Abstract
Background
Biologic therapies represent a significant advance in the treatment of psoriasis. However, no studies have examined the patient characteristics predictive of biologic treatment of psoriasis. The purpose of this study was to ascertain the frequency and predictors of treatment of psoriasis with biologics in three European countries, ie, France, Spain, and the UK.
Methods
This was a cross-sectional analysis of physician-recorded demographic and clinical data on patients receiving either conventional or biologic treatments for psoriasis. Data were drawn from the Adelphi 2007 Psoriasis Disease Specific Program (DSP®), a multinational, real-world survey of patients with psoriasis consulting practicing dermatologists. The numbers of patients treated with biologic and nonbiologic agents were recorded. Data were subjected to bivariate analysis according to treatment regimen (biologic versus nonbiologic). Predictors of treatment with biologics were identified by logistic regression analysis.
Results
A total of 2,509 psoriasis patients were included in this study (1,374 from France, 561 from Spain, and 574 from the UK). Biologic use was most prevalent in Spain (19.4% of patients), followed by the UK (9.1%), and France (8.4%). In the logistic regression analysis, psoriatic arthritis was a statistically significant predictor of increased biologic use in France (odds ratio [OR] 5.38, 95% confidence interval [CI] 3.32–8.77), Spain (OR 2.71, 95% CI 1.16–6.33), and the UK (OR 8.70, 95% CI 3.65–20.83). Physician-assessed moderate-to-severe disease was also a statistically significant predictor of increased biologic use in France (OR 5.08, 95% CI 2.01–12.82), Spain (OR 11.11, 95% CI 4.33–28.57), and the UK (OR 8.55, 95% CI 1.11–66.67).
Conclusion
In this study, an average of about one tenth of psoriasis patients enrolled in Spain, France, and the UK were treated with biologics in 2007. Physician-assessed moderate-to-severe disease and presence of psoriatic arthritis were significantly associated with biologic use in all three countries.
Introduction
Psoriasis is a chronic, genetically based, and immune-mediated inflammatory disorder affecting 2%–3% of the Caucasian population in western countries.Citation1 These plaques may be localized or widespread across the body.Citation2 The fingernails and toenails are involved in 40% of psoriasis cases, with thickening and/or pitting of the nails as common manifestations.Citation3–Citation5
The chronic inflammation underlying psoriasis affects the joints as psoriatic arthritis. The prevalence of psoriatic arthritis among patients with plaque psoriasis ranged from 5.9% to 23.9% (median 11.2%) in eight US and European studies using rheumatologically validated criteria.Citation6 Cardiovascular risk factors such as obesity, metabolic syndrome, and hypertension are frequent comorbidities of psoriasis,Citation7–Citation10 possibly due to common inflammatory pathways.Citation11
In limited (mild) disease, the most commonly used therapy is topical with the addition of phototherapy in refractory cases. In moderate-to-severe psoriasis, phototherapy alone combined with systemic therapy or systemic therapy alone is recommended. Recent guidelines present the level of evidence for the efficacy of the available therapies and give recommendations for their use in daily practice.Citation12
Significant advances have been made in the treatment of psoriasis with the availability of biologic therapies. Biologics approved for the treatment of psoriasis in Europe are the tumor necrosis factor-alpha antagonists, ie, infliximab, etanercept, and adalimumab, and the anti-p40 agent, ustekinumab.Citation2
European and UK guidelines recommend biologics only for patients with moderate-to-severe psoriasis who have not responded to or are intolerant of conventional therapies.Citation13–Citation17 In a recent European consensus, moderate-to-severe psoriasis was defined as a body surface area >10 or psoriasis area and severity index (PASI) >10 and dermatology life quality index (DLQI) >10.Citation18 The British Association of Dermatologists recommends biologics only for patients who have severe psoriasis, defined as a PASI score ≥10 and a DLQI score >10.Citation18 These criteria are less stringent than those of the UK National Institute for Health and Clinical Excellence for use of infliximab in psoriasis, which recommend infliximab only for very severe psoriasis, defined as a PASI score ≥20 and a DLQI score >18.Citation19
These guidelines provide insight into which patients are eligible for treatment with biologics, but there is little information in the literature about the determinants of treatment of psoriasis with biologics in clinical practice. This study was designed to examine variables associated with biologic treatment in populations of psoriasis patients from France, Spain, and the UK.
