Abstract
Objective
The purpose of this study was to determine the prevalence of adverse drug reactions (ADRs) reported in emergency departments (EDs) and carry out a thorough characterization of these to assess preventability, seriousness that required hospitalization, subsequent 30-day mortality, and economic burden.
Methods
This was a retrospective cohort study of data from an active pharmacovigilance project at 32 EDs in the Lombardy region collected between January 1, 2010 and December 31, 2011. Demographic, clinical, and pharmacological data on patients admitted to EDs were collected by trained and qualified monitors, and deterministic record linkage was performed to estimate hospitalizations. Pharmacoeconomic analyses were based on Diagnosis-Related Group reimbursement.
Results
8,862 ADRs collected with an overall prevalence rate of 3.5 per 1,000 visits. Of all ADRs, 42% were probably/definitely preventable and 46.4% were serious, 15% required hospitalization, and 1.5% resulted in death. The System Organ Classes most frequently associated with ADRs were: skin and subcutaneous tissue, gastrointestinal, respiratory thoracic and mediastinal, and nervous system disorders. The most common Anatomical Therapeutic Chemical classes involved in admissions were J (anti-infectives and immunomodulating agents), B (blood and blood-forming organs), and N (nervous system). Older age, yellow and red triage, higher number of concomitantly taken drugs, and previous attendance in ED for the same ADR were significantly associated with an increased risk of hospitalization. The total cost associated with ADR management was €5,184,270, with a mean cost per patient of €585. Fifty-eight percent of the economic burden was defined as probably/definitely preventable.
Conclusion
ADRs are a serious health/economic issue in EDs. This assessment provides a thorough estimation of their seriousness, preventability, and burden impact in a large population from a representative European region.
Introduction
Adverse drug reactions (ADRs) are a leading cause of mortality and morbidity in health care and a significant burden on health care resources.Citation1,Citation2 Estimates of the prevalence of ADRs in the literature vary depending on the definition of ADR used, the study setting, and the study population.Citation3 In addition, the incidence of ADR-related admissions may be underestimated due to lack of documentation in patient medical notes,Citation4–Citation6 on average being 0.1%–54% of all hospital admissions. ADRs are a significant cause of emergency department (ED) visits,Citation7,Citation8 and many are preventable, eg, due to drug treatment, lack thereof, or therapies inconsistent with current best practice.Citation9,Citation10 EDs are ideal places to study ADRs because they are an essential part of health care system, serve as an interface between hospitals and communities, and constitute the most important source of information about the incidence, seriousness, and costs of ADRs.Citation11–Citation13
Previous studies of ED visits associated with ADRs have been limited to one hospital setting,Citation14–Citation16 a specific population,Citation17–Citation21 specific classes of drugCitation22,Citation23 or types of ADRs,Citation24,Citation25 a retrospective study design,Citation26,Citation27 short periods of observation,Citation13,Citation28–Citation32 or did not provide information on preventability.Citation33–Citation35 More extensive studies of ED visits for outpatient ADRs are thus crucial and needed. We have determined the prevalence, preventability, seriousness requiring hospitalization, subsequent 30-day mortality, and economic impact of ADRs presenting to multiple EDs serving a large proportion of the Lombardy region over a 2-year period.
Materials and methods
Setting
This was a retrospective cohort analysis based on ADR charts collected between January 1, 2010 and December 31, 2011 as part of the prospective active pharmacovigilance project Monitoring of ADRs in ED (MEREAFaPS) that is collecting ADRs reported in EDs. The study involves 32 EDs in 16 general hospitals serving different catchment areas of Lombardy, the largest region in Italy with a population of almost 10 million, and altogether accounts for 37.9% of ED visits in the region in the study period. All ADRs reported from patients having at least one suspected ADR, except for those from vaccines, were included in the analysis. Patients who developed an ADR while in the ED for any other reason were included. The local institutional ethics committee of the coordinating center, Niguarda Ca’ Granda Hospital, was informed of the study according to the legal requirements concerning observational studies.
