Abstract
Background
Intravenous patient-controlled analgesia (PCA) equipment and opioid cost analyses on specific procedures are lacking. This study estimates the intravenous PCA hospital cost for the first 48 postoperative hours for three inpatient surgeries.
Methods
Descriptive analyses using the Premier database (2010–2012) of more than 500 US hospitals were conducted on cost (direct acquisition and indirect cost for the hospital, such as overhead, labor, pharmacy services) of intravenous PCA after total knee/hip arthroplasty (TKA/THA) or open abdominal surgery. Weighted average cost of equipment and opioid drug and the literature-based cost of adverse events and complications were aggregated for total costs.
Results
Of 11,805,513 patients, 272,443 (2.3%), 139,275 (1.2%), and 195,062 (1.7%) had TKA, THA, and abdominal surgery, respectively, with approximately 20% of orthopedic and 29% of abdominal patients having specific intravenous PCA database cost entries. Morphine (57%) and hydromorphone (44%) were the most frequently used PCA drugs, with a mean cost per 30 cc syringe of $16 (30 mg) and $21 (6 mg), respectively. The mean number of syringes used for morphine and hydromorphone in the first 48 hours were 1.9 and 3.2 (TKA), 2.0 and 4.2 (THA), and 2.5 and 3.9 (abdominal surgery), respectively. Average costs of PCA pump, intravenous tubing set, and drug ranged from $46 to $48, from $20 to $22, and from $33 to $46, respectively. Pump, tubing, and saline required to maintain patency of the intravenous PCA catheter over 48 hours ranged from $9 to $13, from $8 to $9, and from $20 to $22, respectively. Supplemental non-PCA opioid use ranged from $56 for THA to $87 for abdominal surgery. Aggregated mean intravenous PCA equipment and opioid cost per patient were $196 (THA), $204 (TKA), and $243 (abdominal surgery). Total costs, including for adverse events, complications, and intravenous PCA errors, ranged from $647 to $694.
Conclusion
Although there is variation between different types of surgery, the hospital cost of intravenous PCA after major surgery is substantial. Novel technology should demonstrate cost-effectiveness in addition to clinical superiority.
Introduction
Although intravenous patient-controlled analgesia (PCA) with opioids has been utilized for over 40 years to manage postoperative pain, the cost of providing this method of pain control for patients is not insignificant. This treatment modality continues to be used because there is little debate in the literature that patients having control over their pain medication have lower pain scores and higher patient satisfaction compared with nurse-administered modalities.Citation1,Citation2 The rationale for PCA is that better pain control is achieved by use of small, frequent doses of opioids, while avoiding the risks of overdosing and analgesic gaps that can occur with large, infrequent bolus administration of opioids by health care providers.
While the patient-controlled aspect of intravenous PCA is a clear advantage, the invasive nature of the delivery system and the programming required to match the selected drug and dosing parameters with the delivery device (intravenous PCA pump) are obvious disadvantages. The requirement of a patent intravenous line, necessitating an indwelling intravenous catheter and intravenous tubing that tethers the patient to a computerized pump attached to an intravenous pole, results in a risk of infection, reduced mobility, and analgesic gaps due to infiltration of the intravenous catheter or obstruction of the tubing. Programming of the pump, which is performed by the nurse setting up the device, can result in dosing errors.Citation3–Citation6 Furthermore, the frequency of patient dosing with intravenous PCA is usually 1–3 times per hour, which is not frequent enough to maintain patency of the intravenous catheter. This necessitates additional set-up of an intravenous infusion pump, tubing set, and saline bag to run the intravenous fluid at a “to keep open” rate throughout use of intravenous PCA therapy. Lastly, following major surgeries that result in moderate-to-severe postoperative pain, such as joint replacement or open abdominal surgery, patients utilizing intravenous PCA opioids often have inadequate analgesia and attempt to redose during lockout, which would then require additional nurse-administered opioid treatment as rescue analgesia.Citation7
The purpose of this study was to analyze the equipment and drug costs for US hospitals providing intravenous PCA opioid treatment for patients following major surgery using the Premier hospital database, which is the largest inpatient resource and drug utilization database and contains complete billing and coding history on more than 45 million hospital discharges for acute care, ambulatory, surgery center and clinic visits.Citation8 In addition, a literature analysis of the additional costs due to adverse events occurring in patients using intravenous PCA opioids was included to estimate a complete total cost from the hospital payer perspective.
