Abstract
Background
Preexisting gastrointestinal (GI) events may deter the use of pharmacologic treatment in patients diagnosed with osteoporosis (OP). The objective of this study was to examine the association between preexisting GI events and OP pharmacotherapy initiation among women diagnosed with OP.
Methods
The study utilized claims data from a large US managed care database to identify women aged ≥55 years with a diagnosis code for OP (index date) during 2002–2009. Patients with a claim for pharmacologic OP treatment in the 12-month pre-index period (baseline) were excluded. OP treatment initiation in the post-index period was defined as a claim for bisphosphonates (alendronate, ibandronate, risedronate, zoledronic acid), calcitonin, raloxifene, or teriparatide. During the post-index period (up to 12 months), GI events were identified before treatment initiation. A time-dependent Cox regression model was used to investigate the likelihood of initiating any OP treatment. Among patients initiating OP treatment, a discrete choice model was utilized to assess the relationship between post-index GI events and likelihood of initiating with a bisphosphonate versus a non-bisphosphonate.
Results
In total, 65,344 patients (mean age 66 years) were included; 23.7% had a GI event post diagnosis and before treatment initiation. Post-index GI events were associated with a 75% lower likelihood of any treatment initiation (hazard ratio 0.25; 95% confidence interval 0.24–0.26). Among treated patients (n=23,311), those with post-index GI events were 39% less likely to receive a bisphosphonate versus a non-bisphosphonate (odds ratio 0.61; 95% confidence interval 0.54–0.68).
Conclusion
GI events after OP diagnosis were associated with a decreased likelihood of OP treatment initiation and an increased likelihood of treatment initiation with a non-bisphosphonate versus a bisphosphonate.
Introduction
Recent estimates suggest that 15.4% of US women aged 50 years or older have osteoporosis (OP) and an additional 51.4% have evidence of low bone density.Citation1 An increased risk of fracture is the most significant consequence of OP and low bone density. There were an estimated 2 million OP-related fractures in the USA in 2005 and the number of fractures is projected to increase to more than 3 million annually by 2025.Citation2 Pharmacologic treatment for OP can reduce fracture risk, and is appropriate for many patients with OP. A previous study concluded that 30% of US women aged 50 years or older warrant consideration for OP treatment, based on their risk for fracture.Citation3 Currently, several therapies are approved for OP treatment by the US Food and Drug Administration to reduce fracture risk, including bisphosphonates (alendronate, ibandronate, risedronate, zoledronic acid), calcitonin, raloxifene, teriparatide, and denosumab.Citation4
Despite the availability of multiple treatment options, recent studies indicate a substantial proportion of US women at risk of fracture remain untreated. In the US cohort of the Global Longitudinal Study of Women, only 65% of women with a self-reported diagnosis of OP reported taking anti-resorptive treatment.Citation5 Studies based on electronic medical records or administrative claims documentation of pharmacologic OP treatment initiation suggest under-treatment is considerably greater.Citation6,Citation7 In a Pennsylvania study, less than 20% of women who had experienced a fracture were receiving oral bisphosphonate in the year following the fracture, and only 49% of women with an OP diagnosis or T-score indicative of OP received bisphosphonate treatment within 1 year of their diagnosis or bone mineral density test result.Citation6 A study of a national US claims database reported that only 36% of women had evidence of receipt of any OP treatment in the first year following their OP diagnosis.Citation7
The barriers to treatment initiation are not well understood. Certain patient characteristics appear to decrease the likelihood of treatment, including younger age, lower education level, higher bone mineral density T-score, higher body mass index, and lack of concomitant corticosteroid use.Citation6,Citation8,Citation9 Patient concern over OP medication side effects has also been linked with nontreatment initiation.Citation10 Physicians may also be unwilling to prescribe OP treatment for their patients with OP due to concurrent medical conditions. Bisphosphonates are the most widely prescribed therapy and contraindicated for patients with severe renal insufficiency. Yet, a study of electronic medical records found that the most common reason (28%) for not prescribing bisphosphonates to patients diagnosed with OP was a preexisting gastrointestinal (GI) diagnosis.Citation11 Among patients who do initiate OP treatment, GI intolerance has also been cited as a common cause for OP treatment discontinuation.Citation12–Citation14 However, the risk of GI events while on OP treatment is also a function of preexisting GI conditions.Citation15
The relationship between preexisting GI events and treatment initiation has not been well explored in a managed care population in the USA. The objective of this study was to examine the association between preexisting GI events and likelihood of treatment initiation among women diagnosed with OP within a managed care setting in the USA.