Data source
For this study we used two sources of data, ie, ADRs in ED data collected prospectively as part of the MEREAFaPS project and hospitalization data from the hospital discharge database.
The physicians in each ED were informed about the aims of the MEREAFaPS study, and a monitor was assigned for each hospital. The monitors were medical doctors, pharmacists, and biologists, and underwent an intensive course on the theoretical and practical aspects of pharmacovigilance in an ED. For each ADR notified, the following information were recorded: demographic characteristics (age, gender, ethnic group, deduced from the place of birth if not otherwise indicated); patient clinical status on ED visits; triage code; ongoing therapy, (suspected and concomitant drugs, route, duration, and dosage) codified according to the Anatomical Therapeutic Chemical (ATC) classification system and therapeutic indication for the suspected drug; a description of the ADR according to diagnosis and symptoms, codified as detailed by the Medical Dictionary for Regulatory Activities (MedDRA) dictionary and organized by System Organ Class (SOC)Citation15 and its degree of seriousness, classified according to the World Health Organization criteria as fatal, life-threatening, or requiring hospitalization of the patient, or causing serious/permanent disability;Citation36 history of previous presentation to an ED for the same ADR; and preventability. The diagnosis of ADR and investigation of the relationship between development of the ADR and the drug used were always done by the ED physicians in collaboration with the monitor.
The hospitalization database contains the following information: demographic characteristics (age, sex), 30-day mortality, and Diagnosis-Related Group (DRG) reimbursement rate. To estimate which of the ADRs observed at ED admission or during ED stay led to hospitalization, we performed a deterministic linkage between two data sources using the unique ID anonymous patient code existing in both databases.
Outcome measure
The primary outcome of the study was to determine the rate of ADRs presenting in ED, regardless of whether the ADR was a reason for the visit. In accordance with new European Medicine Agency legislation, diagnosis of an ADR is based on the following definition: a response to a medicinal product that is noxious and unintended, arising from use of a medicinal product within the terms of the marketing authorization as well as from use outside the terms of the marketing authorization, including overdose, misuse, abuse and medication errors, and suspected adverse reactions associated with occupational exposure.Citation37 The causality assessment was done using the Naranjo algorithm.Citation38 Each ADR was characterized in terms of the SOC and ATC classes most frequently involved. We also assessed ADR preventability (defined as definitely or probably preventable, or not preventable), using the criteria devised by Schumock and Thornton.Citation39,Citation40 We evaluated seriousness, estimated potential predictors of ADRs requiring hospitalization using a multivariate model, and analyzed 30-day mortality. In this group, only patients who died during hospitalization or within the 30 days following hospital discharge were considered. Finally, we estimated the economic burden of ADR-related ED visits by calculating direct medical costs in two stages. Stage 1 included the average cost of an ED visit at each hospital, and stage 2 included costs related to patient hospitalization following an ADR, calculated from the DRG reimbursement present in the hospitalization data. Costs are reported in euros.
Statistical analysis
Descriptive statistics are shown as frequencies and percentages for categorical data and as means with standard deviations for continuous data. We used univariate and multivariate logistic regression to estimate the odds ratios (ORs) with 95% confidence intervals (CIs) of potential predictors of hospitalization among total ADRs. Economic estimates of cost are presented as means with standard deviations (SD).
All results were considered to be statistically significant at P<0.05. Data management and statistical analysis were carried out using SAS version 9.2 software (SAS Institute, Cary, NC, USA).
Results
In the 2-year study period, a total of 2,561,400 ED visits were made, of which 8,862 were ADR-related, with an overall prevalence rate of 3.5 per 1,000 ED visits (). The characteristics of patients with ADRs are reported in . Patients with an ADR had a mean (± SD) age of 55.9±24.3 years, most are female and European, and more than half received two or more drugs at the time of their ED visit (). There were 4,111 serious ADRs (46.4%), and among these 1,332 (15% of total ADRs) led to hospitalization. The fatality rate for all ADRs reported in the ED was 1.5%.