Materials and methods
Descriptive analyses using the Premier database (2010–2012) for more than 500 US hospitals were conducted to determine the hospital cost of intravenous PCA after total knee arthroplasty (TKA), total hip arthroplasty (THA), and open abdominal surgery. The International Classification of Diseases-9 (ICD-9) codesCitation9 used to identify surgeries were: 81.54 and v43.65 for TKA; 81.51 and v43.64 for THA; 54.11, 51.21, 51.22, 68.49, 45.73, 45.75, 17.33, 17.35, 55.5x, 45.76, 45.74, 45.8x, and 41.5 for abdominal surgery. Current Procedural Terminology codesCitation10 applied were 27130 for TKA, 27447 for THA, and 49000, 47600, 47605, 47610, 58150, 44140, 44204, 50220, and 38100 for abdominal surgery. Direct acquisition and indirect costs (eg, labor and pharmacy) were included in all costs. The study estimated the cost of the first 48 hours of postoperative management by cost of day 0 to day 2 from the surgery. The key search term “PCA” in Premier standard charge codes was conducted to identify PCA-related charges. The four most commonly used intravenous PCA opioid drugs (morphine, hydromorphone, fentanyl, and meperidine) were used to search for PCA drug cost entries. The term “intravenous” was explicitly specified only in bills of extension set and PCA opioid drugs but not in pump-related entries. In order to focus on intravenous PCA patients, a patient was categorized as an epidural-only patient if he or she had only an epidural PCA pump but no intravenous extension or intravenous opioid drugs recorded, so was excluded from the analysis population. Not all patients have complete entries for each component in the database, so each component (pump, set, and drug) was analyzed separately, and the weighted average for total PCA-related costs was analyzed.
The cost of supplemental non-PCA opioid drugs as well as non-PCA intravenous infusion pumps, intravenous tubing, and saline bags (to maintain the patency of the PCA catheter) during the first 48 hours postoperatively were also analyzed separately as part of the total cost. The cost of supplemental opioid drugs on day 1 after surgery was multiplied by two to approximate the cost of the first 48 hours given that the cost incurred on day 0 might include intraoperative opioid use and the cost on day 2 might include use beyond 48 hours. It was further assumed that for non-PCA carrier supplies, one infusion pump, one intravenous tubing set, and two 1,000 mL saline bags (50 mL/hour) were used over 48 hours.
In order to calculate the total cost of intravenous PCA, the medical literature was used to obtain the frequency and costs of specific adverse events and complications associated with intravenous PCA. The average per patient cost of intravenous PCA complications was calculated using a bottom-up microcosting approach, with the frequency of specific events multiplied by a literature-based cost. The costs of the specific complications from the literature were then added to the drug and equipment costs derived from the Premier database. Costs from the literature were inflated to a common year of 2012 (the end year of the Premier database) for consistency using the medical component of the Consumer Price Index.Citation11
Results
Premier database analysis
Of 11,805,513 patients with at least one inpatient stay, 272,443 (2.3%), 139,275 (1.2%), and 195,062 (1.7%) had TKA, THA, and abdominal surgery, respectively. Patient attrition counts by surgical procedure are shown in . Approximately 20% of orthopedic patients and 29% of abdominal surgery patients had specific intravenous PCA database cost entries.
Figure 1 Patient attrition flow chart.