Materials and methods
Study design
A retrospective cohort study was conducted using a large national claims database known as the Optum Insight Clinformatics Data Mart. This database was designed to primarily support health care outcomes research and other research initiatives. The database includes demographic, medical, and pharmacy claims for more than 80 million managed care enrollees in more than 45 health plans throughout the USA. Over 3 million patients with OP were included in the database during the study period from January 2001 to December 2010. All study data were de-identified and accessed using techniques compliant with the Health Insurance Portability and Accountability Act; therefore, Institutional Review Board approval was not required.
Within the study period – January 1, 2001 to December 31, 2010 – an index date was assigned during January 1, 2002 to December 31, 2009. The index date was defined as the date of the first OP diagnosis observed. The 1-year period before the index date represented the pre-index period, while the post-index period began the day after the index date and lasted for 12 months or until the date that OP medications were initiated, if less than 12 months. Therefore, patients who did not initiate OP treatment had a full 12 months of follow-up, while those who initiated treatment had less than 12 months of follow-up.
Study sample
Women aged 55 years or older on the index date with a primary or secondary diagnosis of OP (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.0x) were included. Patients were required to be continuously enrolled in the health plan for at least 1 year before and 1 year after the index date, and to be OP treatment-naïve, defined as no claims for OP medications prior to the index date. Patients were excluded if they had evidence of estrogen therapy in the pre-index period, a diagnosis of malignant neoplasm (ICD-9-CM codes 140–170, 173–208, 230–239) in the pre-index period or in the 1 year after the index date, or any claim for Paget’s disease (ICD-9-CM code 731.0) ever in the claims history.
Measures
The occurrence of a GI event was defined by the presence of a claim with a GI-related ICD-9-CM diagnostic or Current Procedural Terminology code (see ), including esophagitis, esophageal reflux, ulcer, stricture, perforation, hemorrhage of esophagus, gastric ulcer, duodenal ulcer, peptic ulcer, acute gastritis, duodenitis, GI hemorrhage, nausea or vomiting, and dysphagia. GI events were separately captured for the pre-index and post-index periods.
Patients who received at least one OP medication during the post-index period were characterized as “treated”, while all others were considered to be “untreated”. OP medications were identified based on National Drug Codes and Healthcare Common Procedure Coding System codes, and included all forms (oral, injectable, infused) of bisphosphonates (alendronate, ibandronate, risedronate, zoledronic acid) and all forms of non-bisphosphonates (calcitonin, raloxifene, and teriparitide). Denosumab was excluded because it was not approved until June 2010, near the end of the study period. Classifications of OP medications were determined by National Drug Codes (). Patient age was determined on the index date. The pre-index Charlson Comorbidity Index (CCI) score was computed.Citation16 The CCI is used to predict the mortality of a patient based on the occurrence of 19 predefined comorbid conditions. Higher CCI denotes higher mortality. The presence of comorbid conditions, fracture history, and medication use (gastroprotective agents, nonsteroidal anti-inflammatory drugs [NSAIDs], and glucocorticoids) were also determined during the pre-index period. Fractures during the pre-index period were identified from ICD-9-CM codes ().
Statistical analysis
Patient characteristics were summarized for the entire cohort as well as by the presence or absence of a pre-index GI event. For continuous variables (age and CCI score), means and standard deviations were calculated. The number and proportion of patients with pre-index comorbid conditions, pre-index fractures, pre-index medication use, and pre-index and post-index GI events were tabulated. Comparisons of baseline characteristics between patients with and without a pre-index GI event were performed using χ2 tests for categorical variables and t-tests for continuous variables.
To determine the timing and type of OP treatment initiation, prescription claims after the index date were searched, and used to classify patients as receiving “no treatment”, “bisphosphonates”, or “non-bisphosphonates”. The proportion of patients in each classification was calculated and compared between patients with and without post-index GI events. χ2 tests were used to compare the treatment distributions of patients with and without GI events.
