Abstract
Background
The objective of this study is to identify and review the methodological quality of health economic evaluations of medical devices performed in the People’s Republic of China. To our knowledge, no such investigations have been performed to date.
Methods
A systematic literature review involving searches of Medline, Medline In-Process, the National Health Service Economic Evaluation Database, the Cost-Effectiveness Analysis Registry of the Tufts Medical Center, and the Wanfang Database was performed. The search spanned the period from 1990 to 2013. Studies on health economic evaluations of medical devices, in-vitro diagnostics, procedures, and the use of medical devices in Chinese health care settings were included. Full-text articles and conference abstracts in English and Chinese were included.
Results
Fifty-seven publications were included, 26 (46%) of which were in English and 31 (54%) of which were in Chinese. The included publications covered a wide range of clinical areas, such as surgery (n=23, 40%), screening (n=9, 16%), imaging use (n=6, 11%), kidney intervention (n=4, 7%), and nine other technological areas. Most of the studies (n=31, 54%) were cost analyses. Among the others, 13 (50%) studies used modeling, and another 13 (50%) were within-trial evaluations. Among studies that used modeling, eleven (85%) conducted sensitivity analyses, six of which had one-way sensitivity analysis, whereas one conducted both one-way and two-way sensitivity analyses; four of these eleven modeling-based analyses included probabilistic sensitivity analyses. The incremental cost-effectiveness ratio was reported in ten (18%) studies, eight of which were screening studies. The remaining two modeling studies were in areas of imaging and oncology.
Conclusion
This study indicates that there are major limitations and deficiencies in the health economic evaluations on medical devices performed in the People’s Republic of China. Further efforts are required from different stakeholders – academic, governmental, and privatized – to improve health economic research capacity and to put it to use when informative decisions are made in the health care setting.
Introduction
Health economics helps to compare different health technologies, taking into account clinical and cost consequences, and supports resource allocation decisions.Citation1 After safety, efficacy, and effectiveness, cost-effectiveness has been recognized as the major “fourth hurdle” to secure market access in many developed countries around the world.Citation2,Citation3 Health economics is successfully used to support decision making in medical device use, in-vitro diagnostics, and medical procedure areas.Citation4
While having a long history of use in the United States, Europe, Canada, Australia, and other countries, health economics has only recently emerged as a decision-making supportive tool in Asian countries.Citation5–Citation8 The People’s Republic of China, with a population of >1.3 billion people and with a growing economy, is an attractive market for manufacturers of medical devices and pharmaceuticals.Citation9 The People’s Republic of China’s medical device market has become the world’s second largest in 2010, with a market size having exceeded 15.8 billion US dollars (USD).Citation10 However, reimbursement for medical devices is limited and complex, especially for innovative products.Citation11 With the growing role of health economic evaluations, it is important to evaluate the status of this area in the People’s Republic of China in terms of quantitative data, characteristics, and methodological approaches for evaluations of devices.
There is a lack of information concerning how the People’s Republic of China performs as a stakeholder in terms of health economic evaluations revolving around medical devices and whether or not, or how cost-effectiveness is addressed in the Chinese health care setting. Therefore, the objective of this study was to review the methodological quality of health economic evaluations of medical devices performed in the People’s Republic of China.
Methods
Literature search and citation screening
A systematic literature search was performed in the following databases: Medline, Medline In-Process, the National Health Service Economic Evaluation Database (NHS EED), and the Cost-Effectiveness Analysis (CEA) Registry of the Tufts Medical Center,Citation12 as well as the Wanfang DatabaseCitation13 for studies published in Chinese. The Wanfang Database is an affiliate of the Chinese Ministry of Science and Technology and provides access to a wide range of data, including medical and scientific areas. The full-search strategy for each specific database is presented in the Supplementary materials. The searches were conducted on February 20, 2013 for Medline, on February 25, 2013 for the NHS EED and the CEA Registry, and on March 1, 2013 for the Wanfang database. The search spanned the period from January 1, 1990 to January 31, 2013.
Screening of abstracts and evaluations of full-text publications was performed by a single reviewer using the inclusion/exclusion criteria provided below. A second reviewer checked the appropriateness of inclusion of studies. Disagreements were resolved by consensus.
Study selection
The following inclusion criteria were used:
Type of studies: CEA, cost–utility (CUA), cost–benefit (CBA), cost-minimization (CMA), cost–consequences (CCA), and budget impact analyses. Economic evaluations as a part of published health technology assessments were also considered.