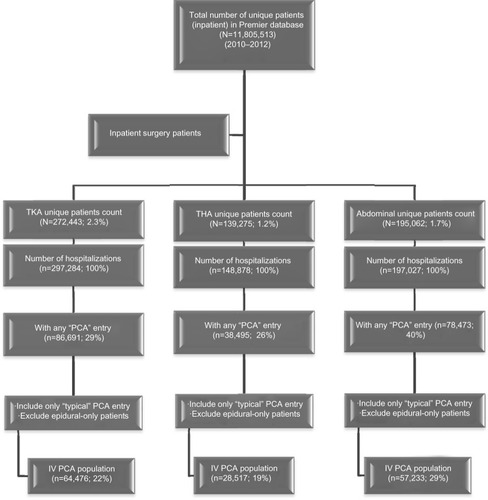
Of the patients in the three separate surgical groups, morphine (56%–57%) and hydromorphone (42%–44%) were the most frequently used PCA drugs. The cost per syringe (30 cc) of drug was consistent across the three types of surgery, with mean values at approximately $16 (30 mg) and $21 (6 mg) for morphine and hydromorphone, respectively. The respective numbers of syringes used for morphine and hydromorphone were 1.9 and 3.2 for TKA, 2.0 and 4.2 for THA, and 2.5 and 4.0 for abdominal surgery ().
Table 1 Cost per syringe of intravenous PCA opioid drugs (day 0 to 2 from surgery)
In , the cost of each individual resource item is listed and weighted to obtain the average cost for the PCA pump, intravenous extension set, and intravenous PCA opioid drugs. “Pump PCA per day” (77%–83%) and “set intravenous extension PCA” (42%–47%) were the most frequently billed terms for the PCA pump and extension set, respectively. It was assumed that a typical intravenous PCA patient would incur the average cost of each component mentioned above plus the cost of non-PCA intravenous fluid carrier supplies (ie, intravenous infusion pump, tubing, and saline bags) to maintain patency of the intravenous PCA catheter. Average costs for the PCA pump, intravenous tubing set, and opioid drugs ranged from $46 to $48, from $20 to $22, and from $33 to $46, respectively. Use of supplemental non-PCA opioids during intravenous PCA ranged from a low of $56 for THA to $86 for abdominal surgery. The additional cost of the non-PCA pump, tubing, and saline over 48 hours ranged from $9 to $13, from $8 to $9, and from $20 to $22, respectively. The aggregated mean total intravenous PCA/opioid costs per patient were $196 for THA, $204 for TKA, and $243 for abdominal surgery.
Table 2 Per patient total cost of equipment and opioid drugs (day 0 to 2 from surgery)
Published literature on costs of complications of intravenous PCA
Peripheral line infection and needle-stick injury
Assessment of the risk of infection due to peripheral venous lines has been performed using the Cochrane database.Citation12 The risk of phlebitis ranged from 7% to 9% depending on routine replacement versus replacement for clinical indication, while the risk of bacteremia ranged from 0.2% to 0.4%. Treatment for catheter-related peripheral venous phlebitis requires replacement of the intravenous catheter and tubing to a new site, and this cost has been estimated at $24.50 (2008 US dollars), including cost of equipment and nursing time.Citation13 Published costs of treatment for bacteremia due to an indwelling vascular catheter range from $24,034 to $36,441 per episode.Citation14,Citation15 While the addition to average overall hospitalization costs of intravenous PCA patients due to development of phlebitis is low ($2.18), the incremental cost due to bacteremia is higher ($106.76).
The cost of needle-stick injury in health care workers is also a concern when utilizing intravenous PCA over noninvasive analgesic modalities. The rate of hollow-bore intravenous tubing needle-stick injuries is reported at 36.7 per 100,000 devices purchased.Citation16 The direct medical costs associated with initial and follow-up treatment of exposed health care workers has been documented to be $3,437 based on the treatment provided,Citation17 which results in a relatively small incremental cost of $1.26 compared with the average overall hospitalization costs for intravenous PCA patients.
