Abstract
The burden of methicillin-resistant Staphylococcus aureus (MRSA) nosocomial pneumonia in the People’s Republic of China is high, with methicillin-resistance rates greater than 80% reported for patients with S. aureus pneumonia treated in intensive care units. Historically, vancomycin was the treatment of choice for patients with hospital-acquired MRSA infections. Recent evidence suggests that the minimum inhibitory concentration for vancomycin is increasing. Additionally, patients treated with vancomycin require monitoring of vancomycin trough concentrations and can develop nephrotoxicity. Linezolid is a treatment option for patients with hospital-acquired MRSA infections that can be administered either intravenously or orally. Analysis of data from a worldwide linezolid surveillance program initiated in the year 2004 shows no evidence of increasing linezolid minimum inhibitory concentrations. The clinical efficacy of linezolid for patients with gram-positive, including MRSA, nosocomial pneumonia, was evaluated in numerous studies. In general, results from these studies show higher or similar clinical success with no mortality difference for linezolid compared to vancomycin treated patients. Results from a Phase IV study enrolling patients with MRSA-confirmed nosocomial pneumonia suggest higher clinical cure rates for linezolid compared to vancomycin treated patients. Although acquisition costs are higher for linezolid compared to vancomycin therapy, evidence suggests similar overall medical costs. Cost-analysis results from a Chinese perspective show that linezolid dominated vancomycin therapy for MRSA nosocomial pneumonia in ∼35% of bootstrap simulations whereas vancomycin dominated linezolid in less than 2% of bootstrap simulations. In summary, results from both clinical and economic studies, including studies conducted from a Chinese perspective, support the use of linezolid for the treatment of patients with MRSA nosocomial pneumonia.
Introduction
Globally, antibacterial resistance negatively affects patient outcomes and health care costs.Citation1 The ability to treat many common health care- and community-associated infections is threatened by the development of resistant bacteria.Citation1 Staphylococcus aureus is an example of a pathogenic, gram-positive organism that over time developed resistance to many available antibiotics, including penicillin and beta-lactamase stable penicillins such as methicillin and oxacillin.
S. aureus is frequently found on the skin and in the nose and is a common cause of skin and soft tissue, bone, bloodstream, and postoperative wound infections. The percentage of S. aureus resistant to methicillin is greater than 20% in many geographical regions and exceeds 80% in some World Health Organization regions.Citation1 Evidence suggests an increasing role of methicillin-resistant S. aureus (MRSA) in patients with hospital-acquired pneumonia (HAP).Citation2 Results from several studies specific to the People’s Republic of China or the Asia region show that S. aureus plays a major role in patients with HAP, including ventilator associated pneumonia (VAP).Citation3–Citation5 Results from a systematic review and meta-analysis show that S. aureus is the fourth most frequently isolated pathogen, and 82.9% of S. aureus isolates were methicillin-resistant in patients with ICU-acquired pneumonia or VAP in the People’s Republic of China.Citation5 Whereas community-acquired S. aureus strains tend to maintain sensitivity to non-beta-lactam antibiotics such as clindamycin and trimethoprim/sulfamethoxazole, hospital-acquired strains are often multidrug-resistantCitation3,Citation5–Citation7 with few treatment options.
Vancomycin and linezolid are treatment options for patients with gram-positive infections including those caused by MRSA. Results from a published Phase IV trial (Zyvox® in the Treatment of Subjects with Nosocomial Pneumonia Proven to be Due to Methicillin-Resistant Staphylococcus aureus [ZEPHyR]) suggest higher clinical success and similar mortality in patients with MRSA nosocomial pneumonia treated with linezolid compared to vancomycin therapy.Citation8 Although acquisition costs are higher for linezolid than vancomycin, analysis of health care resource utilization from a Chinese perspective using outcomes data from the ZEPHyR trial found no difference in overall medical costs between linezolid and vancomycin treated patients.Citation9 Results from a multicenter, retrospective, observational study similarly show higher clinical success and similar mortality in patients with MRSA nosocomial pneumonia treated with linezolid compared to vancomycin therapy.Citation10 In contrast, results from a retrospective study conducted in the People’s Republic of China show similar clinical success but higher mortality in patients empirically treated with linezolid compared to vancomycin for HAP; total hospital costs trended lower for vancomycin compared to linezolid treated patients although this difference was not significant.Citation11 The objective of this review is to summarize the available clinical and economic information on the treatment of MRSA nosocomial pneumonia with a focus on linezolid and vancomycin as well as economic and clinical data available for the People’s Republic of China.