Type of interventions: Medical devices, in-vitro diagnostics, and procedures using medical devices were considered.
Language: Publications in English or Chinese were included.
Type of publication: Full-text publications in peer-reviewed journals and abstracts of conference proceedings were included.
Setting: The study should have been conducted in a Chinese setting.
Data extraction and analysis
Data from included publications were extracted by one reviewer and presented in the format outlined below. The following information was extracted: title, first author, payer perspective, population/settings, intervention, comparator, time horizon, type of economic evaluation, type of study (economic evaluation alongside clinical trial, modeling), type of analysis (CEA, CUA, etc), cost and cost categories (direct, indirect, etc), source of unit cost, year of costing, resources used and their quantity reported separately (yes/no), clinical outcomes, economic outcomes, discounting, sensitivity analysis (one-way, two-way, etc), regression analysis of cost, source of funding, results (incremental cost-effectiveness ratio [ICER] or total cost per intervention), main conclusion, and language of publication. A second reviewer assessed the quality of the data extraction.
Summary statistics were calculated. No formal statistical analysis was used due to the descriptive nature of this study.
Results
Literature search and citation screening
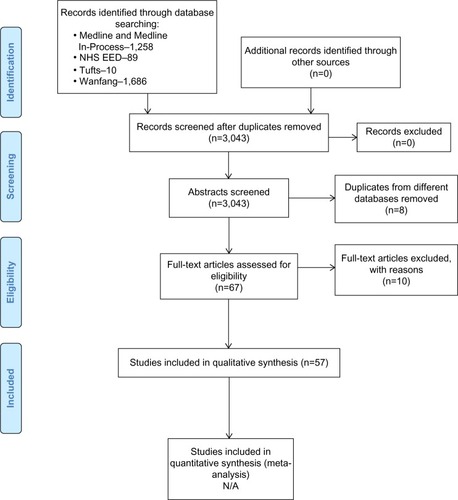
The literature search of electronic databases returned 3,043 initial hits. In total, 57 publications were included in the study. Detailed information about the search process is presented in . A list of excluded publications with the reasons for exclusion is presented in the Supplementary materials.
Description of identified studies
Fifty-seven studies in total were available, 26 (46%) of which were in English and 31 (54%) of which were in Chinese. Methodological characteristics of included publications are presented in .
Table 1 Methodological characteristics of publications included in the analysis
Design and methodology of economic evaluations
Most of the studies (n=31, 54%) were cost analyses of routine care. Among the others (n=26, 46%), 13 (50%) used decision analytic modeling, whereas the other 13 were trial-based evaluations. Thirty-two studies (56%) adopted a hospital perspective, whereas five (9%) adopted a third-party payer perspective, while the remaining studies (n=20, 35%) were from a societal perspective.
Time horizon was reported in 36 (63%) articles. Most studies (n=25, 44%) used the respective study’s follow-up as the time horizon. There were 22 (39%) and three (5%) studies that used hospital stay and lifetime as time horizons, respectively.
Discounting was applied in 12 (21%) studies; four of these discounted future costs with 3% of the annual rate, one discounted future costs with 5% of the annual rate, and seven discounted both future costs and benefits with 3% of the annual rate.
The source of funding was reported in 19 (33%) studies. Of these, three articles had funding from academia, ten studies had funding by the government, three articles had funding by both government and academia, one study had personal support, and two studies specified that there was no supporting funding. There was no single study reporting funding from the industry.
Among all studies involving a health economic analysis, 40 (69%) used a CCA; seven (12%), both a CCA and a CEA; five (9%), a CEA; three (5%), a CUA; one (2%), a CBA; and one (2%), both a CCA and a CMA.
Concerning studies in clinical areas, 23 (40%) involved surgery, nine (16%) involved screening, six (11%) involved imaging, four (7%) involved kidney intervention, three (6%) involved bone surgery, two each involved cardiac surgery/vascular surgery/respiratory support/ophthalmology (3% each), and one each involved oncology/life support/obstetrics/airway surgery (2% each).
