good practice for Budget Impact Analysis (BIA) published in 2007.Citation8
The analytic framework was designed according to results of the local RCT performed by Nafar et al (reference clinical trial).Citation7 Consequently, an Excel® (Microsoft Corporation, Redmond, WA, USA) based model was constructed in which probabilities, health outcomes, and resource utilization were derived from the reference RCTCitation7 as well as national and international literature and standard local guidelines in RTT.
In the present study, authors decided to define “health outcomes” as “adverse events” due to the fact that the main outcomes were treatment-related complications. Clinical data was obtained on the following key events: immunosuppressive drug use, graft failure, AR, CMV infection, hyperlipidemia, hypertension, and thrombocytopenia. Other adverse events which were quite similar in the two groups were excluded. Cost calculations were based on the standard tariffs for drugs and medical services used by Iranian insurance organizations in making payments over the study year (2011–2012).
Estimates of patient population and data sources
Population-based incidence data were obtained from a central registry system in the Management Center for Transplantation and Special Diseases (MCTSD), affiliated with the Ministry of Health (MOH).Citation9 Prevalence data were not considered in this study. Tariffs and expenditure data of RTT and dialysis were extracted from the databases of insurance organizations and hospital-based registries. Regarding required probabilities, apart from the reference RCT, hospital-based registries, local clinical trials, and related literature were reviewed. Estimates on out-patient details of the immunosuppressive regimen were obtained from Helal-e-Ahmar pharmacy records and databases of social security organizations (SSO). Health services (eg, physician or specialist visits, laboratory tests, nursing services, etc) and treatment options, which were similar in the two groups, were excluded. Model components and data sources have been summarized in .
Table 1 Model inputs and data sources for the budget impact analysis
According to MCTSD and insurance organizations’ databases, the prevalence and incidence of ESRD were 357 per million population (pmp) and 66 pmp, respectively, and almost 17,000 patients received RTT in the study year (from March 2011 to March 2012), out of which about 2,200 patients (aged between 18–70 years old) had their renal transplantation operation over the mentioned time horizon.
There are two main semi-public hospitals in Tehran performing renal transplantation, which are in charge of almost half of kidney transplantation cases in the country (25% at the Baghiatollah Hospital and 25% at the Shahid Labbafinejad Hospital). The reference RCT was conducted in Shahid Labbafinejad Hospital, one of the major centers for renal transplantation in Iran with more than 550 transplantation cases per year. The hospital is the property of SSO, which is the largest insurance organization in Iran, and all the tariffs and guidelines are practically applied in exact accordance with the national standards under MOH supervision and regulations. To be in line with the reference RCT, only patients having their transplantation operation within the study year were included in the model (n=2,200).
Time horizon and perspective
To make RTT and dialysis more affordable and accessible for eligible patients from all socioeconomic statuses, there are special facilities and reimbursement processes provided by the MOH as well as insurance organizations in Iran. There are several governmental and quasi-governmental organizations engaged in ESRD issue: a) MOH as the main sponsor; b) SSO; c) Medical Service Insurance Organization (MSIO); d) Armed Forces Medical Service Organization (AFMSO); e) Imam Khomeini Relief Foundation (IKRF); and f) special organizations such as oil companies, radio and television broadcasters, and banks.Citation10,Citation11 In the current study, the model used the perspective of insurance organizations (mainly SSO, MSIO, AFMSO, and IKRF) with an annual number of about 17,000 patients receiving RTT relative to a 12-month time horizon while considering the following two assumptions: 1) tariffs for drugs and medical services were the same in all the considered insurance organizations, and 2) using SRL and CsA was stable throughout the period. Moreover, costs of care for patients were estimated based on the mean duration of treatment in the reference clinical trial.Citation7