Figure 1 Flow chart of selection criteria of adverse drug reactions in emergency department and outcomes of interests, MEREAFaPS Hospitals, 2010–2011.
Abbreviations: ADR, adverse drug reaction; ED, emergency department; MEREAFaPS, Monitoring of ADRs in ED.
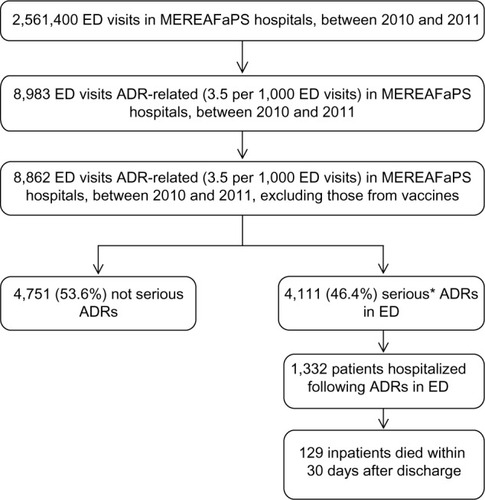
Table 1 Characteristics of patients with adverse drug reactions in emergency departments at MEREAFaPS hospitals, 2010–2011
Most patients with a serious ADR were taking more than one drug (64%) compared with overall ADR (54.3%); similarly, the amount of polypharmacy increased with the degree of seriousness, being 77.9% in patients hospitalized following ADR in ED and 82.2% in patients who died within 30 days of discharge.
Preventable ADRs, a definition which includes both the “definitely” and “probably” preventable ADRs, were in total 42%; of these 51.6% were serious, 59.6% were hospitalized, and 61.2% died.
SOCs most frequently associated with ADRs of significant severity were: skin and subcutaneous tissue, followed by gastrointestinal, respiratory thoracic and mediastinal, and nervous system disorders (). The ATC classes most commonly involved in admissions () were J (anti-infectives for systemic use), B (blood and blood-forming organs), and N (nervous system). In terms of seriousness of ADRs, the ATC class most often involved was V (various), followed by A (alimentary tract and metabolism) and C (cardiovascular).
Table 2 Distribution of adverse drug reactions in emergency departments at MEREAFaPS hospitals, 2010–2011, according to first ten SOC classifications
Table 3 Distribution of adverse drug reactions in emergency departments at MEREAFaPS hospitals, 2010–2011, according to ATC level 1
The most commonly involved drugs (see ) were acetylsalicylic acid (34.5% being preventable), amoxicillin/clavulanic (32.1% preventable), and warfarin (48.6% preventable). The most frequent suspect drugs in serious ADRs and in inpatients who died within 30 days after discharge are reported in Table S1.
Table 4 Ten most frequent suspect drugs for ADRs in ED and patients hospitalized following ADRs in EDs at MEREAFaPS hospitals, 2010–2011
With regard to multivariate predictors of hospitalization, older age (OR 2.76, 95% CI 2.38–3.2), male sex (OR 1.2, 95% CI 1.06–1.37), yellow and red triage (OR 3.62, 95% CI 3.18–4.12), increasing number of concomitant drugs taken (from OR 1.81, 95% CI 1.54–2.13 in patients taking 2–4 drugs, to OR 5.5, 95% CI 3.65–8.29 in those taking ≥10 drugs) and previous attendance in ED for the same ADR (OR 2.04, 95% CI 1.45–2.88) were associated with a significantly increased risk of hospitalization ().