Resource use and cost inputs
Among 44 nonmodeling studies, only 14 (32%) reported resources used and the quantity used for each resource. Thirty studies reported the source of unit cost, 16 (53%) of which adopted local unit costs from hospitals, ten (33%) used regional unit costs, one (3%) used national unit cost as a reference, and three (10%) used both local and regional sources. There was only one study (2%) with a regression analysis on cost available. All (n=57) studies reported direct medical costs. Direct nonmedical costs were reported in 19 (33%) studies, and indirect costs were reported in 14 (25%) studies.
Sensitivity analysis
Among the 13 studies that used modeling, eleven (85%) conducted sensitivity analyses; six (55%) of these had a one-way sensitivity analysis, one (9% of 11) conducted both one-way and two-way sensitivity analyses, and four (36% of 11) reported a probabilistic sensitivity analysis.
Among nonmodeling studies that conducted one-way sensitivity analyses, there were two (4%) that conducted trial-based evaluations and four (7%) that conducted cost analyses of routine care.
Outcomes of economic evaluations
The ICER was presented in ten (18%) studies, eight (80%) of which were screening studies, while the remaining two (20%) were studies in imaging and oncology areas. The reported ICER (with year of costing available) ranged from 12 USD per life-year saved (USD/LYS) for the single colonoscopy screening strategy to 6,014 USD/LYS for an additional positron emission tomography/computed tomography (CT) screening when compared with conventional CT staging.
Detailed information on population, settings, comparators, results, and conclusions is provided in the Supplementary materials.
Discussion
The present study is one of the first attempts to evaluate the status of health economic evaluations of medical devices in the People’s Republic of China. This study complements existing literature on methodological quality of the health economic evaluations in regions by raising the issue of application of this concept in decision making.Citation71–Citation73 While other systematic reviews are focused on health economic evaluations in general and mainly include evaluations of pharmaceuticals, our review is aimed on research in medical device and in-vitro diagnostic areas. Specifics of economic evaluation of medical devices in comparison with pharmaceuticals have been extensively reviewed elsewhere.Citation74 This includes difficulties in conducting randomized controlled trials, “learning curve” and usability aspects, wider organizational implications, a shorter life cycle, fast price erosion, etc. Moreover, reimbursement and funding for medical devices differ significantly than for pharmaceuticals, as a special approach is required for the evaluation of medical device studies.
This study reveals that literature on health economics of medical devices in the People’s Republic of China is limited (57 publications in total from the year 1990), and the majority of the publications (53%) include cost or CCAs of routine care. Decision analytic modeling and within-trial evaluations have been used equally.
To include the majority of relevant studies, the search was also conducted using a local Chinese bibliographic database. Although locally published studies bring value in terms of having a complete picture of available research, they may have different methodological quality. Interestingly, the majority of the studies published in Chinese (24 out of 31) used CCAs, mainly at the hospital level. On the other hand, nine out of the ten studies that reported ICER and nine out of 17 studies that conducted sensitivity analyses were published in English, while all the Chinese studies with sensitivity analyses conducted only one-way sensitivity analysis. Among the 13 studies that applied modeling approaches, ten studies were published in English. In addition, among 42 studies with study follow-ups longer than 1 year, only 13 had discounting available. Among these 13 studies, nine were published in English. This may reflect both interests from authors to increase exposure to international literature and to have the influence of international recommendations on good reporting practice on content of published articles. The development of clear guidelines for economic evaluations is clearly considered part of the strategy to increase application of these studies in decision making.Citation75 Recently, Consolidated Health Economic Evaluation Reporting Standards were issued by the International Society for Pharmacoeconomics and Outcomes Research Task Force, which may help improve the quality of reporting of economic evaluations.Citation76
A sensitivity analysis was also conducted to account for the time that passed after the primary analysis was completed (January 2013 to December 2014). The analysis was performed using Medline and Medline In-Process on January 15, 2015, with the same search strategy that was used during the primary analysis. Among the 181 hits generated from the search, eight studies were identified.Citation77–Citation84 Most of the studies (n=4, 50%) were trial-based evaluations, and the remainder were cost analyses of routine care (n=2, 25%) and decision analytic modeling (n=2, 25%). Discounting was not applied in any article (it was not applicable to one study). Apart from one study published in Chinese, the remaining studies were published in English. Six studies (75%) used CCA, and both CEA and CUA were used in two studies (25%). In the two studies that used modeling, both conducted sensitivity analyses – one study conducted only one-way sensitivity analyses, while the other performed one-way, two-way, and probabilistic sensitivity analyses. However, it is worth mentioning that the study that conducted a CEA and CUA with decision analytic modeling and sensitivity analyses (one-way, two-way, and probabilistic) was conducted in the setting of Hong Kong,Citation83 which runs a different health care system and medical device funding system than Mainland China. With these results, it appears that over the extent of the latest time period specified, the quality of the health economic studies performed in the People’s Republic of China remains the same.
