Abstract
Background
As HIV infection turned into a chronic treatable disease, now ranking as one of the most costly in medicine, long-term sustainability of highly active antiretroviral treatment (HAART) expenses became a major issue, especially in countries with universal access to care. Identification of determinants of higher HAART costs may therefore help in controlling costs of care, while keeping high levels of retention in care and viral suppression.
Methods
With this aim, we enrolled a large multicentric sample of consecutive unselected human immunodeficiency virus (HIV) patients followed at five sites of care in Italy, and evaluated annual individual HAART costs in relation to a number of sociodemographic, clinical, and laboratory variables.
Results
We enrolled 2,044 patients, including 1,902 on HAART. Mean HAART costs were €9,377±€3,501 (range 782–29,852) per year, with remarkable site-based differences, possibly related to the different composition of local assisted populations. Percentages of patients on viral suppression were homogeneously high across all study sites. The factors identified by cross-validation were line of HAART, diagnosis of acquired immune deficiency syndrome, current CD4 T-cell count, and detectable HIV viremia >50 copies/mL. In the final multivariable model, HAART costs were independently directly associated with more advanced HAART line (P<0.001) and inversely correlated with current CD4 T-cell count (P=0.024). Site of care held independent prediction of higher costs, with marked control of expenses at sites 2 (P=0.001) and 5 (P<0.001).
Conclusion
Higher costs of HAART were strongly associated with previous treatment failures, detectable HIV viremia, and lower CD4 T-cell count at the time of evaluation, with no correlation at all with sex, age, hepatitis C virus coinfection, and nadir CD4 T-cell counts. Newer drugs, which are typically those associated with high prices, at the time of the analysis were still prevalently prescribed to rescue and maintain viral suppression in patients with more complex treatment history. Further analyses of the contribution of the single drug/regimen to the estimated cost are warranted.
Introduction
With the advent of combined highly active antiretroviral therapy (HAART), human immunodeficiency virus (HIV) turned into a chronic treatable disease, compatible with near normal lifespan expectancy.Citation1–Citation3 As a consequence of increased survival of both early and advanced HIV infections, expenditure for HIV care is now ranking as one of the most costly chronic diseases.Citation1,Citation4 Indeed, even though costs for HIV hospital admissions have somewhat decreased,Citation5,Citation6 especially in patients with early diagnosis, total costs for HIV care are getting higher and higher in line with the following trends: increasing costs of newer antiretrovirals, larger numbers of patients on chronic therapy, guidelines suggesting early prescription of HAART, and early access to treatment as prevention.Citation5,Citation7–Citation10
The majority of industrialized countries strive to guarantee long-term sustainability for lifelong antiretroviral treatment of HIV patients. Universal access to care seems to be indeed helpful for patients’ retention in care in some European countries like Italy and Denmark, where physicians may prescribe antiretrovirals in the absence of immediate control of expenses and current results regarding retention in care and HAART efficacy are suggestive of adequacy.Citation11–Citation14
As the costs of HAART regimens are relatively easy to determine and analyze, in the present study we set up a research protocol to retrospectively assess the issue of which factors are associated with the use of more costly HAART regimens in clinical practice. We wondered whether costs might be presently associated with sociodemographic and clinical features of our patients, or rather with other conditioning factors.
Materials and methods
A retrospective unselected sample of consecutive HIV outpatients aged >18 years and followed at five sites of care well spread throughout Italy was collected for a cross-sectional evaluation of cost determinants in 2012. We considered sociodemographic characteristics (age, sex), risk factors for HIV infection (heterosexual, homosexual, intravenous drug use, blood transfusion), current HIV viral load (copies/mL, Roche Amplicor®), current (at the time of cost evaluation) and nadir CD4 T-cell counts (cells/mm3), time from HIV diagnosis, acquired immune deficiency syndrome (AIDS)-defining events (ever reported during clinical history), current HAART line, and hepatitis C virus (HCV) coinfection, as already defined elsewhere.Citation15 Years from HIV diagnosis were calculated as the difference between 2012 and the year when HIV infection was first diagnosed. Line of HAART was defined on the basis of the number of treatment changes due to virological failure; simplification or switch for side effects were not considered as a change of HAART line. Annual costs of individual HAART regimens were calculated using pharmacy data retrieved at each distribution site as of December 2012.