Scenarios to compare
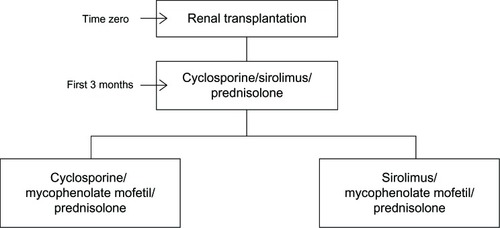
According to the reference RCT conducted by Nafar et al,Citation7 100 kidney transplant recipients were randomly divided into two groups of 50 patients and received CsA, SRL, and steroids over the first 3 months posttransplantation and, from the fourth month on, CsA and SRL were replaced with mycophenolate mofetil (locally produced branded generic dosage form) in the SRL and CsA groups, respectively (). Mean age ± standard deviation in each group was 38.5 ± 12.5 years and 42.5 ± 14.3 years in the SRL and CsA groups, respectively. After 3 years of follow-up, 36 patients remained in the SRL group and 28 patients in the CsA group. Two patients dropped out of the SRL group because of leucopenia and anemia and four patients died in the CsA group because of sepsis and cerebrovascular accident (CVA). One patient missed the follow-up in each group. According to the results of this study, biopsy proven AR (as the main health outcome followed in the study) occurred in nine patients in the CsA group (34 episodes) and in our patients (20 episodes) in the SRL group and thus the rate of AR in the CsA group was 1.7 (34/20) fold higher than in the SRL group which corresponded to an incidence rate of almost 18% versus 8% AR in the CsA and SRL groups, respectively, over 12 months posttransplantation. Rates of other adverse events were not published but registered in the follow-up database for a 1-year period of time from the transplantation operation. There was no statistically significant difference in graft and patient survival after 1-year between the two groups.
The costs and health outcomes (adverse events) were compared from the 4th month on, during which therapy regimens were different between the two groups (270 days was the duration of treatment).
Cost of immunosuppressive agents
The mean SRL dose was calculated based on the recommended dose of 2 mg/day (two tablets per day) using the regulated market price for SRL of 10,000 Iranian Rials (IRR) (US$0.82) for each tablet (US$1.64 per patient per day). The recommended dose for MMF administered along with SRL was 1 g/day, yielding almost 22,000 IRR (US$1.79) per patient per day.
The mean CsA dose was 150 mg/day per patient which was available in three dosages (25, 50, and 100 mg oral tablets). CsA cost was acquired from the insurance organizations’ databases and, considering the total number of patients using each dosage (according to Helal-e-Ahmar pharmacy records), the cost of CsA per day was estimated as almost 5,700,000 IRR (US$470). Cost of MMF in patients receiving CsA was almost double compared to the SRL administered group and prednisolone was the same dose and cost in both groups ().
Table 2 Cost of immunosuppressive agents in SRL versus CsA based therapies in Iran (2011–2012)
Cost of adverse events
Major adverse events were taken into account for SRL versus CsA during 12 months after transplantation, which were mainly AR (8% versus 18%),Citation7 thrombocytopenia (45% versus 8%), hyperlipidemia (44% versus 14%), hypertension (78% versus 67%), CMV infection (6% versus 23%),Citation3,Citation6,Citation12,Citation13 and graft failure (7.5% versus 10.5%), respectively.Citation7 Regarding CMV infection, ganciclovir was administered over a 7-day hospitalization with an average treatment dose of 5 mg/kg every 12 hours. In the case of AR episodes, methylprednisolone (with daily boluses of 250–1000 mg), antithymocyte globulin (ATG) with a daily dose of 10–20 mg/kg, and ganciclovir for CMV infection prophylaxis (5 mg/kg every 24 hour until hospitalization) were administered in renal transplant recipients. Other adverse events, their treatment details and related cost per each individual patient, are summarized in . Total cost of adverse events and a comparison between SRL and CsA based regimens is shown in .