This systematic review reveals several areas in which improvements in methodology and reporting are possible for Chinese health economic studies. These include reporting of resource used, sensitivity analysis, presentation of study’s results with ICER, both internal and external validation of decision analytic models, transparency on source of funding, etc.
The limited role of economic evaluations in reimbursement for medical devices in the People’s Republic of China should be taken into account while interpreting the findings of our study. On the national level, the health care system was financed through out-of-pocket payments (35%), social insurance schemes (35%), and government subsidies (30%).Citation85 However, tier III hospitals (highest tier with highly specialized services) dominated 70% of the medical device market among the top 12 cities (including Shanghai, Beijing, and Guangzhou). They have a different purchasing pattern due to a different source of revenue than tier II hospitals.Citation86 Tier III hospitals are expected to generate at least 40% of their revenue from out-of-pocket payments, while tier II hospitals have 20% of revenue coming from out-of-pocket payments.Citation86 This also drives tier III hospitals to procure the most advanced products to boost demand for high-end medical devices, as well as to attract complex disease treatment to enhance out-of-pocket revenue. Thus, with a demand for top medical products, the scarcity of health economic evaluations in the People’s Republic of China needs to change, to determine which of these, eg, are cost-effective so that the rural areas could also consider which of these products may be accessible for them. Fee-for-service remains the main and basic payment mechanism. In most cases, reimbursement does not cover the cost of medical devices completely and there is no established mechanism for evaluating the long-term benefits and cost-effectiveness of technologies. If the role of reimbursement from public sources grows in future, it may lead to increased demand for clinical and economic evidence of benefits of medical technologies.
Conclusion
This study indicates that there are a limited number of economic evaluations of medical devices existing in the People’s Republic of China, and these are mainly focused on cost analysis and have methodological deficiencies. Further efforts are required from different stakeholders, including academia, state institutions, and the private industry, to improve health economic research capacity and to put the results of analyses into practice to ensure that decisions in health care are based on the best available clinical and economic evidence.
Disclosure
The authors report no conflicts of interest in this work.
References
- DrummondMFSculpherMJTorranceGWO’BrienBJStoddartGLMethods for the Economic Evaluation of Health Care Programmes3rd edOxfordOxford University Press2005
- PaulJETruemanP‘Fourth hurdle reviews’, NICE, and database applicationsPharmacoepidemiol Drug Saf200110542943811802589
- GulácsiLBonczIDrummondMIssues for countries considering introducing the “fourth hurdle”: the case of HungaryInt J Technol Assess Health Care200420333734115446763
- GirlingAYoungTBrownCLilfordREarly-stage valuation of medical devices: the role of developmental uncertaintyValue Health201013558559120412542
- LiuGGEgglestonKHuTWEmerging health economics and outcomes research in the Asia-Pacific regionValue Health200811Suppl 1S1S218387052
- TarnYHHuSKamaeIHealth-care systems and pharmacoeconomic research in Asia-Pacific regionValue Health200811Suppl 1S137S15518387058
- YangBMLeeKGrowing application of pharmacoeconomics and outcomes research in health-care decision-making in the Asia-Pacific regionValue Health200912Suppl 3S1S220586968
- KulsomboonVYangBMHuSBridging the gap in pharmacoeconomics and outcomes research between researchers, policymakers, and practitioners in the Asia-Pacific regionValue Health2012151 SupplS1S222265053
- CIAThe World Factbook2013 Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/ch.htmlAccessed August 13, 2013
- Chinese Association for Medical Device IndustryMarket Report2013 Available from: http://www.camdi.org/news!look.action?entityId=2480Accessed August 13, 2013