Statistical analysis
The outcome was the cost of the regimen currently used by the patients. Costs were modeled in the original raw scale as the distribution was approximately normal and the log transformation only marginally reduced the skewness. Univariable analyses for determinants of higher HAART costs were set up for all the investigated variables. The basic set of covariates were: line of HAART, age, AIDS event, current CD4 T-cell count, time since HIV diagnosis, coinfection with HCV, nadir CD4 T-cell count, risk for HIV infection, sex, and detectable HIV RNA >20 copies/mL. Initially, the potential independent predictors of HAART costs were evaluated using a multivariable regression model, including all covariates that were significantly associated with costs in the univariable analyses. Statistical significance was defined as a two-sided P-value <0.05 for all analyses, and multiple regression models were fit using Stata version 10.1 (Stata Corporation, College Station, TX, USA). In addition to this approach, three different criteria for the selection of covariates in the model were used, similar to what was done by Cozzi-Lepri et al:Citation16 best subset least squared estimations, least absolute shrinkage and selection operator,Citation17 and a mixed approach using both least angle regression and least absolute shrinkage and selection operator.Citation18 In all these methods, model sequencing is determined by the original algorithm, while the coefficients of the parameters at each step are determined using ordinary least squares. Both least absolute shrinkage and selection operator and least angle regression are shrinkage methods for linear regression that minimize the sum of squared errors though with a bound on the sum of the absolute values of the coefficients given by a parameter K. This parameter K was chosen to minimize the average squared error based on a 10-fold cross-validation. Briefly, to perform a 10-fold cross-validation means to randomly divide the dataset into ten portions of the same size. The method then fits the model for a range of values of K to 9/10th of the data and computes the prediction error on the remaining 1/10th. This is done, in turn, for each of the 1/10 portions, and eventually the mean of these ten obtained estimates of the prediction error is calculated. Using this approach, we obtained an estimated prediction of the 10-fold cross-validation error (CV PRESS) curve as a function of the model evolution steps. This was used to establish at which step to stop the inclusion of the covariates. Specifically, the “one-standard-error” rule was used to select the final model: the model with the smaller number of included covariates providing a CV PRESS within one standard error of its minimum value. Cross-validation was applied to our database (by randomly splitting 50% as training, 25% as validation, and 25% as test set) to select K for all estimation procedures. Of note, the training set is used to determine the coefficients but not to decide when to stop. The results of the cross-validation in terms of selection of the most important predictors were similar when using the costs in the raw or the log scale. All these additional analyses were performed using the procedure GLMSELECT in SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
We included 2,044 patients, 69.0% of whom were male. The mean patient age was 46.9±10.0 years, and 33.4% of patients were coinfected with HCV. The risk for HIV infection was heterosexual contact (881 patients, 43.7%), homosexual contact (519 patients, 25.7%), intravenous drug use (591 patients, 29.3%), and blood transfusion (25 patients, 1.2%). Mean time since HIV diagnosis was 13.6±7.9 years. Mean nadir CD4 T-cell counts were 230.0±169.3 cells/mm3, mean current CD4 T-cell counts were 579.0±299.0 cells/mm3. An AIDS diagnosis was recorded for 30.8% of patients. Significant differences in demographic and clinical features were observed between patients cared for at different sites, as shown in .
Table 1 Demographic and viro-immunological characteristics of the sample overall and by site
There were 1,902 (93.0%) evaluable patients on HAART. Among them, viremic (≥20 copies/mL) patients were 31.2% (635 patients); only 19.0% (387 patients), however, had HIV RNA values >50 copies/mL. Median HAART line was 3.47±2.08. Mean individual HAART costs were €9,377±€3,501 (range 782–29,852) per year. Costs were significantly different at the five sites of care, as indicated in .
Univariable analyses for higher HAART costs were set up for all the investigated variables. All of them were associated with higher HAART costs with the exception of sex, as shown in .