Intravenous PCA errors
The morbidity and mortality due to intravenous PCA errors, mainly due to human factors such as misprogramming, has been well documented over the years.Citation3–Citation6 Meissner et alCitation5 have performed the most extensive analysis to date regarding the cost of such intravenous PCA errors as incorrect flow rates, missing decimal places, dosage strength errors (μg versus mg), and malfunctions of the intravenous PCA device itself. We evaluated both the MEDMARX and Manufacturer and User Facility Device Experience (MAUDE) datasets for errors related to intravenous PCA. The average cost per error event was $733 (MEDMARX) and $552 (MAUDE) which, annualized in 2006 dollars, resulted in an additional $388 million for medication-related errors (MEDMARX data) and $12 million for device-related errors (MAUDE data) for the cost of health care in the USA. Based on the reported rate of 407 intravenous PCA medication errors and 17 device-related errors per 10,000 intravenous PCA patients annually, the addition to the average overall hospitalization costs for intravenous PCA patients due to these errors is $35.52.
Summary of total costs of intravenous PCA
Based on the data available from the Premier database and the complications directly attributable to the invasive and programmable nature of intravenous PCA reported in the literature, the total cost of postoperative delivery of opioids in the first 48 hours after surgery via a regimen involving intravenous PCA is tabulated in . The average cost of each component of total hospital cost is also shown in .
Figure 2 Average hospital cost component of IV PCA.
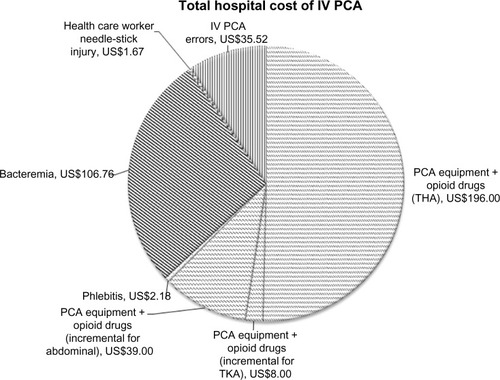
Table 3 Total average per patient cost of postoperative pain management via intravenous PCA
Adverse events due to decreased mobility
Although not directly attributable to intravenous PCA per se, the cost of certain adverse events related to decreased ambulation after surgery should not be ignored. Common adverse events are deep venous thrombosis, pulmonary embolism, and postoperative pneumonia. A recent assessment of the relative cost of these events in patients utilizing intravenous PCA opioids for postoperative pain control found that 2.1% of intravenous PCA patients, compared with 1.6% of patients using nonintravenous PCA opioids, reported these complications over the first 2 days postoperatively. The addition to the average overall hospitalization costs of intravenous PCA patients due to development of deep venous thrombosis was $18.17, for pulmonary embolism was $43.19, and for postoperative pneumonia was $265.02.Citation18
Discussion
Analysis of the Premier database demonstrates that the cost of intravenous PCA equipment and opioid drugs in the first 48 hours following major surgery ranges from $196 to $243. Hospitals have been willing to cover these costs, as studies have shown the advantages of providing a patient-controlled option for patients suffering from acute pain in the hospital setting. As hospitals look to decrease costs and as governmental agencies increase their awareness of the morbidity and mortality of infusion pump errors,Citation19 the additional costs due to intravenous PCA complications, both economically and in terms of patient lives, cannot be ignored. While this paper has highlighted recent published analyses of the costs of such adverse events, quantifying these costs is difficult, and our estimates are rough at best. Costs associated with troubleshooting of intravenous PCA equipment by health care professionals or the effect of reduced mobility on physical therapy and time to discharge were not assessed in this study.
The finding that patients undergoing abdominal surgery have overall higher postoperative opioid costs in the first 48 hours than patients undergoing TKA is somewhat surprising, given the higher levels of pain usually experienced following knee replacement. It is likely that use of femoral and/or sciatic nerve blocks may decrease opioid consumption in TKA patients to some degree. In fact, the use of nonopioid analgesics (local anesthetic blocks, anti-inflammatory agents, gabapentanoids) to supplement opioids in a multimodal analgesia approach has increased over the years. Blinded, placebo-controlled studies of nonopioid adjuvants show either no effect on opioid consumption in the postoperative period or various levels of opioid-sparing effects ranging from 20% to 50%,Citation20–Citation23 with combinations of adjuvants producing slightly higher opioid-sparing effects.Citation24,Citation25 While these nonopioid analgesics can reduce opioid requirements, patients still use a significant amount of opioid analgesics in the first 48 hours after major surgery.