- Medical Product OutsourcingA look at the Medical Reimbursement System in China2007 Available from: http://www.mpo-mag.com/issues/2007-07/view_breaking-news/a-look-at-the-medical-reimbursement-system-in-china/Accessed August 13, 2013
- Cost-effectiveness Analysis Registry2013 Available from: https://research.tufts-nemc.org/cear4/Home.aspxAccessed February 20, 2013
- Wanfang Data2011 Availabla from: http://www.wanfangdata.com.cn/Accessed February 20, 2012
- WangYTHuangGIs FDG PET/CT cost-effective for pre-operation staging of potentially operative non-small cell lung cancer? – from Chinese healthcare system perspectiveEur J Radiol2012818e903e90922698711
- ZhangBYangBCombined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancerJ Med Screen19996210811010444731
- WeiQWangJGLiLBLiJDManagement of choledocholithiasis: comparison between laparoscopic common bile duct exploration and intraoperative endoscopic sphincterotomyWorld J Gastroenterol20039122856285814669352
- KimJJLeungGMWooPPGoldieSJCost-effectiveness of organized versus opportunistic cervical cytology screening in Hong KongJ Public Health (Oxf)200426213013715284314
- WooPPKimJJLeungGMWhat is the most cost-effective population-based cancer screening program for Chinese women?J Clin Oncol200725661762417308266
- WongIOKuntzKMCowlingBJLamCLLeungGMCost effectiveness of mammography screening for Chinese womenCancer2007110488589517607668
- WongIOKuntzKMCowlingBJLamCLLeungGMCost-effectiveness analysis of mammography screening in Hong Kong Chinese using state-transition Markov modellingHong Kong Med J201016Suppl 3384120601733
- WongIOTsangJWCowlingBJLeungGMOptimizing resource allocation for breast cancer prevention and care among Hong Kong Chinese womenCancer2012118184394440322359352
- ChenZChenLWuLTranscatheter amplatzer occlusion and surgical closure of patent ductus arteriosus: comparison of effectiveness and costs in a low-income countryPediatr Cardiol200930678178519365653
- ChengMMLuBHuSSOptimizing CAD diagnosis in China with CT angiographyJ Cardiovasc Comput Tomogr20093315315819394919
- GongKZhangNLuYComparison of the open tension-free mesh-plug, transabdominal preperitoneal (TAPP), and totally extraperitoneal (TEP) laparoscopic techniques for primary unilateral inguinal hernia repair: a prospective randomized controlled trialSurg Endosc201125123423920552368
- FengBZhuQLXiaYDirect and indirect costs and long-term survival of laparoscopic anterior resection for rectal cancerMed Sci Monit20101612H97H102
- ChenQChenLWCaoHZhangGCChenDZZhangHIntraoperative device closure of atrial septal defects with inferior vena cava rim deficiency: a safe alternative to surgical repairJ Thorac Cardiovasc Surg2011141363163621236449
- ZangJZhangCZhouHGaoJEarly laparoscopic cholecystectomy after endoscopic common bile duct stone extraction: the experience from a developing countrySurg Laparosc Endosc Percutan Tech201121212012221471806
- GurbanovEMengXCuiYEvaluation ECMO in adult cardiac transplantation: can outcomes of marginal donor hearts be improved?J Cardiovasc Surg (Torino)2011523419427
- ZhangJShiQWangGZWangFJiangNCost-effectiveness analysis of ureteroscopic laser lithotripsy and shock wave lithotripsy in the management of ureteral calculi in eastern ChinaUrol Int201186447047521597268
- LiuXJiaXGuoWUltrasound-guided foam sclerotherapy of the great saphenous vein with sapheno-femoral ligation compared to standard stripping: a prospective clinical studyInt Angiol201130432132621747350
- WongEMRainerTHNgYCChanMSLopezVCost-effectiveness of Dermabond versus sutures for lacerated wound closure: a randomised controlled trialHong Kong Med J201117Suppl 648
- XieYFuQChenZQComparison between two pedicle screw augmentation instrumentations in adult degenerative scoliosis with osteoporosisBMC Musculoskelet Disord20111228622188765
- LuZYiXFengWCost-benefit analysis of laparoscopic surgery versus laparotomy for patients with endometrioid endometrial cancer: experience from an institute in ChinaJ Obstet Gynaecol Res20123871011101722487546
- YangJWeiWQNiuJLiuZCYangCXQiaoYLCost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of ChinaWorld J Gastroenterol201218202493250122654446