Table 5 Factors associated with hospitalizations for patients with 8,862 adverse drug reactions in emergency department at MEREAFaPS hospitals, 2010–2011
The total cost incurred by the National Health Service because of ADR-related ED visits and subsequent hospitalizations in MEREAFaPS hospitals for the 2010–2011 period was estimated to be €5,184,270, with an average cost of €585±2,149 per patient (). The average estimated cost was €1,166±3,054 to treat patients with serious ADRs, €3,422±4,613 for ED visits leading to hospitalization, and €4,147±3,789 in ADRs that proved fatal. The proportion of preventable cases identified in this study indicates a potential saving of €3,009,800 for the National Health Service, representing 58% of the total expenses incurred in the treatment of ADRs in the ED.
Table 6 Cost of illness associated with ADRs in EDs at MEREAFaPS hospitals, 2010–2011
Discussion
Our study is the first to contribute useful information on the clinical and economic impact of ADRs in Italy via a long-term survey of several EDs that included a substantial number of patients over a 2-year period. Ours is also one of the largest such studies. We present a clear picture, reporting on the prevalence, seriousness, and preventability of drug-related visits to the ED and their real economic impact over a long period in a large number of EDs. Previous studies addressing the issue of ADRs in the ED were limited to either single hospitals with small numbers of patients, or to short periods of observation or a specific population without insight into clinical or economic impact.Citation14,Citation29,Citation31,Citation34,Citation35,Citation41 They also differ in the criteria used to identify ADR, collection periods, and study design.Citation28,Citation42,Citation43 Therefore, data from these studies cannot be extrapolated reliably to a general population.
In our study, the prevalence of ADR-related ED visits was less than 1%, as already reported for France.Citation44 However, this value is strikingly different from that observed in other studies.Citation14,Citation26,Citation42,Citation43 The difference may be due in part to the different ADR inclusion criteria used in the various studies, ie, reported to EDs versus identified in ED or leading to ED visits, and/or due to the known dissimilarity in the incidence of medication-related ED visits between prospective and retrospective studies.Citation42,Citation29,Citation43
Wiffen et alCitation45 observed that patients in their ADR group were prescribed more drugs on average than those in the non-ADR group, a situation known to be associated with an increased prevalence of ADRs.Citation43,Citation46 In accordance with these data, our study shows a correlation between the risk of a serious ADR and the number of drugs being taken; regarding the effect of risk factors on the incidence of ADR-related hospitalization, multiple regression analysis showed a statistically significant association between use of a large number of medications and the risk of presenting with an ADR that would require hospitalization. Likewise, we add further information on the issue of polypharmacy as a cause of ADRs; previous studies were only in elderly people, where polypharmacy is common, and showed that polypharmacy is a reliable predictor of rehospitalization and a prolonged length of hospital stay during which at least one ADR occurred.Citation47
Consistent with previous work,Citation48–Citation51 we found a high rate of preventability of ADRs requiring hospitalization and those resulting in death at 30 days. Reasons for such high rates may include errors in prescribing and/or monitoring, or poor compliance, underscoring the importance of developing strategies to improve the quality and safety of prescribing.