Methods
A PubMed search was performed to identify studies reporting the incidence of MRSA in the People’s Republic of China for the years 2004 through 2014. Of the 179 citations identified, ten studies reported incidence data for MRSA isolates in the People’s Republic of China.Citation6,Citation7,Citation12–Citation19 A PubMed search was also performed to identify comparative studies and meta-analyses evaluating linezolid and vancomycin therapy in adult patients with nosocomial pneumonia. Of the 35 citations identified, 24 studies or meta-analysesCitation8,Citation10,Citation11,Citation31–Citation51 compared the economic or clinical outcome of patients treated with either linezolid or vancomycin therapy and were included in this review. An additional economic study was identified through a Google search.Citation9
MRSA incidence in the People’s Republic of China
Overall
Results from studies conducted throughout the People’s Republic of China from the year 2004 through the year 2011 suggest that the burden of MRSA is high, ranging from ∼27% to 65% ().Citation6,Citation7,Citation12–Citation19 Analysis of S. aureus resistance in Chinese cities shows considerable variability ranging from a low of less than 40% at Beijing Union Medical College Hospital and Sir Run Run Shaw Hospital of Zhejiang University Medical College to a high of more than 80% at Beijing Hospital.Citation20
Table 1 Summary of results for studies reporting Staphylococcus aureus resistance to methicillin in the People’s Republic of China
Nosocomial pneumonia
The prevalence of MRSA in the People’s Republic of China may be higher in patients with respiratory than other types of infections.Citation3,Citation5–Citation7 Results from a meta-analysis and systematic review of studies published between January 2007 and May 2012 show that S. aureus was the fourth most common cause of pneumonia in patients hospitalized in intensive care units in the People’s Republic of China; 82.9% of S. aureus isolates were methicillin-resistant.Citation5 Results from a prospective surveillance study conducted in ten Asian countries by the Asian Network Surveillance of Resistant Pathogens (ANSORP) group for the years 2008 to 2009 were similar.Citation3 For the ten Asian locales included in this study, S. aureus was the most common cause of HAP accounting for 15.8% of all HAP cases and the third most common cause of VAP accounting for 12.2% of all VAP cases; 82.1% of S. aureus isolates were resistant to methicillin. Analysis of data from the People’s Republic of China only found that S. aureus was the second most common cause of HAP accounting for 16.0% of all HAP cases and the third most common cause of VAP accounting for 24% of all VAP cases; 82.4% of isolates were resistant to methicillin. Lastly, results from a prospective multicenter study performed in 13 Chinese urban tertiary hospitals showed that S. aureus was the third most common cause of HAP accounting for 13.4% of all HAP cases, and the second most common cause of VAP accounting for 21.4% of all VAP cases; 87.8% of isolates were resistant to methicillin.Citation4
MRSA pneumonia treatment
Treatment recommendations
Recommendations for the treatment of HAPCitation22 and S. aureus nosocomial pneumoniaCitation23 for the Asia region are available in the medical literature. These guidelines account for not only differences in HAP epidemiology and microbiology but also the use, availability, and cost of antibiotic therapies between the Asia region and those covered by other published guidelines such as the USCitation24,Citation25 and England.Citation26
Asian HAP Working Group
The Asian HAP Working Group published treatment recommendations for patients with HAP in Asian countries in the year 2008.Citation22 These guidelines state that inclusion of glycopeptide or linezolid therapy is a matter of clinical judgment and local sensitivity profiles but should be considered for patients with late-onset HAP or early- or late-onset VAP. Vancomycin and teicoplanin are the recommended first-line therapies; linezolid and tigecycline are the recommended second-line therapies. The working group suggested reserving linezolid for second-line MRSA therapy to avoid selection of resistant strains.
Asian Consensus Taskforce on MRSA Nosocomial Pneumonia
More recently, the Asian Consensus Taskforce on MRSA Nosocomial Pneumonia published treatment recommendations in the year 2014 to provide clinicians guidance on treating adult patients with MRSA nosocomial pneumonia in Asia taking into account regional data on MRSA colonization and vancomycin minimum inhibitory concentrations (MICs).Citation23 These recommendations stress the importance of timely appropriate antibiotic therapy with either linezolid or vancomycin for patients with suspected MRSA nosocomial pneumonia. Linezolid is recommended for patients with a high risk of vancomycin failure or intolerance defined as the following: prevailing local vancomycin MIC of 1.5 mg/L or greater, age of 65 years or greater, reduced renal function, concurrent administration of nephrotoxic drugs, body mass index greater than 30 kg/m2, or prior vancomycin therapy. Vancomycin is recommended for patients not at risk of vancomycin failure or intolerance.