- ShengQSLinJJChenWBHand-assisted laparoscopic versus open right hemicolectomy: short-term outcomes in a single institution from ChinaSurg Laparosc Endosc Percutan Tech201222326727122678326
- WangZHGaoQYFangJYRepeat colonoscopy every 10 years or single colonoscopy for colorectal neoplasm screening in average-risk Chinese: a cost-effectiveness analysisAsian Pac J Cancer Prev20121351761176622901118
- HuangLHZhangLTobeRYCost-effectiveness analysis of neonatal hearing screening program in China: should universal screening be prioritized?BMC Health Serv Res2012129722510223
- WuJXLiBLiuTEighty-six cases of laparoscopic vaginoplasty using an ileal segmentChin Med J2009122161862186619781361
- XiaWSheSLamTHMedical versus surgical abortion methods for pregnancy in China: a cost-minimization analysisGynecol Obstet Invest20117225726321997301
- ChenMLiZHeQA prospective cost-utility study of early renal replacement therapyChin J Evid Based Med200447453459
- ChenLHuangRXiaoJCost analysis of laparoscopy in treatment of acute abdomenZh J Traumatic2012174436438
- FanHValidity evaluation and cost-effectiveness of CT and MRI for diagnosis of cerebrovascular disease, ThesisShanxi Medical UniversityShanxi2007
- GuALiuJSunXShiYHuangGCost-effective analysis of PET application in NSCLCChin J Nucl Med20062628992
- FuMGuYSongDLengBWangQYingXComparison of outcome and cost of endovascular coiling versus surgical clipping in the treatment of ruptured anterior or posterior communicating artery aneurysm aneurysmsInt J Cerebrovasc Dis2011194269274
- GuanYCaoBLiuXZhaoXLiuJHuangLComparison in health economics between laparoscopic and open myomectomyChin Med Factory Mine2007203253254
- GuoXLiuMYanRLiWZhangJGuoYEconomic analysis of the imaging diagnosis for small hepatomaChin Med Dev2009247913
- HanQQiMJiaYZhaoHCost-effectiveness analysis of long-time domiciliary noninvasive positive pressure ventilation in the treatment of chronic obstructive pulmonary disease complicated with chronic respiratory failureInt J Respir2011315348350
- HeTLiuLLiangXZhouJXieXDaiQAnalysis of effectiveness and health economics of myomectomy using laparoscopy and laparotomyJ Gannan Med Uni20123217879
- HouXYangXZenQHuangHHuangJCost-effectiveness of dual-source CT in the evaluation of patients with chest painChin Mod Med201118258384
- GenYRetrospective economic analysis in the cost and disease burden of hemodialysis and peritoneal dialysis, ThesisTianjin Medical UniversityTianjin2008
- GuoYPercutaneous transhepatic metal versus plastic stent implantation for treating malignant biliary obstruction: influence factors of clinical efficacy and cost-effectiveness analysis (A multiple center research), ThesisFirst Military Medical UniversityGuangzhou2001
- LanHHuangBWangSTanXLiaoYQinTAnalysis of care costs of tube-humidifying systems in different breathing machinesJ Nur200714614
- LiWLiuYCost analysis of laparoscopy in treatment of tubal pregnancyAcad J Sec Mil Med Univ2003243282284
- LiuJLiuHLiTCoflex interspinous dynamic reconstruction and 360° fusion for single level lumbar degenerative disease: a cost-utility analysisChin J Evid Based Med2011118893898
- MaCBuXZhouWEfficacy and cost analysis of microsurgical clipping and vascular embolization in treatment of patients with cerebral aneurysmsChin J Neuromed2012117709712
- PengLTanCChenGStudies on the methods of cost-utility analysis for cholelithiasis surgery patientsJ Chin Pharm2007182922452247
- TanXJLangJHShenKOperative approaches, indications, and medical economics evaluation of 4180 cases of hysterectomyActa Acad Med Sin2003254406409
- TanHLaparoscopic versus open common bile duct exploration and choledocholithotomy: a review study of outcomes, quality of life, and costs, ThesisPeking Union Medical CollegeBeijing2007
- WangDWangMLiuKCost-effectiveness analysis on surgical treatment for intracerebral hemorrhageChin J Med Guide200682127130
- WangXLuJYuWWeiYFangMOuyangJComparison of clinical effect and costs between percutaneous vertebroplasty and kyphoplasty for the treatment of acute osteoporotic vertebral mild compression fracturesCli Orthop2012152125128