Among the various drug classes that are particularly worthy of attention in terms of preventable ADRs are the classical anticoagulants. We observed that most of the patients who died within 30 days of discharge had suffered from anticoagulant-associated ADRs, 40.3% of which were attributable to warfarin. This evidence is consistent with the findings of previous studies indicating that anticoagulants are among the drugs that need to be more closely monitored because of their propensity to lead to preventable hospitalizations.Citation4,Citation11,Citation52
We found that several organ systems were affected by ADRs, with the highest frequency in the dermatological system, which is consistent with previous reports in the literature.Citation13,Citation44 Likewise, our results in terms of therapeutic categories more frequently involved in ADR (antibiotics, anticoagulants, digoxin, diuretics, hypoglycemic agents, and nonsteroidal anti-inflammatory drugs) are not dissimilar from those in the literature.Citation53
Another aspect of this study is the estimate of the economic impact associated with ADRs, information not yet available on ADRs observed in EDs and in such a large population.Citation54 Calculation of the impact of ADRs on costs is indeed complex.Citation55 ADRs may increase costs because of increased likelihood of hospitalization, prolongation of hospital stay, and the additional clinical investigations needed in more serious cases. Further, ADRs may trigger prescription cascade when new medications are prescribed for conditions that are a consequence of another medication, conditions which are often an unrecognized ADR.Citation56–Citation58 This may explain in part why our analysis in an Italian setting estimated the costs for treatment of serious ADRs to be significantly higher than those observed in France, ie, €3,422 versus approximately €2,500, respectively.Citation59
Studies carried out in general hospitals or specialist units suggest that the cost of an ADR depends on the nature of both the ADR and the culprit drug, and this has to be taken into account when designing programs to control costs and minimize ADRs.Citation10,Citation16 Our economic analysis highlights two relevant issues. The first is that ATC class A is associated with a high number of serious ADRs, and about 20% of the drugs dispensed were to treat diabetes. Antidiabetic drugs (ATC code A10) typically require periodic monitoring, and our data strengthen previous evidence of a progressive and significant increase in health care costs for patients in whom diabetes is not properly monitored.Citation60 The second issue relates to preventability of ADRs. Our data show that 42% of reported ADRs were preventable, indicating a total cost-saving potential of €3 million, representing 58% of the total economic impact of ADRs. Likewise, a study in Germany showed the total cost of treating ADRs to be €434 million per year; considering the proportion of preventable cases (20.1%), this represented a potential cost saving of €87 million per year.Citation61 Certain strategies are known to reduce the impact of ADRs, such as improving adherence to therapeutic guidelines, educational programs, identification of risk groups, and associate therapeutic drug monitoring, whenever available, to the evaluation of reliable biomarkers of safety and efficacy.Citation16,Citation62–Citation64 The heavy burden of preventable ADRs may translate into potentially significant cost savings if these strategies can be implemented further. In this respect, cooperation should be encouraged between clinicians, clinical pharmacologists, and pharmacists, who may play a significant role in preventing and decreasing the burden of ADRs.Citation65
Limits and strengths
This study was of a retrospective nature, so may have underestimated the prevalence of drug-related ED visits as a result of missing or inaccurately documented information. Indeed, retrospective studies cannot identify all reported ADR-related ED visits due to the limitations of the ICD codes used, which do not cover all illnesses potentially caused by ADRs. To address this limitation, we included only cases that were recognized and documented by emergency physicians as ADRs. Another limitation is that the ADRs in MEREAFaPS are the results of spontaneous reports of ADRs. The real prevalence of ADRs is not known and a control group (with no ADRs) was not available; and this is also a limitation with regard to assessment of causality between ADRs and hospitalization. The DRG in Lombardy reflects reimbursement of hospitals by the National Health Service and not the actual costs incurred, although is designed to represent actual costs as far as possible. We could not extract from the DRG those costs associated with management of conditions not strictly related to the ADR.
This study has some important strengths. For example, we used computerized monitoring programs and trained professionals to detect ADRs, and physicians working in EDs to identify ADRs. In addition, this is the first retrospective analysis within a prospective study evaluating the reporting of ADRs in a large number of EDs over a long period. Further, the study recorded mortality occurring up to 30 days after discharge. This is recognized as a good patient outcome indicator,Citation66 although we cannot exclude as a confounding factor the possibility that mortality in some cases was due to other reasons. Moreover, this is the first study assessing hospitalization-related ADR costs in terms of seriousness and preventability, making it possible to estimate the potential cost savings in relation to the preventable cases observed.
Author contributions
VP and VC conceptualized and designed the study, interpreted the data, drafted the manuscript, and revised and approved the final manuscript as submitted. SS, DS, and LP participated in the design of the study and interpretation of the data, revised the manuscript, and approved it as submitted. LDE supervised data collection, analysis, and interpretation, revised the manuscript, and approved it as submitted. MV, SR, EC, and GV conceptualized and designed the study, participated in analysis and interpretation of the data, coordinated and supervised data collection, and finalized and approved the manuscript as submitted. All authors had full access to the data, including the statistical results and tables for this study, and take responsibility for the integrity of the data and the accuracy of the data analyses. GV is the study guarantor.