Materials and methods
Study design
This was a cross-sectional analysis of physician-recorded demographic and clinical data on patients receiving treatment for psoriasis. Data were drawn from the Adelphi 2007 Psoriasis Disease Specific Program (DSP®), a multinational, real-world survey of patients with psoriasis consulting practicing dermatologists. Physicians were identified from the public lists of health care professionals and were screened for eligibility based on the criteria of whether they were licensed physicians between 1970 and 2006, specialized in dermatology, and actively managing ten or more psoriasis patients per month. Each physician was asked to recruit 8–10 consecutive patients presenting with psoriasis in his/her office. One hundred and forty-three physicians were recruited from France and 61 physicians were recruited from both Spain and the UK (n=265) to complete a detailed patient record form for patients presenting with psoriasis and receiving a prescription for topical agents, phototherapy, or systemic treatment during routine visits in the calendar year 2007. Patients who were 18 years or older and diagnosed as having psoriasis were recruited. Patients who had a missing value for age or gender were excluded from the data analysis.
Variables
The patient record form included demographics, body mass index, disease symptoms and severity determined by PASI scores, current and previous treatments, patient-reported disease management strategies, the physician’s assessment of current severity and severity at diagnosis, the patient’s PASI score, and physician-reported reasons for clinical decisions and prescribing. PASI scores were based on the extent of psoriasis in four specific body surface areas (head, trunk, upper limbs, and lower limbs) and the degree of plaque erythema (redness), scaling, and thickness as described by Fredriksson and Pettersson.Citation20 The numeric PASI score ranges from 0 (absence of disease) to 72 (maximal disease). The severity of disease was determined from the PASI scores, and the proportions of patients with mild (PASI score <10) and moderate-to-severe (PASI score ≥10) disease. Obesity was defined as a body mass index ≥30. Nonbiologic treatments in this study included topical (eg, corticosteroids, vitamin D3), phototherapy (eg, natural sunlight or ultraviolet A or B irradiation), and traditional systemic (eg, methotrexate, cyclosporine). Biologics in this study included infliximab, etanercept, adalimumab, and efalizumab.
Statistical analysis
Patient characteristics were analyzed descriptively for the total study samples from each country and then assessed in a bivariate analysis according to treatment with biologics (yes or no). The statistical significance of the distributions across treatment was determined by Student’s t-test for continuous data (age and PASI score) and by chi-square or Fisher’s Exact tests for categorical data (sex [male, female], obesity [yes, no], nail involvement [yes, no], and psoriatic arthritis [yes, no]). The availability of PASI scores in the Adelphi database was <20%, so we developed an algorithm to impute the PASI score for missing values. Potential predictors of the PASI score, ie, severity of body areas affected (11 specific body areas), symptom severity (15 specific symptoms), and percentage of body surface area affected, were introduced stepwise into a multiple fractional polynomial model to derive the polynomials that best predicted the PASI score for each variable. A generalized estimating equations model was used to adjust for clustering of the predictors (severity of body areas affected, symptom severity, and percentage of body surface area affected). After applying this algorithm, the correlation between actual and predicted PASI scores was R=0.51.