Time-varying Cox regression (which takes into consideration the timing of the post-index GI events before treatment initiation) was used to estimate the likelihood of treatment initiation for any OP treatment, stratified by the presence of pre-index GI events. For treated patients, a discrete choice model was used to estimate the likelihood of receiving a bisphosphonate versus a non-bisphosphonate. In the Cox model and the discrete choice model, the independent variable of interest was the presence of a post-index GI event; models were also adjusted for the presence of pre-index GI events (discrete choice model only), age group, pre-index medication use (gastroprotective agents, NSAIDs, glucocorticosteroids), pre-index CCI, and selected pre-index comorbidities (chronic inflammatory bowel disease, chronic inflammatory joint disease, celiac disease, diabetes, depression, chronic kidney failure, hypertension, GI mucositis and urination problems, hyperparathyroidism, vitamin D deficiency, and fatigue). The Cox regression model separately quantified the effects of a post-index GI event for those with and without pre-index GI events.
Results
Patient characteristics
A total of 65,344 women were identified for analysis (). The average age at diagnosis was 65.7 years, and 17,828 patients (27.3%) experienced a GI event during the pre-index period (). Among all patients, the most common comorbid conditions were hypertension (48%), chronic inflammatory joint disease (21%), and fatigue (16%). Patients with a pre-index GI event were more likely to have each of the comorbid conditions evaluated than patients without a pre-index GI event (P<0.001). Patients with a pre-index GI event were more likely to use gastro-protective agents (37.8% versus 7.3%), NSAIDs (26.4% versus 19.8%), and glucocorticoids (18.8% versus 11.7%) than patients without a pre-index GI event (P<0.001). The occurrence of a GI event during the pre-index GI event was also associated with a higher mean CCI score (1.01 versus 0.52, P<0.001) and a higher rate of fractures (9.0% versus 6.0%, P<0.001).
Table 1 Patient characteristics in the pre-index periodTable Footnotea
Osteoporosis treatment
The frequency of OP treatment within this cohort has been previously reported.Citation7 Briefly, 42,033 (64.3%) patients received no OP medication within the first year following the OP diagnosis. A total of 23,311 (35.7%) patients received treatment: 20,200 (30.9%) with a bisphosphonate and 3,111 (4.9%) with a non-bisphosphonate.Citation7
GI events and OP treatment initiation
Overall, 24% of all patients experienced a GI event in the post-index period, although GI events were more common among those who had experienced a pre-index GI event (43.4% versus 16.2%, ). The rate of pre-index GI events was similar among patients who did and did not initiate treatment (bisphosphonate or non-bisphosphonate, ). However, the occurrence of post-index GI events appeared to be associated with OP treatment initiation (). The proportion of patients receiving no OP treatment was 85.1% among patients with a post-index GI event versus 57.9% among patients with no post-index GI event. Among treated patients, 11.7% of patients with a post-index GI event initiated treatment with a bisphosphonate compared with 36.9% of patients without a post-index GI event.
Figure 2 Treatment with medications for osteoporosis in the post-index period by (A) pre-index GI events and (B) post-index GI events.
Abbreviation: GI, gastrointestinal.
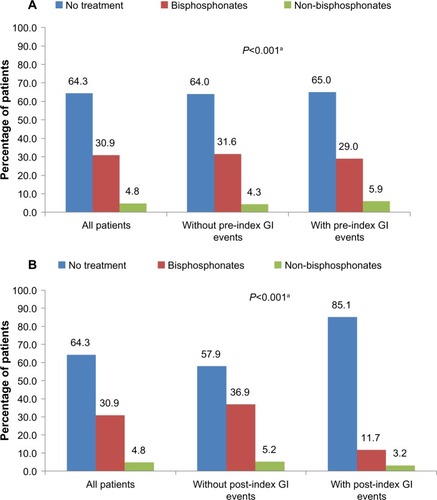
Table 2 Distribution of GI events in the pre-index and post-index periods
Multivariate analysis for association between GI events and OP treatment initiation
The results of the time-varying Cox regression model for the association between post-index GI events and any OP treatment (bisphosphonate or non-bisphosphonate) initiation are shown in . Among all covariates included in the analysis, the occurrence of post-index GI events had the greatest impact on OP treatment initiation. Patients who experienced post-index GI events were 75% less likely to initiate OP treatment than patients without post-index GI events (hazard ratio 0.25; 95% confidence interval [CI] 0.24–0.26; P<0.0001). Hypertension, chronic inflammatory joint disease, and diabetes were also significantly associated with a lower likelihood of OP treatment initiation (P≤0.04), while depression and use of gastroprotective agents, NSAIDs, and glucocorticoids were associated with a higher likelihood of treatment initiation (P<0.001).