Table 2 Individual annual HAART costs according to selected variables
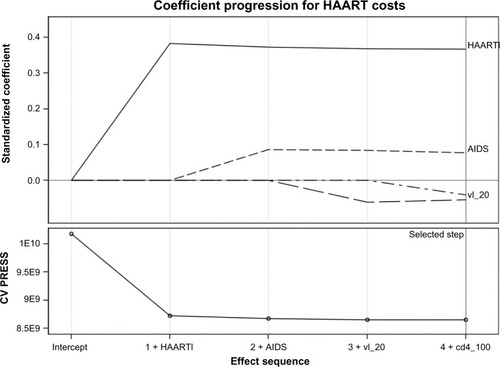
When we used the best subset selector least squared estimates of the coefficients and 10-fold cross-variation to identify our best multivariable predictive model, starting from the full set of measured variables, the following factors were selected: line of HAART (parameter estimate = +€557 per additional regimen), AIDS diagnosis (estimate = +€351 compared with asymptomatic), current CD4 T-cell count (estimate = −€629 for those having >200 and <500 cells/mm3 current CD4 count comparing with those having ≤200 cells/mm3, and −€1,135 for those having >500 cells/mm3 current CD4 count) and HIV viral load >50 copies/mL (estimate = +€405 compared with viral load ≤50 copies/mL). shows the relative importance of these factors at each step of the selection process, and provides information as to when effects entered the model (the estimates above are derived directly from the standardized coefficients at final step number 4; a negative estimate means a negative impact on costs). Thus, for example, the line of HAART is the first factor to be entered in the model with an estimated coefficient close to 0.4 (step 1). In the following three steps, a diagnosis of AIDS, having a viral load ≤50 copies/mL and current CD4 count were added into the model, respectively. For further analyses, see Supplementary material and .
Figure 1 Coefficient progression for individual HAART costs, according to the GLMSELECT procedure. Factors were included in the analysis as follows: HAART line (haartl), AIDS event (AIDS), detectable HIV viral load (vl_20), and current CD4 count (cd4_100). Deflections from the starting point are directly correlated with the impact of each determinant on HAART costs, ie, the more the deflection is, the more HAART costs are influenced from such factor. HAART line is the most important factor influencing HAART costs.
Finally, we also fitted the full model including all factors measured and collected in the database, which were found to be significant at univariate analyses. This was done to verify whether the factors identified through cross-validation had an independent effect on costs after controlling for all other variables. The results of this full regression model are shown in . HAART costs were independently directly correlated with the number of HAART lines used (the cost was €592 higher per each additional line of therapy used, 95% confidence interval 518–665, P<0.001); in contrast, they were inversely correlated with current CD4 T-cell count (€674 less expensive for patients having a current CD4 count >200 and <500 cells/mm3 versus those having a current CD4 count ≤200 cells/mm3, 95% confidence interval –1,281, –68, P=0.029; and €1,136 less expensive for those having a current CD4 count of >500 cells/mm3, 95% confidence interval –1749, –523, P<0.001) and HCV coinfection (€527 less expensive than HCV-negative patients). Independent prediction of higher costs was confirmed for the site of care in the full regression model, with marked control of expenses at site 2 (€743 less expensive, 95% confidence interval –1139, –347, P<0.001) and site 5 (€1,224 less expensive, 95% confidence interval –1,675, –774, P<0.001).
Table 3 Multivariable regression predicting individual annual HAART costs
Discussion
We evaluated the raw costs of HAART, calculated as at December 2012, in a multicenter Italian sample of HIV outpatients, to derive a comparative estimate at different sites of care and identify potential determinants of more costly HAART regimens. In our search for such determinants, we investigated the possible correlation between HAART costs and many clinical and demographic factors, including the burden of toxicities and comorbidities affecting the single patient as accounted for by a multifactorial risk score.Citation19 Preliminary analyses, performed for patients followed at site 1 only, unexpectedly revealed that HAART costs were not correlated at all with comorbidities and HAART toxicities, as evaluated by such a score; in that analysis, costs were indeed significantly associated with other measurable factors, such as more advanced HAART lines, lower nadir and current CD4 T-cell counts, AIDS events, HCV coinfection, age, years from HIV diagnosis, and detectable HIV viremia. We therefore evaluated all these factors in this final multicenter sample, in order to better estimate the independent weight of each determinant of HAART costs.