Table 3 Cost of adverse events per patient in renal transplantation therapy in Iran (2011–2012)
Table 4 Total adverse events cost related to SRL versus CsA based therapies in renal transplantation therapy in Iran (2011–2012)
Analysis
The model was constructed using Microsoft Excel® 2010. The total cost included both cost of immunosuppressive agents and cost of adverse events. The total cost difference between two scenarios was also reported in per-member per-month (PMPM) value, expressed in both IRR and US dollars (US$). According to the official exchange price in the study year, 1 US$ was equal to 12,260 IRR ( and ).
Figure 2 Budget impact of using SRL to replace the current conventional therapy with CsA in Iran (2011–2012).
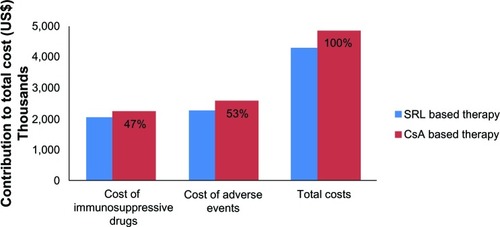
Table 5 Budget impact results of conversion from CsA to SRL in renal transplantation therapy for insurance organizations in Iran (2011–2012)
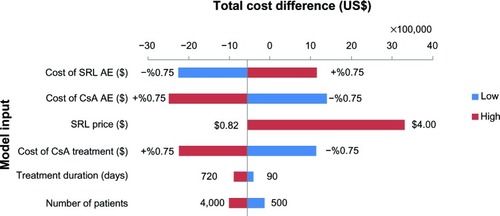
One-way sensitivity analysis was performed to determine robustness of the assessment through evaluation of changes in important variables.Citation8,Citation16 These variables included number of eligible patients per year, SRL market price, treatment duration, cost of immunosuppressive agents in the CsA based therapy, and costs of adverse events for SRL and CsA based regimens. Model parameters were varied by 75% from the base-case value, except treatment duration (which was from 90 to 720 days) and SRL market price (which varied between prices before and after subsidization).
Results
Base-case
According to the model, almost 2,200 patients with ESRD underwent renal transplantation over the study year. The estimated expected 1-year cost of RTT for receiving CsA was almost 60 billion IRR (US$4,850,000) versus 53 billion IRR (US$4,300,000) for patients receiving SRL in their RTT regimen.
These figures included both the cost of immunosuppressive agents (47% of total costs) and the cost of adverse events (53% of total costs) in SRL and CsA based therapies (). These costs corresponded to a cost savings of almost 7 billion IRR (US$550,000) or 33,000 IRR (US$3) PMPM for insurance organizations ().
One-way sensitivity analysis
Sirolimus market price is highly influential and could dramatically change the budget of the new strategy. The results showed that purchasing the drug at price up to almost US$1.2–1.3 per tablet would result in cost savings for the payers (). In addition, the cost of CsA based immunosuppressive therapy and its relative adverse events showed an essential role in the total cost difference. Regarding “number of patients”, the results showed that, even with double the number of patients, the budget savings would also be almost twice greater than the base-case condition. In case of “treatment duration”, by varying from 90 to 720 days, budget savings increased over time ().
Figure 3 Sensitivity analyses results for SRL market price in Iran (2011–2012). At a price between $1.22 and $1.31, the budget difference would be zero.
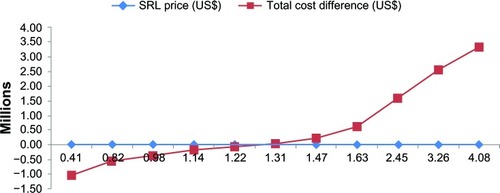
Figure 4 Sensitivity analyses: total BI difference between SRL and CsA based therapies (2011–2012).
Abbreviations: AE, adverse events; BI, budget impact; CsA, cyclosporine a; SRL, sirolimus.