Acknowledgments
The study was supported by grants from the Regional Pharmacovigilance Centre of Lombardy and the Italian Medicines Agency (AIFA).
Supplementary materials
Table S1 Ten most frequent suspect drugs in: seriousTable Footnote* ADRs in ED at MEREAFaPS hospitals, 2010–2011 and in inpatients who died within 30 days after discharge
Disclosure
The authors declare that they have no competing interests in this work.
References
- MooreNBriffautCNobletCNormandCAThuillezCIndirect drug-related costsLancet199534589495885897776802
- LazarouJPomeranzBHCoreyPNIncidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studiesJAMA199827915120012059555760
- BeijerHJde BlaeyCJHospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studiesPharm World Sci2002242465412061133
- PirmohamedMJamesSMeakinSAdverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patientsBMJ20043297456151915231615
- PatelHBellDMolokhiaMTrends in hospital admissions for adverse drug reactions in England: analysis of national hospital episode statistics 1998–2005BMC Clin Pharmacol20077917894876
- LeendertseAJVisserDEgbertsACvan den BemtPMThe relationship between study characteristics and the prevalence of medication-related hospitalizations: a literature review and novel analysisDrug Saf201033323324420158287
- HohlCMNosykBKuramotoLOutcomes of emergency department patients presenting with adverse drug eventsAnn Emerg Med2011583270279. e27421354651
- BudnitzDSLovegroveMCShehabNRichardsCLEmergency hospitalizations for adverse drug events in older AmericansN Engl J Med2011365212002201222111719
- BatesDWSpellNCullenDJThe costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study GroupJAMA199727743073119002493
- ZedPJAbu-LabanRBBalenRMIncidence, severity and preventability of medication-related visits to the emergency department: a prospective studyCMAJ2008178121563156918519904
- HowardRLAveryAJHowardPDPartridgeMInvestigation into the reasons for preventable drug related admissions to a medical admissions unit: observational studyQual Saf Health Care200312428028512897361
- LagnaouiRMooreNFachJLongy-BoursierMBegaudBAdverse drug reactions in a department of systemic diseases-oriented internal medicine: prevalence, incidence, direct costs and avoidabilityEur J Clin Pharmacol200056218118610877014
- BudnitzDSPollockDAMendelsohnABWeidenbachKNMcDonaldAKAnnestJLEmergency department visits for outpatient adverse drug events: demonstration for a national surveillance systemAnn Emerg Med200545219720615671977
- PatelKJKediaMSBajpaiDMehtaSSKshirsagarNAGogtayNJEvaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: a prospective studyBMC Clin Pharmacol20077817662147
- AndreazzaRSSilveira De CastroMSippel KochePHeineckICauses of drug-related problems in the emergency room of a hospital in southern BrazilGac Sanit201125650150621835509
- WasserfallenJLivioFBuclinTTilletLYersinBBiollazJRate, type, and cost of adverse drug reactions in emergency department admissionsEur J Intern Med200112544244711557331
- RashedANNeubertAAlhamdanHDrug-related problems found in children attending an emergency department in Saudi Arabia and in the United KingdomInt J Clin Pharm201335332733123549774
- ZedPJHaughnCBlackKJMedication-related emergency department visits and hospital admissions in pediatric patients: a qualitative systematic reviewJ Pediatr2013163247748323465404
- JouiniSDjebbiOSouissiSBouhajjaBIatrogenic adverse drug reaction in the emergency consultation of elderly people: epidemiological observational prospective studyLa Tunisie Medicale2013913200204 French23588635
- ChenYCFanJSChenMHRisk factors associated with adverse drug events among older adults in emergency departmentEur J Intern Med2014251495524200546