The mean annual cost of HAART was €9,377±€3,501 per single patient, in line with the only comparable Italian data, reported by Rizzardini et al, who quantified such costs in the Lombardy region, finding a mean of €8,471 in 2009 and a constant trend to annual increase.Citation8,Citation20
It is worth noting that each site faced with a specific population of patients, as shown in : site 3 took care of a higher percentage of previous intravenous drug users with a consequent higher percentage of HCV-coinfected patients with a lower CD4 T-cell nadir; in contrast, site 4 had a higher percentage of sexually infected patients with a higher CD4 T-cell nadir. The variability in the assisted population may partly determine the observed differences in HAART costs; such differences, however, might also reflect different prescribing attitudes at different sites,Citation21 in spite of excellent rates of virological suppression at all sites. Detailed analysis of the individual regimens, not included in this retrospective study, may help understanding of local differences.
Analysis of the larger patient population studied here shows that the most powerful determinant of HAART costs is the line of HAART. Similar results were shown in a recent evaluation of HIV costs in Germany, which reported on increased antiretroviral experience as related to higher expenses.Citation22 Given that previous treatment failures compel physicians to resort to newer and more expensive drugs and regimens, such data are easy to interpret. However, the entity of this association was somewhat surprising, as it emerges by our additional statistical analyses and appears as one of the most relevant findings of the present study.
In the German evaluation of HIV costs,Citation22 Mostardt et al reported that a low CD4 T-cell count at evaluation was a strong predictor of more intensive health care utilization in the following year, as also shown by other authors.Citation21,Citation23 Immune recovery at the time of cost calculation was indeed significantly related to HAART costs in our sample too, as patients with a good immunological response to HAART may be switched to less expensive regimens. Once more, future detailed analysis of the individual regimens (dual therapy, monotherapy, or others) may pinpoint specific preferences for simpler and less expensive strategies in such patients.
A detectable HIV viremia at the time of drug cost calculation was similarly associated with higher HAART costs. This is in contrast with a previous report from Rizzardini et al,Citation8 indicating undetectable HIV viremia as associated with higher total and HAART costs for HIV care. The present finding, however, seems rather easy to explain, ie, virological failures are indeed more frequent in more advanced HAART lines and in patients with a longer duration of HIV infection,Citation24 where many drugs have already been prescribed. However, viral load ≥50 copies/mL was associated with higher costs independently of the line of HAART. This might be explained by the fact that persistence or relapse of low level HIV viremia and subsequent risk of virological failureCitation25 make it difficult to simplify more complex and expensive rescue HAART regimens, independently contributing to the costly management regardless of number of HAART lines used. However, this association of HIV viremia with HAART costs did not remain significant in the final multivariable model.
HAART costs were higher in patients with a lower CD4 T-cell nadir. In our analyses, however, the correlation of costs with nadir CD4 T-cell count only showed a trend for significance in the multivariable models. Immune status at HIV diagnosis has been repeatedly associated with higher HIV care costs; in a different investigational model, Krentz et alCitation7 showed that patients presenting with CD4 T-cell counts <350 cells/mm3 had 2.5 times greater total costs than less advanced patients, with higher costs persisting as much as 5 years after presentation. The CD4 T-cell nadir was also evaluated by Rizzardini et al.Citation8 In their multivariable analysis, patients with a CD4 T-cell nadir <200 cells/mm3 had both higher total costs and HAART costs.
HCV coinfection has been identified as a determinant of higher total costs for HIV care, as well as for HAART and hospital admission costs.Citation20 Even though there was also a direct association between HCV coinfection and higher HAART costs in univariable analyses in our study, all multivariable models surprisingly showed an inverse correlation, ie, HCV-positive patients have lower HAART costs. In fact, patients with HCV coinfection had more frequently advanced HAART lines, and lower nadir and current CD4 T-cell counts, and therefore this association remains surprising.