- WeiXLuYYangHCost-effectiveness and outcome analysis of laparoscopic modity hysterectomy for cervical carcinoma in early stageChin J Endoscopy2011173243246
- WenJJiYZhengZMaZPengYYuXCost-effectiveness analysis of hemodialysis, CAPD and kidney transplantationChin J Nephrol20052110616619
- XiaJEfficacy and cost analysis of laparoscopic and open surgery for colon cancerGuide Chin Med2010822141143
- YuNAssessment of laparoscopic hernia repair: its safety, effectiveness and economy, ThesisFudan UniversityShanghai2007
- ZhouSLiuQGongLComparison of laparoscopic and open anterior resection for rectal cancer with anal sphincter preservationChin J Endoscopy2011177695698
- WuSZenZHealth economic evaluation of the small incision phacoemulsification cataract surgery by the clinical pathway in the primary hospitalInt J Ophthalmol2010101121712173
- LiuBChenXLinXThe cost-effective comparison of laparoscopic splenectomy by two different pedicle division strategy for the treatment of ITPJ Hepatopancreatobil Surg20112313134
- HanYHaoGChenBZenLLiuQClinical economical evaluation for three surgery methods of angle-closure glaucoma combined cataractJ Clin Ophthalmol2006143238240
- LiuCLiJGZhouQThe cost-efficiency and safety of bedside forceps dilatational tracheostomy in the intensive care unitChin Crit Care Med2010229537539
- LvFTangJLuoYCost-effectiveness analysis of the focal injection treatment of the severe abdominal parenchymal organs traumaChin J Ultrasonogr20112013437
- GarattiniLDe CompadriPClementeRCornagoDEconomic evaluations in Italy: a review of the literatureInt J Technol Assess Health Care200319468569115095774
- Al-AqeelSAState of health economic evaluation research in Saudi Arabia: a reviewClinicoecon Outcomes Res2012417718422826634
- MachadoMIskedjianMEinarsonTRQuality assessment of published health economic analyses from South AmericaAnn Pharmacother200640594394916670369
- SorensonCTarriconeRSiebertMDrummondMApplying health economics for policy decision making: do devices differ from drugs?Europace201113Suppl 2ii54ii5821518751
- YothasamutJTantivessSTeerawattananonYUsing economic evaluation in policy decision-making in Asian countries: mission impossible or mission probable?Value Health200912Suppl 3S26S3020586976
- HusereauDDrummondMPetrouSISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task ForceConsolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task ForceValue Health201316223125023538175
- KangMZhaoYHuangYLiJLiuLLiHAccuracy and direct medical cost of different screening modalities for breast cancer among Chinese womenZhonghua Zhong Liu Za Zhi2014363236240 Chinese24785288
- ZhangXGZhangZLHuSYWangYLUltrasound-guided ablative therapy for hepatic malignancies: a comparison of the therapeutic effects of microwave and radiofrequency ablationActa Chir Belg20141141404524720137
- TaoMFRongRShaoHFXiaJThe cost-effectiveness analysis of laparoscopic treatment of ectopic pregnancy: a single-center review of a five-year experienceClin Exp Obstet Gynecol2014411242724707677
- ChengYJiangZSXuXPLaparoendoscopic single-site cholecystectomy vs three-port laparoscopic cholecystectomy: a large-scale retrospective studyWorld J Gastroenterol201319264209421323864785
- ShenDYeHWangYComparison of short-term outcomes between laparoscopic greater curvature plication and laparoscopic sleeve gastrectomySurg Endosc20132782768277423443480
- KeSDingXMGaoJA prospective, randomized trial of Roux-en-Y reconstruction with isolated pancreatic drainage versus conventional loop reconstruction after pancreaticoduodenectomySurgery2013153674375223601899
- WongCLukIWIpMYouJHPrevention of gram-positive infections in peritoneal dialysis patients in Hong Kong: a cost-effectiveness analysisAm J Infect Control201442441241624679568
- TobeRGMoriRHuangLXuLHanDShibuyaKCost- effectiveness analysis of a national neonatal hearing screening program in China: conditions for the scale-upPLoS One201381e5199023341887
- China Ministry of Health2012 China Healthcare Development Report2013 Available from: http://www.moh.gov.cn/mohwsbwstjxxzx/s7967/201306/fe0b764da4f74b858eb55264572eab92.shtmlAccessed August 19, 2013
- YarwoodJGrowth status and market environment of medical supplies in China4th China International Medical Device Summit2013Shanghai, China