- NickelCHRuedingerJMMessmerASDrug-related emergency department visits by elderly patients presenting with non-specific complaintsScand J Trauma Resusc Emerg Med2013211523497667
- RendePPalettaLGallelliGRetrospective evaluation of adverse drug reactions induced by antihypertensive treatmentJ Pharmacol Pharmacother20134Suppl 1S47S5024347982
- SeeIShehabNKeglerSRLaskarSRBudnitzDSEmergency department visits and hospitalizations for digoxin toxicity: United States, 2005 to 2010Circ Heart Fail201471283424300242
- JonesSCBudnitzDSSorbelloAMehtaHUS-based emergency department visits for fluoroquinolone-associated hypersensitivity reactionsPharmacoepidemiol Drug Saf201322101099110623963962
- BanerjiARuddersSClarkSWeiWLongAACamargoCAJrRetrospective study of drug-induced anaphylaxis treated in the emergency department or hospital: patient characteristics, management, and 1-year follow-upJ Allergy Clin Immunol Pract201421465124565768
- WuCBellCMWodchisWPIncidence and economic burden of adverse drug reactions among elderly patients in Ontario emergency departments: a retrospective studyDrug Saf201235976978122823502
- HohlCMKuramotoLYuERogulaBStausbergJSobolevBEvaluating adverse drug event reporting in administrative data from emergency departments: a validation studyBMC Health Serv Res20131347324219303
- RaschettiRMorguttiMMenniti-IppolitoFSuspected adverse drug events requiring emergency department visits or hospital admissionsEur J Clin Pharmacol1999541295996310192758
- AhernFSahmLJLynchDMcCarthySDetermining the frequency and preventability of adverse drug reaction-related admissions to an Irish University Hospital: a cross-sectional studyEmerg Med J2014311242923389832
- CalderLAForsterANelsonMAdverse events among patients registered in high-acuity areas of the emergency department: a prospective cohort studyCJEM201012542143020880432
- CapuanoAIrpinoAGalloMRegional surveillance of emergency-department visits for outpatient adverse drug eventsEur J Clin Pharmacol200965772172819294371
- FriedmanSMProvanDMooreSHannemanKErrors, near misses and adverse events in the emergency department: what can patients tell us?CJEM200810542142718826729
- RouletLBallereauFHardouinJBAssessment of adverse drug event recognition by emergency physicians in a French teaching hospitalEmerg Med J2013301636722366041
- TrifiroGCalogeroGIppolitoFMAdverse drug events in emergency department population: a prospective Italian studyPharmacoepidemiol Drug Saf200514533334015682429
- CapuanoAMotolaGRussoFAdverse drug events in two emergency departments in Naples, Italy: an observational studyPharmacol Res200450663163615501703
- The Uppsala Monitoring Centre, WHO Collaborating Centre for International Drug MonitoringGlossary of terms used in pharmacovigilance Available from: http://www.who-umc.org/graphics/25301.pdfAccessed February 16, 2014
- European Medicines AgencyDirective 2010/84/eu of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use Available from: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0074:0099:EN:PDFAccessed October 19, 2014
- NaranjoCABustoUSellersEMA method for estimating the probability of adverse drug reactionsClin Pharmacol Ther19813022392457249508
- SchumockGTThorntonJFocusing on the preventability of adverse drug reactionsHosp Pharm19922753810118597
- LauPMStewartKDooleyMJComment: hospital admissions resulting from preventable adverse drug reactionsAnn Pharmacother200337230330412549969
- KongkaewCNoycePRAshcroftDMHospital admissions associated with adverse drug reactions: a systematic review of prospective observational studiesAnn Pharmacother20084271017102518594048
- TafreshiMJMelbyMJKabackKRNordTCMedication-related visits to the emergency department: a prospective studyAnn Pharmacother199933121252125710630823