Physician-assessed severity was classified as mild, moderate, or severe, and the frequencies of each level of severity were determined by country. A chi-square test was used to determine the statistical significance of differences in the frequency distributions. The correlation between PASI scores (measured or imputed) and physician-assessed severity (mild, moderate, or severe) was then assessed by a Spearman analysis in patients for whom both variables were available. We used a logistic regression model to estimate the adjusted odds of receiving treatment with a biologic. Independent variables were age, sex, obesity, physician-assessed severity, PASI score, nail involvement, and psoriatic arthritis (defined as in the descriptive analysis). Finally, the severity of disease was determined from the PASI scores of patients, and the proportions with mild (PASI score <10) and moderate-to-severe (PASI score ≥10) disease were compared between patients with psoriatic arthritis and those with psoriasis only. For all analyses, P<0.05 was considered to be statistically significant.
Results
Patient characteristics
A total of 2,588 patients with psoriasis were included in this study. Seventy-nine patients were excluded due to missing values for age or gender. Finally a total of 2,509 psoriasis patients were included in the statistical analysis (1,374 from France, 561 from Spain, and 574 from the UK, ). The mean age ranged from 42.4 years (Spain) to 46.6 years (France), and men accounted for 52.4% (UK) to 58.7% (Spain) of the population. Patients had been diagnosed with psoriasis at a mean of 9.2 (Spain) to 14.8 (UK) years earlier. The mean PASI score ranged from 9.6 (Spain) to 10.4 (UK). The proportion of patients with psoriatic arthritis ranged from 6.2% (Spain) to 13.0% (UK). Approximately one quarter of patients in France (25.8%) and Spain (29.4%) had nail involvement, but the percentage was numerically higher in the UK (42.5%). In our bivariate assessment of patient characteristics associated with biologic use, psoriatic arthritis was significantly associated with biologic use in all three countries. Obesity, PASI score, and nail involvement were also found to be significantly associated with biologic use in patients from France and Spain.
Table 1 Patient characteristics according to treatmentCitation3
Patient characteristics associated with biologic use
Use of biologics was reported in 11.2% (n=282) of the total patient population (). Biologic use was most prevalent in Spain (19.4%), followed by France (9.1%) and the UK (8.4%). Among patients with physician-assessed mild disease, biologic use was 8.2% in France, 12.8% in Spain, and 7.1% in the UK (). Among patients with physician-assessed moderate-to-severe psoriasis, biologic use was 27.8% in Spain, followed by France (9.7%) and the UK (9.3%). Physician-assessed moderate-to-severe disease was also significantly associated with biologic use in all three countries (all comparisons P<0.0005).
Table 2 Patient treatment by country
Table 3 Patient biologic treatment according to physician-assessed disease severity
Distribution of psoriasis severity and correlation with PASI score
The distribution of physician-assessed psoriasis severity varied significantly between countries (P<0.001, ). There were more patients with mild disease than moderate disease and severe disease in Spain (55.6%, 37.6%, and 6.6%, respectively) and the UK (43.9%, 39.4%, and 16.7%, respectively). There were more patients with moderate disease than mild disease and severe disease in France (44.8%, 40.6%, and 14.6%, respectively). Physician-assessed psoriasis severity correlated strongly with PASI scores in each country (Spearman coefficient R=0.699, ).
Table 4 Physician-assessed current severityTable Footnotea
Table 5 PASI score by physician-assessed current severityTable Footnotea
Predictors of biologic use
In the logistic regression analysis (), psoriatic arthritis was a statistically significant predictor of increased biologic use in France (odds ratio [OR] 5.38,95% confidence interval [CI] 3.32–8.77), Spain (OR 2.71, 95% CI 1.16–6.33), and the UK (OR 8.70, 95% CI 3.65–20.83). Consistent with this is our finding that patients with psoriatic arthritis had a greater prevalence of PASI score-based moderate-to-severe disease as compared with those patients with psoriasis only ().
Figure 1 Distribution of psoriasis severity* in patients with (A) psoriatic arthritis and (B) psoriasis only.
Abbreviation: PASI, Psoriasis Area and Severity Index.