Our evaluation has some limitations. It is a cross-sectional analysis that is prone to reverse causality (difficult to establish whether using costly drugs had an impact on patients viro-immunological status or if, in contrast, are the level of the markers which are inducing different costs); moreover, the effect of dynamic changes in treatment usage and of surrogate markers of prognosis, fitted as time-dependent factors during the year of observation, could not be evaluated. Moreover, costs were calculated as an estimate from medical and pharmacy records, and may not reflect the exact expenses for the Italian health system.
Conclusion
Our study provides a novel insight into the strength of different independent determinants of HAART costs in a large representative sample of Italian HIV outpatients. Higher costs of HAART were strongly associated with previous treatment failures, and lower CD4 T-cell count at the time of evaluation. Further analyses to clarify the contribution of the single drug/regimen to the annual costs of HAART are warranted.
Acknowledgments
We are very grateful to the Fondazione Onlus Camillo de Lellis per l’Innovazione e la Ricerca in Medicina, Pescara, Italy, which supported us throughout this study.
Supplementary material
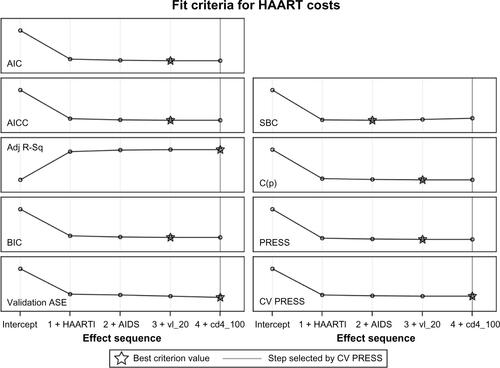
The average squared error (ASE) evolved at each step until final step 4 was reached and inclusion of factors terminated because the ten error estimates computed by cross-validation (CV PRESS) stopped showing a decrease using the “one-standard-error” rule. The values for the adjusted R-square at the various steps were the following: 0.146, 0.152, 0.154 and 0.155 at step 4 which was the optimal value criterion, indicated with a star symbol in the plot. The values for the standard R-square were similar (not shown). Step 4 was the chosen closing step for the chosen fit criteria of CV PRESS and for most of other fit criteria considered (eg, the adjusted R-square) or the previous step, removing current CD4 count (Akaike’s Information Criterion [AIC], Bayesian Information Criterion [BIC], see ). The ASE was greatly reduced when the line of highly active antiretroviral therapy (HAART) first entered the model. The plot of the improvements in ASE after the introduction of the various factors is key as it shows that the model including the line of therapy alone is a substantial improvement compared to the null model (with intercept only) but that adding any of the other factors determines only further small improvements to the predictive performance. These minor changes of the ASE were seen on the training, validation, and test datasets (data not shown). Results were similar when we used other methods of selection, such as least absolute shrinkage and selection operator (LASSO) or least angle regression (LAR).
Figure S1 Evolution of the ASE at each step of the GLMSELECT procedure. Factors were included in the analysis as follows: HAART line (HAARTl), AIDS event (AIDS), detectable HIV viral load (vl_20), and current CD4 count (cd4_100).
Abbreviations: Adj R-Sq, adjusted R-squared; ASE, average squared error; AIC, Akaike’s Infor ma tion Criterion; BIC, Bayesian Information Criterion; HAART, highly active antiretroviral therapy; LASSO, least absolute shrinkage and selection operator; LAR, least angle regression; AICC, corrected Akaike’s information criterion; C(p), Mallows statistic; SBC, Schwarz Bayesian information criterion.
Disclosure
The authors report no conflicts of interest in this work.