Discussion
Currently, Iran has one of the most successful transplantation programs in the Middle East.Citation14 Notwithstanding renal replacement therapies (transplantation and hemodialysis), which have been grouped as special diseases and receive subsidy along with full reimbursement coverage in Iran, out-of-pocket expenditure is considerable for both of the aforementioned therapies. MOH as the main sponsor and, along with health insurance organizations, they cover almost all medicines and medical services included in the standard guidelines approved to be used in RTT.
However, regarding new expensive medicines and interventions, the cost of medical expenditures in renal replacement therapy is rapidly growing and becoming quite unaffordable for the government; therefore, out-of-pocket payment is dramatically increasing over time. In order to improve quality of care in terms of patient and graft survival, policy makers of insurance organizations should make a choice between newly introduced drugs, which are quite expensive, and current alternatives.Citation15 This condition has led to an increased interest in health economic and financial evaluation of health care programs (cost effectiveness and BIA).Citation16,Citation17
The present study was the first BIA performed in Iran, introducing financial analyses as effective practical policy making tools to the Iranian health budget holders and was conducted in accordance with ISPOR standard guideline for good practice in BIA. It aimed to evaluate the financial consequence of adding mTOR inhibitors to the drug formulary of insurance organizations.Citation8,Citation17,Citation18
Regarding different RTT strategies, in order to improve long-term graft survival and reduce CNI (CsA) toxicity, in recent years, attempts have been made to change immunosuppressive regimen by reducing CNI dosage or replacing them with other agents such as mTOR inhibitors.Citation7
CsA reduction or replacement improves glomerular filtration rate (GFR) by 10%–20%. In addition, studies have shown fewer cases of posttransplantation malignancies and CMV infections in patients receiving mTOR inhibitors compared to CsA.Citation6,Citation7 A common approach is to advise a CNI-free regimen for maintenance therapy based on mTOR inhibitors (such as SRL and everolimus).Citation1,Citation19 Additionally, according to an economic evaluation study performed by McEwan et al in 2005, SRL was cost-effective compared to CsA for 10 to 20 years after renal transplantation in the UK.Citation20
The present model was developed to estimate the financial effect (budgetary impact) of switching from CsA to SRL in RTT from the perspective of Iranian health insurance organizations. Based on the results of this study, considering 2,200 patients had their renal transplantation operation during the study year, the budget impact of conversion from CsA to SRL was minus 7 billion IRR (US$550,000) or minus 33,000 IRR (US$3) PMPM.
The results showed that, although SRL is relatively much more expensive than CsA, mainly because of the lower required doses of MMF (which is also quite expensive) and leading to fewer cases of graft failure, AR, and CMV infection over 12 months after renal transplantation, an SRL based regimen would be less costly compared to conventional CsA based therapies.
In addition, the analysis showed that, with an increase in the number of eligible patients and duration of treatment, the amount of cost saving would consequently increase. Also, considering the preventive effect of mTOR inhibitors in the incidence of posttransplantation malignancies (which commonly occur after 1-year posttransplantation), even more financial savings regarding less resource utilization (eg, hospitalization) are expected if a time horizon of more than 1 year is taken.
The regulated company price of SRL in Iran is almost 50,000 IRR (US$4) per tablet; it has been currently subsidized by MOH at almost US$3 per tablet and the rest (US$1 per tablet) is paid by patients (out-of-pocket), which is not still quite affordable for many patients. It is recommended to health insurers to simply cover the rest of expenditures related to SRL with no additional expenses.
Conclusion
In the Iranian health care system, the MOH is responsible for the performance and also financing of the entire system through subsidization. For health care financing, there are also quasi-governmental health insurance organizations.
mTOR inhibitors as immunosuppressive agents are increasingly administered in RTT maintenance therapy and both patients and physicians are truly satisfied in practice. However, due to quite high out-of-pocket expenditure, many patients cannot afford to receive SRL and continue to use CsA.