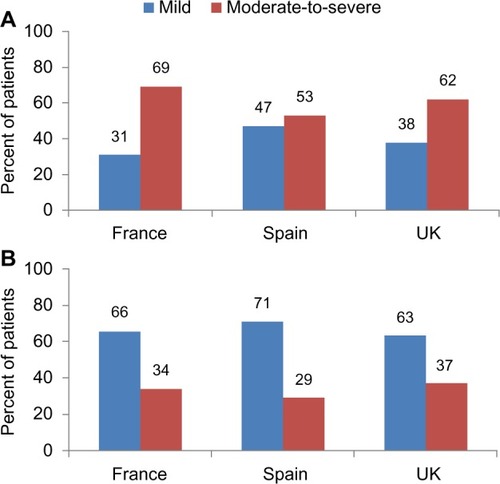
Table 6 Predictors of current biologic treatment
Physician-assessed moderate-to-severe disease was also a statistically significant predictor of increased biologic use in France (OR 5.08, 95% CI 2.01–12.82), Spain (OR 11.11, 95% CI 4.33–28.57), and the UK (OR 8.55, 95% CI 1.11–66.67).
No other variables were consistent across countries, although higher PASI scores increased the odds of using biologics in France (OR 1.05, 95% CI 1.02–1.08) and Spain (OR 1.02, 95% CI 1.00–1.05). Obesity was found to be associated with increased odds of biologic use only in France (OR 2.29, 95% CI 1.33–3.95).
Discussion
In this study, an average of about one tenth of psoriasis patients enrolled in Spain, France, and the UK were treated with biologics in 2007. The European Medicines Agency requires that only those patients with moderate-to-severe psoriasis and an inadequate response to conventional systemic medications (or adverse effects/contraindication to these agents) are eligible for prescription of biologics in the European Union.Citation13–Citation16 The primary caveat to the assessment of patient eligibility for biologic treatment in this study is the lack of information in the Adelphi database on patient history regarding response to conventional therapies. Although a determination of moderate-to-severe disease implies prior therapies had been ineffective, the proportion of patients who had failed to respond to conventional therapies was unknown, and therefore the true percentage of eligible patients cannot be determined. However, the mean PASI score was found to be significantly higher among patients on biologic treatment than in those with nonbiologic treatment in France (P<0.0001) and in Spain (P<0.0005). There was no significant difference in PASI score between patients with biologic treatment and those with nonbiologic treatment in the UK. In support of our assumption that this determination was accurate is the median PASI score, which was 8.2 (mean 9.5), indicating that nearly 50% of patients had moderate-to-severe psoriasis on the basis of PASI score. In comparison, 39.0% of patients with psoriasis had moderate-to-severe disease based on a PASI score ≥10 in a study of patients attending dermatology clinics in Germany.Citation21 Similarly, a multinational survey in Eastern Europe found that 37% of patients had severe disease, defined as a PASI score ≥10 and a DLQI >10 (the same as the British Association of Dermatologists’ criteria for moderate-to-severe psoriasis), and were thus considered eligible for treatment with biologics.Citation22 Determinations of the prevalence of moderate-to-severe psoriasis based on the percentage of body surface area affected are available from the USCitation23 and Canada,Citation24 but they vary widely (17%–95% of patients) and may not correlate well with PASI-based determinations. However, a survey of California dermatologists in which physician-assessed severity was reported showed that 41% of psoriasis patients had moderate-to-severe disease.Citation25
Large differences between the adjusted OR and unadjusted OR can happen with confounding factors introduced. In this study, greater psoriasis severity, as indicated by PASI score, was not independently associated with biologic treatment. This suggests that European and UK guidelines may not strongly influence treatment decisions. However, physician-assessed severity was found to be a significant predictor of biologic use in all three countries. It is interesting to note that a subjective assessment of physician severity, which was done by dermatologists presumably familiar with the diagnosis and management of psoriasis, was found to be a significant predictor, and not PASI score, which is an objective measure. A physician’s severity assessment may be driving the treatment decision to prescribe biologics rather than an objective measure in clinical practice. However, the lack of association might speak to the imputation method used to calculate PASI score for those patients where it was missing.