References
- MagoniMScarcellaCVassalloFThe evolving burden of HIV infection compared with other chronic diseases in northern ItalyHIV Med20111212913720666848
- LevyARJamesDJohnstonKMThe direct costs of HIV/AIDS careLancet Infect Dis2006617117716500598
- SullivanRPeppercornJSikoraKDelivering affordable cancer care in high-income countriesLancet Oncol20111293398021958503
- SloanCEChampenoisKChoisyPNewer drugs and earlier treatment: impact on lifetime cost of care for HIV-infected adultsAIDS201226455622008655
- KrentzHBAuldMCGillMJfor the HIV Economic Study GroupThe changing direct costs of medical care for patients with HIV/AIDS, 1995–2001CMAJ200316910611012874156
- MeritoMBonaccorsiAPammolliFEconomic evaluation of HIV treatments: the ICONA cohort studyHealth Policy20057430431316226140
- KrentzHBGillJDespite CD4 cell count rebound the higher initial costs of medical care for HIV-infected patients persist 5 years after presentation with CD4 cell counts less than 350 microLAIDS2010242750275320852403
- RizzardiniGRestelliUBonfantiPThe cost of HIV disease in Northern Italy: the payer’s perspectiveJ Acquir Immune Defic Syndr20115721121721546850
- CohenMSChenYQMcCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapyN Engl J Med201136549350521767103
- Center for Disease Control and PreventionPreexposure prophylaxis for the prevention of HIV infection on the United States 2014 Available from: http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdfAccessed June 8, 2014
- ForemanCGazzardBJohnsonMSharottPCollinsSMaintaining cost-effective access to antiretroviral drug therapy through a collaborative approach to drug procurement, consensus treatment guidelines and regular audit: the experience of London HIV commissioners and providersSex Transm Infect20128811211522345023
- GazzardBMoecklinghoffCHillANew strategies for lowering the costs of antiretroviral treatment and care for people with HIV/AIDS in the United KingdomClinicoecon Outcomes Res2012419320022888265
- HellebergMEngsigFNKronborgGRetention in a public healthcare system with free access to treatment: a Danish nationwide HIV cohort studyAIDS20122674174822156974
- HallHIHalversonJWilsonDPLate diagnosis and entry to care after diagnosis of human immunodeficiency virus infection: a country comparisonPLoS One20138e7776324223724
- ParrutiGVadiniFSozioFPsychological factors, including alexithymia, in the prediction of cardiovascular risk in HIV infected patients: results of a cohort studyPLoS One20138e5455523349927
- Cozzi-LepriAProsperiMCKjærJCan linear regression modeling help clinicians in the interpretation of genotypic resistance data? An application to derive a lopinavir-scorePLoS ONE20116e2566522110581
- TibshiraniRRegression shrinkage and selection via the LassoJ R Stat Soc Series B Stat Methodol199658267288
- EfronBHastieTJohnstoneITibshiraniRLeast angle regressionAnn Stat200432407499
- TontodonatiMSozioFVadiniFA multifactorial risk score to evaluate baseline and antiretroviral-related toxicities relative to the costs of current antiretroviral regimens in a cohort of HIV-infected patientsOral communication OC40 presented at the Ninth Italian Conference on AIDS and RetrovirusesJune 10–12, 2012Naples, Italy
- RizzardiniGRestelliUBonfantiPCost of human immunodeficiency virus infection in Italy, 2007–2009: effective and expensive, are the new drugs worthwhile?Clinicoecon Outcome Res20124245252
- Oliva-MorenoJLópez-BastidaJSerrano-AguilarPPerestelo-PérezLDeterminants of health care costs of HIV-positive patients in the Canary Island, SpainEur J Health Econ20101140541220049503
- MostardtSHanhoffNWasemJCost of HIV and determinants of health care costs in HIV-positive patients in Germany: results of the DAGNA K3A StudyEur J Health Econ20131479980822990377
- FongRChengACVujovicOHoyJFFactors associated with virological failure in a cohort of combination antiretroviral therapy-treated patients managed at a tertiary referral centreSex Health20131044244724119435
- LapriseCde PokomandyABarilJGDufresneSTrottierHVirologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observationClin Infect Dis2013571489149623946221
- KrentzHBAuldMCGillMJThe high cost of medical care for patients who present late (CD4 <200 cells/microL) with HIV infectionHIV Med20045939815012648