To evaluate the financial consequence of adding mTOR inhibitors (SRL as a case) to insurance organizations’ formulary, a BIA was conducted in the current study. According to the results of this analysis, the budget saving of converting from CsA to SRL was almost 33,000 IRR (US$3) PMPM in Iran. Fewer cases of costly adverse events (graft failure, AR, and CMV infection) along with lower required doses of MMF related to SRL based therapies were major reasons for budgetary savings of switching to SRL.
Insurance organizations in Iran could simply purchase the drug with a price range of almost US$0.82 to US$1.30 per tablet with almost no budgetary surplus in RTT. Moreover, with a higher number of patients as well as longer time horizon, savings would be much higher in value compared to the base case condition which is considered the preferred characteristics of drugs recommended for maintenance therapies.
Acknowledgments
The authors would like to thank Dr Zahra Sahraee, Dr Hamid Reza Safikhani, Dr Keyvan Tajbakhsh, Dr Behrang Alipour, Dr Arash Foroutan, and Barakat Pharmaceutical Holding Company for their cooperation in this project. These findings are the result of work supported by Shahid Behashti School of Pharmacy.
Disclosure
The authors report no conflicts of interest in this work.
References
- HanFWuJHuangHConversion from cyclosporine to sirolimus in chronic renal allograft dysfunction: a 4-year prospective studyExp Clin Transplant201191424921605022
- KahanBSirolimus: a new agent for clinical renal transplantationTransplant Proc1997291–248509123092
- GrothCGBäckmanLMoralesJMSirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study GroupTransplantation19996771036104210221490
- MorathCArnsWSchwengerVSirolimus in renal transplantationNephrol Dial Transplant200722Suppl 8viii61viii6517890266
- WebsterACLeeVWChapmanJRCraigJCTarget of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trialsTransplantation20068191234124816699448
- BüchlerMCaillardSBarbierSSPIESSER GroupSirolimus versus cyclosporine in kidney recipients receiving thymoglobulin, mycophenolate mofetil and a 6-month course of steroidsAm J Transplant20077112522253117868057
- NafarMAlipourBAhmadpoorPSirolimus versus calcineurin inhibitor-based immunosuppressive therapy in kidney transplantation: a 4-year follow-upIran J Kidney Dis20126430030622797101
- MauskopfJASullivanSDAnnemansLPrinciples of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices – budget impact analysisValue Health200710533634717888098
- Mahdavi-MazdehMRouchiAHNorouziSAghighiMRajolaniHAhrabiSRenal replacement therapy in IranJ Urol2007426670
- MehrdadRHealth system in IranJMAJ20095216973
- HajizadehMNghiemHSOut-of-pocket expenditures for hospital care in Iran: who is at risk of incurring catastrophic payments?Int J Health Care Finance Econ201111426728521915727
- MacDonaldASRAPAMUNE Global Study GroupA worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allograftsTransplantation200171227128011213073
- CravediPRuggenentiPRemuzziGSirolimus for calcineurin inhibitors in organ transplantation: contraKidney Int201078111068107420703217
- EinollahiBKidney Transplantation in IranIran J Med Sci201035118
- KhosroshahiHTShort history about renal transplantation program in Iran and the world: Special focus on world kidney dayJ Nephropathology201211510
- NuijtenMJMittendorfTPerssonUPractical issues in handling data input and uncertainty in a budget impact analysisEur J Health Econ201112323124120364289
- OrlewskaEMierzejewskiPProposal of Polish guidelines for conducting financial analysis and their comparison to existing guidance on budget impact in other countriesValue Health20047111014720125
- MarshallDADouglasPRDrummondMFGuidelines for conducting pharmaceutical budget impact analyses for submission to public drug plans in CanadaPharmacoeconomics200826647749518489199
- FlechnerSMReviewing the evidence for de novo immunosuppression with sirolimusTransplant Proc200840Suppl 10S25S2819100902
- McEwanPBaboolalKConwayPCurrieCJEvaluation of the cost-effectiveness of sirolimus versus cyclosporin for immunosuppression after renal transplantation in the United KingdomClin Ther200527111834184616368455