The PASI index does not specifically include nail psoriasis or the presence of psoriatic arthritis, which may reflect greater disease severity. Although these other variables related to disease severity were negatively associated with biologic treatment in the bivariate analyses, psoriatic arthritis was predictive of treatment with biologics in the regression model (statistically significant for Spain, numerically so for France), consistent with the greater disease severity observed in patients with psoriatic arthritis. According to Spanish guidelines, the presence of arthritis is a criterion for prescription of biologics.Citation26 Because of the cross-sectional design of this study, we could not tell whether biologic use was the reason for lower PASI scores. We must speculate that the effectiveness of biologic therapy was at least part of the reason for the negative association.
To date there are no reliable predictors of response to tumor necrosis factor-alpha antagonists as a class (regardless of disease),Citation27 and few studies have been performed to identify genetic predictors of response to biologics in psoriasis.Citation28 Studies of variables associated with the clinical response of psoriatic arthritis to tumor necrosis factor-alpha antagonists have identified younger ageCitation29,Citation30 and male sex.Citation29,Citation31 In the present study, both age and female sex were not predictive of treatment with biologics.
Several limitations in this analysis stem from the data source. The data set refers to treatment patterns in 2007, which do not necessarily reflect current trends in biologic use. The proportions of patients seen in this study are not representative of psoriasis patients in general. The majority of mild-moderate patients are seen primarily by primary care practices. Further, as noted above, there was no documentation of prior failure to respond to conventional therapies. Data on PASI scores were not available for all patients. The paucity of PASI scores in the database required imputation of scores for most of the study population. As stated in the Materials and methods section, the correlation between actual and predicted PASI scores was moderate (R=0.51). In addition, we observed no difference between actual and imputed mean PASI scores for patients treated with biologics versus patients treated with nonbiologics (including topicals only, phototherapy, and conventional systemic therapy).
In conclusion, an average of about one tenth of the psoriasis patients enrolled in this study in Spain, France, and the UK were treated with biologics. Physician-assessed moderate-to-severe disease and the presence of psoriatic arthritis were significantly predictive of biologic use in all three countries.
Disclosure
This study was supported by Merck and Co, Inc. The authors thank Lauren Weisenfluh and Melissa Stauffer, in collaboration with Scribco, for medical writing assistance. TF, QD, and NS are employees of Merck and Co, Inc. LP has no conflict of interest to report.
References
- NestleFOKaplanDHBarkerJNPsoriasisN Engl J Med200936149650919641206
- PathiranaDOrmerodADSaiagPEuropean S3-guidelines on the systemic treatment of psoriasis vulgarisJ Eur Acad Dermatol Venereol200923Suppl 217019712190
- de JongEMSeegersBAGulinckMKBoezemanJBvan de KerkhofPCPsoriasis of the nails associated with disability in a large number of patients: results of a recent interview with 1,728 patientsDermatology199619343003038993953
- SalomonJSzepietowskiJCProniewiczAPsoriatic nails: a prospective clinical studyJ Cutan Med Surg20037431732112879333
- AugustinMReichKBlomeCSchäferILaassARadtkeMANail psoriasis in Germany: epidemiology and burden of diseaseBr J Dermatol2010163358058520456340
- PreySPaulCBronsardVAssessment of risk of psoriatic arthritis in patients with plaque psoriasis: a systematic review of the literatureJ Eur Acad Dermatol Venereol201024Suppl 2313520443998
- AugustinMReichKGlaeskeGSchaeferIRadtkeMCo-morbidity and age-related prevalence of psoriasis: analysis of health insurance data in GermanyActa Derm Venereol201090214715120169297
- PreySPaulCBronsardVCardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studiesJ Eur Acad Dermatol Venereol201024Suppl 2233020443997
- DreiherJWeitzmanDDavidoviciBShapiroJCohenADPsoriasis and dyslipidaemia: a population-based studyActa Derm Venereol200888656156519002339
- CohenADWeitzmanDDreiherJPsoriasis and hypertension: a case-control studyActa Derm Venereol2010901232620107721
- TamLSTomlinsonBChuTTCardiovascular risk profile of patients with psoriatic arthritis compared to controls – the role of inflammationRheumatology200847571872318400833
- MenterAKormanNJElmetsCAGuidelines of care for the management of psoriasis and psoriatic arthritis. Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapyJ Am Acad Dermatol201062111413519811850
- European Medicines AgencyUstekinumab (Stelara) Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000958/human_med_001065.jsp&murl=menus/medicines/medicines.jsp&jsenabled=trueAccessed December 5, 2013
- European Medicines AgencyAdalimumab (Humira) Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000481/smops/Positive/human_smop_000184.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d127Accessed December 5, 2013
- European Medicines AgencyInfliximab (Remicade) Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000240/human_med_001023.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124&jsenabled=trueAccessed December 5, 2013
- European Medicines AgencyEtanercept (Enbrel) Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000262/human_med_000764.jsp&mid=WC0b01ac058001d125&murl=menus/medicines/medicines.jsp&jsenabled=trueAccessed December 5, 2013
- SmithCHAnsteyAVBarkerJNBritish Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009Br J Dermatol20091615987101919857207
- MrowietzUKragballeKReichKDefinition of treatment goals for moderate-severe psoriasis: a European consensusArch Dermatol Res2011303111020857129
- National Institute of Health and Clinical ExcellenceInfliximab for the treatment of adults with psoriasis2008NICE technology appraisal guidance 134 Available from: http://www.nice.org.uk/nicemedia/pdf/ta134guidance.pdfAccessed December 5, 2013
- FredrikssonTPetterssonUSevere psoriasis – oral therapy with a new retinoidDermatologica19781574238244357213
- AugustinMReichKReichCQuality of psoriasis care in Germany – results of the national study PsoHealth 2007J Dtsch Dermatol Ges20086864064518801145
- PalotaTSzepietowskiJCPecJA survey of disease severity, quality of life, and treatment patterns of biologically naive patients with psoriasis in central and eastern EuropeActa Dermatovenerol Croat201018315116120887696
- KurdSKGelfandJMThe prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004J Am Acad Dermatol200960221822419022533
- MahlerRJacksonCIjacuHThe burden of psoriasis and barriers to satisfactory care: results from a Canadian patient surveyJ Cutan Med Surg200913628329319919805
- PatelVHornEJLoboscoSJFoxKMStevensSRLebwohlMPsoriasis treatment patterns: results of a cross-sectional survey of dermatologistsJ Am Acad Dermatol200858696496918378352
- PuigLCarrascosaJMCarreteroGSpanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. Part 1: On efficacy and choice of treatmentActas Dermosifiliogr2013104869470924018211
- SfikakisPPThe first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directionsCurr Dir Autoimmun20101118021020173395
- HébertHLAliFRBowesJGriffithsCEBartonAWarrenRBGenetic susceptibility to psoriasis and psoriatic arthritis: implications for therapyBr J Dermatol2012166347448222050552
- GlintborgBØstergaardMDreyerLTreatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor alpha therapy: results from the nationwide Danish DANBIO registryArthritis Rheum201163238239021279995
- IervolinoSDi MinnoMNPelusoRPredictors of early minimal disease activity in patients with psoriatic arthritis treated with tumor necrosis factor-alpha blockersJ Rheumatol201239356857322247361
- Van den BoschFMangerBGoupillePEffectiveness of adalimumab in treating patients with active psoriatic arthritis and predictors of good clinical responses for arthritis, skin and nail lesionsAnn Rheum Dis201069239439919815494