Table 3 Time-varying Cox regression model for the association between post-index GI events and any osteoporosis treatment (bisphosphonate or non-bisphosphonate) initiation
The results of the discrete choice model among patients who initiated OP treatment are shown in . Patients with a post-index GI event were 39% less likely to receive a bisphosphonate than a non-bisphosphonate compared with those without a post-index GI event (odds ratio 0.61; 95% CI 0.54–0.68; P<0.0001). A pre-index GI event, older age, and use of gastroprotective agents also significantly decreased the likelihood of bisphosphonate treatment initiation but to a lesser extent than post-index GI events. Comorbid diabetes predicted a slightly higher likelihood of treatment initiation with a bisphosphonate, and GI mucositis and urinary problems were associated with a lower likelihood of treatment initiation with a bisphosphonate.
Table 4 Discrete choice model examining the association between post-index GI events and receipt of bisphosphonate among patients who initiated osteoporosis treatment
Discussion
In this population of US women diagnosed with OP, a majority (64.3%) received no OP medication in the year following their OP diagnosis. GI events in the post-index period strongly decreased the likelihood of initiating any treatment for OP. Among patients who initiated treatment, the presence of a GI event in the post-index period also decreased the likelihood of treatment with a bisphosphonate versus a non-bisphosphonate.
While published data regarding the association of GI events and OP treatment is limited, some research suggests a possible relationship. In a study of elderly women who had recently experienced a fracture, preexisting GI disease was associated with a modest but nonsignificantly lower odds of receiving treatment.Citation17 Foster et al reported that certain pre existing GI conditions increased the likelihood of treatment with raloxifene versus bisphosphonates in a commercial/Medicare cohort (but not a Medicaid cohort).Citation18 However, the proportion of patients who experienced a post-index GI event prior to treatment initiation in our study was significantly higher than that observed by Foster et al (24% versus 1%–3%). In addition, the definition of what constituted a GI event differed, as we used a more comprehensive series of codes and examined the collective impact of all GI events on choice of OP treatment, rather than individual categories of GI events (eg, peptic ulcer and gastric ulcer). Our study also examined both baseline and post-index GI events. Our results indicate that baseline GI events did not appear to impact treatment initiation, but GI events that more closely coincided with OP diagnosis (ie, post-index GI events) strongly decreased the likelihood of treatment initiation. These results suggest that the clinical management of patients diagnosed with OP should take into account not only GI event history but also GI events that may occur after OP diagnosis.
Twenty-seven percentage of patients in this study had a GI event prior to their OP diagnosis and 23.7% experienced a GI event after their OP diagnosis (but before OP treatment initiation). In the POSSIBLE-US study, approximately 20% of patients initiating OP medication reported GI symptoms at study entry.Citation19 The higher rate of GI events we observed may be a function of the length of the observation period for GI events and the definition of GI events. We captured baseline GI events during the full 12-month period prior to diagnosis and post-OP diagnosis events up until treatment initiation or 12 months for patients who did not initiate treatment. We also relied on medical claims for documentation of a GI event. In contrast, the POSSIBLE-US study identified GI symptoms “at study entry” and was based on patient report. Irrespective of the methodologic differences, the results of both studies indicate that GI events are common among women with OP.
We found other patient characteristics also predicted OP treatment initiation, but to a lesser extent than post-index GI events. Use of gastroprotective agents, NSAIDS, and glucocorticoids prior to OP diagnosis were each significantly associated with an increased likelihood of receiving OP treatment. Previous studies have noted a similar association between glucocorticoid or corticosteroid use and treatment initiation.Citation6,Citation17,Citation20 Although a higher comorbidity score was not associated with lower likelihood of treatment initiation, the presence of specific comorbid conditions (hypertension, chronic inflammatory disease, and diabetes) independently predicted lower likelihood of OP treatment initiation. Considering that diabetes and hypertension are common in the US populationCitation21,Citation22 and that diabetes in particular has been linked with an increased risk of fracture in older women,Citation23–Citation25 the potential impact of comorbid conditions on treatment initiation warrants further research.