Treatments
Vancomycin
Vancomycin, a glycopeptide antibiotic, traditionally was the treatment of choice for patients with MRSA infections including MRSA pneumonia. Recent evidence suggests, however, that the vancomycin MIC for staphylococci is increasing which may ultimately result in an increase in vancomycin-resistant staphylococci.Citation27 Results from a study conducted in six Chinese hospitals suggest that the vancomycin MIC for MRSA isolates is increasing. For the years 2006 to 2011, the MIC for vancomycin significantly increased over time from 0.906 to 1.040 mg/L (P<0.001 for trend). Likewise, the percentage of MRSA with a vancomycin MIC greater than 1 mg/L increased from 26.0% in the year 2006 to 42.8% in the year 2011 (P<0.005). The probability of achieving optimal vancomycin concentrations when the MIC is greater than 1 mg/L at a vancomycin dose of 0.5 to 2 g every 12 hours is unlikely.Citation23 A creep in the MIC for teicoplanin was also reported increasing from 0.749 mg/L in the year 2009 to 0.973 mg/L in the year 2011.
Other disadvantages of vancomycin therapy include slow bactericidal activity, minimal penetration into lung tissue, and nephrotoxicity.Citation23 Current recommendations for patients with nosocomial pneumonia are to achieve vancomycin trough concentrations of 15 to 20 mg/L to maximize pharmacokinetic and pharmacodynamic properties.Citation28 Nephrotoxicity with vancomycin therapy appears to increase with trough concentrations greater than 10 mg/L, especially in patients receiving concurrent nephrotoxic therapies.Citation28
Linezolid
Linezolid is a synthetic oxazolidione antibacterial agent indicated for the treatment of vancomycin-resistant Enterococcus faecium infections, complicated and uncomplicated skin and soft tissue infections caused by methicillin sensitive S. aureus (MSSA) or Streptococcus pyogenes, complicated skin and soft tissue infections caused by MRSA or Streptococcus agalactiae, and nosocomial pneumonia caused by MRSA, MSSA, or Streptococcus pneumonia including multidrug-resistant strains. Linezolid is available in both an intravenous and oral form; the oral form is 100% bioavailable allowing for a seamless transition from intravenous to oral therapy. Hematologic toxicity can occur with linezolid therapy; however, this typically occurs with long-term administration.
Linezolid activity against gram-positive organisms is monitored through the Zyvox® Annual Appraisal of Potency and Spectrum (ZAAPS) program, with data obtained from 73 medical centers in 33 countries (not including the US) from five continents and available for the years 2004 through 2013. Since inception of this worldwide surveillance program, linezolid susceptibility remains greater than 99.9% with no evidence of MIC creep.Citation29,Citation30
Clinical evidence, linezolid versus vancomycin
ZEPHyR trial
The objective of the ZEPHyR trial was to compare the efficacy, safety, and tolerability of linezolid (n=172) compared to vancomycin (n=176) therapy in adult patients with culture-proven MRSA nosocomial pneumonia.Citation8 This multinational, prospective, randomized, double-blind, multicenter study randomized patients to either intravenous linezolid 600 mg every 12 hours or intravenous vancomycin 15 mg/kg every 12 hours for 7 to 14 consecutive days. Vancomycin dosing was adjusted based on renal function and trough concentrations.
More patients treated with linezolid (n=95, 57.6%) compared to vancomycin (n=81, 46.6%) were clinically cured at end of study (P=0.042). In general, clinical success rates were 10% to 15% higher in linezolid compared to vancomycin treated patients in most populations and subgroups evaluated including patients with a vancomyin MIC of 1 μg/mL (61.5% vs 47.8%; 95% confidence interval [CI] for difference, 1.6–25.8) and APACHE II score <20 (61.6% vs 48.8%; 95% CI for difference, 0.2–25.5). Microbiological success (either eradication or presumed eradication) was also greater in linezolid than vancomycin treated patients at both end of study (58.1% vs 47.1%; 95% CI: 0.4–21.5) and end of treatment (81.9% vs 60.6%; 95% CI: 12.3–30.2) suggesting that MRSA clearance may be more complete with linezolid than vancomycin therapy.