Therefore, in patients suffering from moderate-to-severe pain in the hospital setting, providing the flexibility of patient control over opioid dosing should remain a goal of postoperative analgesia for both increased patient satisfaction and better analgesic efficacy.Citation1,Citation2 Novel opioid delivery systems that contain a patient-activated dosing feature while avoiding invasive, cumbersome delivery routes and the risk of dosing errors should be a focus of future hospital-based analgesia regimens. Given the scheduled nature of these drugs (ie, Schedule II Controlled SubstancesCitation26), it is important that these bedside opioid systems have security features, such as locked drug access and/or single-user identification. It will also be important for these newer systems to be cost-neutral compared with the current standard of care for hospitals, since postoperative analgesia is included in third-party payments to hospitals for overall surgical care of the patient. A sublingual microtablet system for sufentanil is one such system currently under review by the US Food and Drug Administration. The delivery of a fixed dose of sufentanil (15 μg) and a preset lockout time of 20 minutes avoids prescribing and programming errors, while the sublingual route avoids mobility and intravenous catheter-related issues. A locked security tether, a locked drug cartridge, and a radiofrequency identification patient thumb tag provide secure access. In an open-label, active-comparator study, postoperative patients randomized to the sufentanil system had superior patient satisfaction, higher ease-of-care scores for both patients and health care providers, and fewer patients with oxygen desaturation events compared with intravenous PCA morphine.Citation27
Conclusion
In summary, patient requirements for opioids are significant after major surgery, and the costs of opioid drugs and intravenous PCA equipment, while varying slightly between the different types of surgery, are just above $100 per day. The added costs of intravenous PCA-related adverse events are difficult to quantify but may significantly increase this cost to over $300 per day. These adverse events are possibly avoidable with the advent of the newer noninvasive patient-activated analgesia systems. In the current health care reimbursement environment, it is optimal when novel hospital-based drug and device technology can demonstrate cost-effectiveness in addition to clinical superiority to the current standard of care.
Disclosure
This research was sponsored by AcelRx Pharmaceuticals, Inc. Pharmerit International received research fees to conduct the database analysis. PP is an employee of AcelRx, the developers of novel pain therapies. XJ and JS are employees of Pharmerit International, a health economics consulting firm. This research was presented in part as a poster at the International Society for Pharmacoeconomics and Outcomes Research 19th Annual International Meeting, May 31 to June 2, 2014, Montreal, Canada. The authors report no other conflicts of interest in this work.
References
- BallantyneJCCarrDBChalmersTCDearKBAngelilloIFMostellerFPostoperative patient-controlled analgesia: meta-analyses of initial randomized control trialsJ Clin Anesth1993531821938318237
- HudcovaJMcNicolEQuahCLauJCarrDBPatient-controlled opioid analgesia versus conventional opioid analgesia for postoperative painCochrane Database Syst Rev20064CD00334817054167
- HankinCScheinJClarkJPanchalSAdverse events involving intravenous patient-controlled analgesiaAm J Health Syst Pharm200764141492149917617499
- HicksRWSikiricaVNelsonWScheinJRCousinsDDMedication errors involving patient-controlled analgesiaAm J Health Syst Pharm200865542944018281735
- MeissnerBNelsonWHicksRSikiricaVGagneJScheinJThe rate and costs attributable to intravenous patient-controlled analgesia errorsHosp Pharm2009444312324
- Institute for Safe Medication PracticesHigh alert medication feature: reducing patient harm from opiatesISMP Medication Safety Alert2222007 Available from: http://www.ismp.org/Newsletters/acutecare/articles/20070222.aspAccessed January 27, 2014