The reasons for not initiating treatment may lie with a physician’s decision to not prescribe or patients not electing to receive treatment. GI disorders have been linked with physician prescribing patterns. A study of electronic medical records revealed that GI diagnoses were the most common (28%) reason for not prescribing oral bisphosphonates to patients diagnosed with OP, followed by functional status (24%) and renal impairment (12%).Citation11 Patients who did not initiate OP treatment are more likely to report a concern over OP medication side effects than patients who begin treatment,Citation10 and the risk of GI side effects in particular may also influence patient treatment preferences.Citation26 In a discrete choice experiment of preferred OP treatments, concern over the risk of GI side effects ranked higher than other side effects evaluated.Citation26 Patients with preexisting GI disorders prior to treatment also have a high risk of experiencing GI events while on treatment,Citation15 and GI side effects while on treatment are associated with worse adherence to treatmentCitation27 and greater likelihood of early treatment discontinuation.Citation19,Citation28,Citation29 Our results coupled with previous research suggest that GI events may pose a significant barrier to effective treatment of OP.
This study is subject to the limitations inherent in a retrospective analysis of a claims database. Claims data are collected primarily for payment purposes, not research, and are subject to coding errors. The presence of a claim for a filled prescription does not necessarily indicate that the medication was taken or that it was taken as prescribed. In addition, this study was designed to assess all bisphosphonates as a group. However, different bisphosphonates may have different adverse effect profiles,Citation30 and the timing (eg, daily versus monthly) and type (oral versus intravenous) of the dosing regimen may be a determinant of these profiles.Citation12 The length of the study period (10 years) includes a span during which clinical patterns of bisphosphonate use may have been changing from daily to weekly regimens.Citation31 From the available data, we are not able to ascertain why OP treatment was not initiated; it may reflect a clinical decision not to prescribe treatment or the failure of a patient to fill a prescription. Over-the-counter drugs such as low-dose NSAIDs and aspirin are not included in this analysis. Only GI events that resulted in medical service utilization were captured in this analysis; therefore, the association observed may reflect more severe GI events. Also, bone mineral density test results to support the claims diagnosis of OP were not available in the database. Finally, there may have been other unmeasured patient and clinician characteristics that influenced OP treatment initiation and receipt of a bisphosphonate versus a non-bisphosphonate treatment.
Conclusion
This study advances the understanding of how GI events impact treatment of OP. In a US managed care population of women with an incident diagnosis of OP, approximately one of four patients diagnosed with OP had a GI event post diagnosis and before treatment initiation, of whom 85% did not receive any treatment. Patients with a post-diagnosis GI event were 75% less likely to initiate treatment (versus those without a GI event). Among patients who initiated OP treatment, post-diagnosis GI events were associated with a 39% lower likelihood of initiation with a bisphosphonate compared with a non-bisphosphonate therapy. New therapies may be needed to address the unmet need for OP treatments for patients with prior and existing GI events.
Acknowledgments
Medical writing assistance was provided by Melissa Stauffer and Lauren Weisenfluh in collaboration with SCRIBCO, and was funded by Merck & Co., Inc., Kenilworth, NJ, USA.
Supplementary material
Table S1 Codes used to identify gastrointestinal events, fractures, and use of bisphosphonates and non-bisphosphonates
Disclosure
Study funding was provided by Merck & Co, Inc., Kenilworth, NJ, USA. Ankita Modi, Shiva Sajjan and Shuvayu Sen are employees of Merck & Co., Inc. Ethel S Siris has done consulting for Amgen, Eli Lilly, Merck, Novartis, AgNovos and Radius. Jackson Tang was a paid consultant to Merck in connection with this study and development of this manuscript.