- MalhotraSJainSPandhiPDrug-related visits to the medical emergency department: a prospective study from IndiaInt J Clin Pharmacol Ther2001391121811204932
- DehoursEBounesVBagheriHValleBDucasseJLMontastrucJLAdverse drug reactions in an emergency medical dispatching centreEur J Clin Pharmacol201470788188724798891
- WiffenPGillMEdwardsJMooreAAdverse drug reactions in hospital patients. A systematic review of the prospective and retrospective studiesBandolier Extra2002116
- Obreli-NetoPRNobiliAde Oliveira BaldoniAAdverse drug reactions caused by drug-drug interactions in elderly outpatients: a prospective cohort studyEur J Clin Pharmacol201268121667167622644345
- NobiliALicataGSalernoFPolypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI studyEur J Clin Pharmacol201167550751921221958
- KanjanaratPWintersteinAGJohnsTEHattonRCGonzalez-RothiRSegalRNature of preventable adverse drug events in hospitals: a literature reviewAm J Health Syst Pharm200360171750175914503111
- HakkarainenKMGyllenstenHJonssonAKAndersson SundellKPetzoldMHaggSPrevalence, nature and potential preventability of adverse drug events – a population-based medical record study of 4970 adultsBr J Clin Pharmacol201478117018324372506
- WintersteinAGSauerBCHeplerCDPooleCPreventable drug-related hospital admissionsAnn Pharmacother2002367–81238124812086559
- ThomsenLAWintersteinAGSondergaardBHaugbolleLSMelanderASystematic review of the incidence and characteristics of preventable adverse drug events in ambulatory careAnn Pharmacother20074191411142617666582
- HowardRLAveryAJSlavenburgSWhich drugs cause preventable admissions to hospital? A systematic reviewBr J Clin Pharmacol200763213614716803468
- ShehabNPatelPRSrinivasanABudnitzDSEmergency department visits for antibiotic-associated adverse eventsClin Infect Dis200847673574318694344
- LundkvistJJonssonBPharmacoeconomics of adverse drug reactionsFundam Clin Pharmacol200418327528015147278
- GyllenstenHHakkarainenKMHaggSEconomic impact of adverse drug events – a retrospective population-based cohort study of 4970 adultsPLoS One201493e9206124637879
- MooreNLecointreDNobletCMabilleMFrequency and cost of serious adverse drug reactions in a department of general medicineBr J Clin Pharmacol19984533013089517375
- KalischLMCaugheyGERougheadREGilbertALThe prescribing cascadeAust Prescr201134162166
- SenstBLAchusimLEGenestRPPractical approach to determining costs and frequency of adverse drug events in a health care networkAm J Health Syst Pharm200158121126113211449856
- DetournayBFagnaniFPouyannePCost of hospitalizations for adverse drug effectsTherapie2000551137139 French10860015
- Degli EspostiLSaragoniSBudaSSturaniADegli EspostiEGlycemic control and diabetes-related health care costs in type 2 diabetes; retrospective analysis based on clinical and administrative databasesClinicoecon Outcomes Res2013519320123696709
- RottenkolberDSchmiedlSRottenkolberMAdverse drug reactions in Germany: direct costs of internal medicine hospitalizationsPharmacoepidemiol Drug Saf201120662663421384463
- GoettlerMSchneeweissSHasfordJAdverse drug reaction monitoring – cost and benefit considerations. Part II: cost and preventability of adverse drug reactions leading to hospital admissionPharmacoepidemiol Drug Saf19976Suppl 3S79S9015073758
- GautierSBacheletHBordetRCaronJThe cost of adverse drug reactionsExpert Opin Pharmacother20034331932612614184
- LeapeLLCullenDJClappMDPharmacist participation on physician rounds and adverse drug events in the intensive care unitJAMA1999282326727010422996
- RouletLBallereauFHardouinJBChiffoleauAPotelGAsserayNAdverse drug event nonrecognition in emergency departments: an exploratory study on factors related to patients and drugsJ Emerg Med201446685786424565882
- PiazzaGNguyenTNCiosDAnticoagulation-associated adverse drug eventsAm J Med2011124121136114222114827