In the intent to treat population, investigator-reported renal events defined as one or more of the following: renal failure, renal impairment, and azotemia, were two times more common in vancomycin (7.3%) compared to linezolid (3.7%) treated patients.Citation8 Investigator-reported rates of anemia, neutropenia, and thrombocytopenia were similar between the linezolid and vancomycin groups.
All-cause, 60-day mortality in both the intent to treat and modified intent to treat populations was similar between the linezolid (15.7%) and vancomycin (17.0%) treatment groups.
Phase III and other sponsor supported randomized trials
Two additional sponsor supported studies were conducted in adult patients with nosocomial pneumonia.Citation31,Citation32 Both of these studies were randomized, double-blind registration studies that compared linezolid with vancomycin for the empiric treatment of nosocomial pneumonia. Patients were randomly assigned to receive either linezolid 600 mg intravenously every 12 hours or vancomycin 1,000 mg intravenously every 12 hours (dose adjusted per an unblinded investigator). Aztreonam therapy was permitted in both treatment groups for gram-negative coverage.
Results from both studies show similar clinical cure and pathogen eradication rates for linezolid and vancomycin treated patients (). Secondary efficacy outcomes evaluated such as clinical signs and symptoms of pneumonia, chest radiograph, temperature, respiratory rate, and white blood cell count were also similar between the treatment groups. There were no significant differences in the number of deaths between the treatment groups in either study.
Table 2 Clinical cure and organism eradication rates for linezolid and vancomycin treatment groups in randomized studies enrolling patients with known or suspected gram-positive nosocomial pneumoniaTable Footnotea
In contrast to the ZEPHyR study in which enrolled patients had culture proven MRSA pneumonia, patients were enrolled in these Phase III studies for the empiric treatment of nosocomial pneumonia. Approximately 30% to 35% of patients had a gram-positive organism identified at baseline; an additional 8% to 13% of patients had both a gram-positive and gram-negative organism identified at baseline. Data for patients with S. aureus pneumonia were retrospectively combined and analyzed.Citation33 Results from this combined analysis showed a significantly higher clinical cure rate for linezolid (36 of 61 patients; 59.0%) compared to vancomycin (22 of 62 patients; 35.5%) treated patients with MRSA-confirmed nosocomial pneumonia (P<0.01); this difference remained significant following adjustment for differences in patient characteristics at baseline (odds ratio [OR], 3.3; 95% CI, 1.3–8.3; P=0.01). Kaplan–Meier analyses showed a survival difference for linezolid (80%) compared to vancomycin (63.5%) therapy in patients with MRSA pneumonia (P=0.03) which remained significant after adjusting for baseline variables (OR, 2.2; 95% CI, 1.0–4.8; P=0.01).
A second retrospective analysis evaluated data from these two Phase III trials for patients with suspected gram-positive VAP (N=544); 91 patients had documented MRSA VAP.Citation34 Clinical cure rates and hospital survival were higher for linezolid compared to vancomycin therapy for all populations evaluated. Results from logistic regression analyses showed that linezolid therapy was a significant predictor of clinical cure and hospital survival for all four populations evaluated.
A randomized, double-blind, Phase III study comparing linezolid and vancomycin therapy in patients with gram-positive infections was also performed at seven hospitals in the People’s Republic of China.Citation35 Patients enrolled had known or suspected gram-positive pneumonia or complicated skin and soft-tissue infections. Overall, S. aureus was the most common organism isolated at baseline; 75% of S. aureus isolated were resistant to methicillin. Results are summarized for patients with pneumonia only. At end of treatment and at the follow-up visit (7 to 28 days post-treatment), clinical cure rates were higher for patients treated with linezolid than vancomycin; microbiological eradication was also higher for patients treated with linezolid compared to vancomycin ().
Lastly, two additional sponsor supported studies randomized patients to linezolid or vancomycin therapy for treatment of known or suspected MRSA infections including patients with known or suspected MRSA pneumoniaCitation36 or MRSA VAP.Citation37 In the known or suspected MRSA infection study,Citation36 there was no difference in clinical cure rate (56.8% vs 55.0%; P=0.74; 95% CI for difference −8.5 to 12.0) or microbiological success rate (50.8% vs 51.7%; P=0.90; 95% CI for difference −13.4 to 11.7) between linezolid and vancomycin treated patients overall. In the MRSA VAP study,Citation37 numerically more patients treated with linezolid than vancomycin achieved microbiologic (56.5% vs 47.4%; P=0.757; 95% CI −21.1 to 39.4) and clinical (66.7% vs 52.9%; P-value not reported) cures. Additionally, more patients treated with linezolid than vancomycin survived (86.7% vs 70.0%).