- BadnerNHDoyleJASmithMHHerrickIAEffect of varying intravenous patient-controlled analgesia dose and lockout interval while maintaining a constant hourly maximum doseJ Clin Anesth1996853823858832449
- FinnTPremier perspectives database: comparative effectiveness research Available from: http://hcmatters.com/2012/04/premier-perspectives-database-comparative-effectiveness-research-cer-validated/#sthash.WPix7UW0.dpufAccessed May 1, 2014
- ICD-9 Code Lookup [webpage on the Internet]BaltimoreCenters for Medicare & Medicaid Services2014 Available from: http://www.cms.gov/medicare-coverage-database/staticpages/icd-9-code-lookup.aspxAccessed May 31, 2014
- CPT – Current Procedural Terminology [webpage on the Internet]ChicagoAmerican Medical Association2014 Available from: http://www.ama-assn.org/ama/pub/physician-resources/solutions-managing-your-practice/coding-billing-insurance/cpt.pageAccessed May 31, 2014
- Consumer Price Index [webpage on Internet]WashingtonUnited States Department of Labor2014 Available from: http://www.bls.gov/cpi/Accessed May 31, 2014
- WebsterJOsborneSRickardCHallJClinically indicated replacement versus routine replacement of peripheral venous cathetersCochrane Database Syst Rev20103CD00779820238356
- WebsterJClarkeSPatersonDRoutine care of peripheral intravenous catheters versus clinically indicated replacement: randomized controlled trialBMJ2008337157160
- EngemannJJFriedmanJYReedSDClinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysisInfect Control Hosp Epidemiol200526653453916018428
- ReedDKemmerlySAInfection control and prevention: a review of hospital-acquired infections and the economic implicationsOchsner J200991273121603406
- JaggerJHuntEHBrand-ElnaggarJPearsonRDRates of needle-stick injury caused by various devices in a university hospitalN Engl J Med198831952842883393183
- HatcherIBReducing sharps injuries among health care workers: a sharps container quality improvement projectJt Comm J Qual Improv200228741041412101553
- SchechterLNHarshawQFryeCBErnstFRKrukasMRShillingtonACCosts associated with intravenous patient-controlled analgesia (IV PCA) in US hospitalsValue Health201316A117
- Association for the Advancement of Medical InstrumentationInfusing patients safely: priority issues from the AAMI/FDA Infusion Device Summit, 2010 Available from: http://www.aami.org/infusionsummit/AAMI_FDA_Summit_Report.pdfAccessed January 27, 2014
- KleinJRHeatonJPThompsonJPCottonBRDavidsonACSmithGInfiltration of the abdominal wall with local anaesthetic after total abdominal hysterectomy has no opioid-sparing effectBr J Anaesth200084224824910743462
- NgAParkerJToogoodLCottonBRSmithGDoes the opioid-sparing effect of rectal diclofenac following total abdominal hysterectomy benefit the patient?Br J Anaesth200288571471612067012
- ReynoldsLWHooRKBrillRJNorthJReckerDPVerburgKMThe COX-2 specific inhibitor, valdecoxib, is an effective, opioid-sparing analgesic in patients undergoing total knee arthroplastyJ Pain Symptom Manage200325213314112590029
- KehletHPostoperative opioid sparing to hasten recovery: what are the issues?Anesthesiology200510261083108515915017
- FayazMKAbelRJPughSCHallJEDjaianiGMecklenburghJSOpioid-sparing effects of diclofenac and paracetamol lead to improved outcomes after cardiac surgeryJ Cardiothorac Vasc Anesth200418674274715650984
- GilronIOrrEDongshengTO’NeillJPZamoraJEBellACA placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib and their combination for spontaneous and movement-evoked pain after abdominal hysterectomyPain20051131–219120015621380
- Electronic Code of Federal Regulations [webpage on the Internet]WashingtonU.S. Government Printing Office2014 [updated May 19, 2014]. Available from: http://www.ecfr.gov/cgi-bin/text-idx?rgn=div5&node=21:9.0.1.1.9Accessed May 31, 2014
- MelsonTBoyerDLMinkowitzHPalmerPPRoyalMSufentanil NanoTab®PCA system versus IV PCA morphine for postoperative pain: a randomized, open-label, active-comparator trial (A3087) Available from: http://www.asaabstracts.com/strands/asaabstracts/abstract.htm;jsessionid=36082DD5A67E4B69D165AC547A95748C?year=2013&index=17&absnum=5065Accessed January 27, 2014