References
- WrightNCLookerACSaagKGThe recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spineJ Bone Miner Res201429112520252624771492
- BurgeRDawson-HughesBSolomonDHWongJBKingATostesonAIncidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025J Bone Miner Res200722346547517144789
- Dawson-HughesBLookerACTostesonANJohanssonHKanisJAMeltonLJ3rdThe potential impact of the National Osteoporosis Foundation guidance on treatment eligibility in the USA: an update in NHANES 2005–2008Osteoporos Int201223381182021717247
- WattsNBBilezikianJPCamachoPMAmerican Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosisEndocr Pract201016Suppl 313721224201
- GugginaPFlahiveJHoovenFHCharacteristics associated with anti-osteoporosis medication use: data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) USA cohortBone201251697598022964142
- AscheCNelsonRMcAdam-MarxCJhaveriMYeXPredictors of oral bisphosphonate prescriptions in post-menopausal women with osteoporosis in a real-world setting in the USAOsteoporos Int20102181427143619798459
- SirisESModiATangJGandhiSSenSSubstantial under-treatment among women diagnosed with osteoporosis in a US managed-care population: a retrospective analysisCurr Med Res Opin201430112313024102262
- BrennanRMWactawski-WendeJCrespoCJDmochowskiJFactors associated with treatment initiation after osteoporosis screeningAm J Epidemiol2004160547548315321845
- ColeRPPalushockSHaboubiAOsteoporosis management: physicians’ recommendations and womens’ compliance following osteoporosis testingWomen Health199929110111510427644
- YoodRAMazorKMAndradeSEEmaniSChanWKahlerKHPatient decision to initiate therapy for osteoporosis: the influence of knowledge and beliefsJ Gen Intern Med200823111815182118787907
- KamnevaONyeAMHamrickIReasons for bisphosphonate exclusion in patients treated for osteoporosisConsult Pharm201126532533121733813
- BlumentalsWAHarrisSTColeREHuangLSilvermanSLRisk of severe gastrointestinal events in women treated with monthly ibandronate or weekly alendronate and risedronateAnn Pharmacother200943457758519318598
- EttingerJPressmanAScheinJChanJSilverPConnollyNAlendronate use among 812 women: prevalence, complaints, noncompliance with patient instructionsJ Managed Care Pharm1998488492
- HamiltonBMcCoyKTaggartHTolerability and compliance with risedronate in clinical practiceOsteoporos Int200314325926212730745
- VestergaardPSchwartzKPinholtEMRejnmarkLMosekildeLGastric and esophagus events before and during treatment of osteoporosisCalcif Tissue Int201086211011519957165
- DeyoRACherkinDCCiolMAAdapting a clinical comorbidity index for use with ICD-9-CM administrative databasesJ Clin Epidemiol19924566136191607900
- BlockAESolomonDHCadaretteSMMogunHChoudhryNKPatient and physician predictors of post-fracture osteoporosis managementJ Gen Intern Med20082391447145118584260
- FosterSAFoleyKAMeadowsESCharacteristics of patients initiating raloxifene compared to those initiating bisphosphonatesBMC Womens Health200882419105828
- WooCGaoGWadeSHochbergMCGastrointestinal side effects in postmenopausal women using osteoporosis therapy: 1-year findings in the POSSIBLE US studyCurr Med Res Opin20102641003100920201623
- PressmanAForsythBEttingerBTostesonANInitiation of osteoporosis treatment after bone mineral density testingOsteoporos Int200112533734211444079
- GillespieCDHurvitzKACenters for Disease Control and PreventionPrevalence of hypertension and controlled hypertension – United States, 2007–2010MMWR Surveill Summ201362Suppl 314414824264505
- Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health Interview StatisticsCrude and age-adjusted percentage of civilian, noninstitutionalized adults with diagnosed diabetes, United States, 1980–2011 Available from: http://www.cdc.gov/diabetes/statistics/prev/national/figageadult.htmAccessed September 22, 2014
- NicodemusKKFolsomARIowa Women’s Health StudyType 1 and type 2 diabetes and incident hip fractures in postmenopausal womenDiabetes Care20012471192119711423501
- SchwartzAVSellmeyerDEEnsrudKEOlder women with diabetes have an increased risk of fracture: a prospective studyJ Clin Endocrinol Metab2001861323811231974
- TaylorBCSchreinerPJStoneKLLong-term prediction of incident hip fracture risk in elderly white women: study of osteoporotic fracturesJ Am Geriatr Soc20045291479148615341549
- HiligsmannMDellaertBGDirksenCDPatients’ preferences for osteoporosis drug treatment: a discrete-choice experimentArthritis Res Ther2014161R3624479410
- ReynoldsKViswanathanHNO’MalleyCDPsychometric properties of the osteoporosis-specific Morisky Medication Adherence Scale in postmenopausal women with osteoporosis newly treated with bisphosphonatesAnn Pharmacother201246565967022510666
- LoJCPressmanAROmarMAEttingerBPersistence with weekly alendronate therapy among postmenopausal womenOsteoporos Int200617692292816609824
- YunHCurtisJRGuoLPatterns and predictors of osteoporosis medication discontinuation and switching among Medicare beneficiariesBMC Musculoskelet Disord20141511224684864
- No authors listedManagement of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause SocietyMenopause2010171255420061894
- GoldDTBonnickSLAmonkarMMKamelHKAgarwalSZaidiMDescriptive analysis of concomitant prescription medication patterns from 1999 to 2004 among US women receiving daily or weekly oral bisphosphonate therapyGend Med20085437438419108810