Retrospective analyses
Four studies retrospectively compared clinical outcomes in patients with MRSA pneumoniaCitation10,Citation11,Citation38,Citation39 including two studies that compared outcomes in patients with MRSA VAP.Citation10,Citation39 Results from a retrospective analysis of data in the US Veterans Health Administration national database (January 1, 2002 to September 30, 2010) reflecting real-world clinical settings found a significantly higher clinical success rate in linezolid (n=231) than vancomycin (n=3,500) treated patients (adjusted hazard ratio, 1.25; CI, 1.07–1.47) with MRSA pneumonia.Citation38 Thirty-day mortality was similar between linezolid (19.5%) and vancomycin (20.9%) treated patients (P=0.56). In contrast, results from a retrospective study using a hospital database from a tertiary care hospital in Shanghai, People’s Republic of China, showed similar clinical response rates (31.7% vs 30.0%) and lower treatment failure rates (45.0% vs 55.0%; P=0.847), but higher pneumonia-related (10.0% vs 1.7%; OR, 6.425; P=0.059) and all-cause mortality (18.3% vs 3.3%; OR, 6.564; P=0.013) at hospital discharge for linezolid compared to vancomycin treated patients.Citation11 Results from this study however, should be evaluated cautiously as differences existed between the linezolid and vancomycin matched treatment groups and enrolled patients were diagnosed with HAP, not exclusively gram-positive HAP.
Results from the two retrospective analyses in patients with MRSA VAP showed higher clinical success with no difference in mortality between linezolid and vancomycin treated patients similar to the results from the prospective studies.Citation10,Citation39
Meta-analyses
Although numerous meta-analyses evaluated the role of linezolid in the treatment of patients with pneumonia,Citation40–Citation49 only five directly compared linezolid and vancomycin therapy.Citation40,Citation41,Citation44,Citation47,Citation49 In general, results from these analyses show similarCitation40,Citation41,Citation47,Citation49 or increasedCitation44 clinical success with linezolid compared to vancomycin therapy (). Only two analyses compared mortality rates between patients treated with vancomycin and linezolid therapy for nosocomial pneumonia; no difference in mortality rates was found.Citation47,Citation49
Table 3 Meta-analyses results for studies comparing linezolid and vancomycin therapy in patients with gram-positive pneumonia
Analysis of clinical evidence
Numerous studies evaluated the efficacy and safety of linezolid in the treatment of patients with suspected or proven gram-positive pneumonia.Citation8,Citation10,Citation31,Citation32,Citation35–Citation37 In general, results from these studies show higher or similar clinical success for linezolid versus vancomycin therapy with no difference in mortality.
Differences in outcomes reported for these studies can potentially be explained by differences in populations evaluated. Similar or higher clinical success for linezolid versus vancomycin therapy was reported in studies that enrolled patients with culture-proven MRSA HAP or at a minimum documented or presumed gram-positive HAP.
Lower clinical response rates were reported in the real-world study conducted in the People’s Republic of China.Citation11 In this study, linezolid and vancomycin therapy were initiated for the empiric treatment of HAP; few patients underwent microbiologic testing. The lower clinical response rates reported for this study suggest that at least some linezolid and vancomycin treated patients had non-gram-positive HAP which is consistent with epidemiological data showing that the majority of HAP cases in the People’s Republic of China are due to gram-negative organisms.Citation4,Citation5 The authors of this study stated that the routine microbiological assessment of patients with HAP is not routinely performed in Chinese tertiary care hospitals. A clearer understanding of the antimicrobial treatment of patients with HAP in Chinese tertiary care hospitals is needed in order to promote the rational use of antimicrobials in this setting.
Economic evidence
ZEPHyR analysis
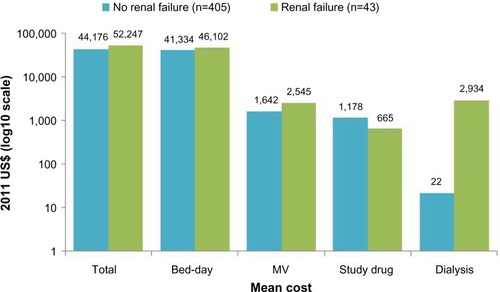
The economic impact of linezolid compared to vancomycin therapy for the treatment of gram-positive nosocomial pneumonia was evaluated in several clinical trials including the ZEPHyR trial. Economic results from the ZEPHyR trial for patients with MRSA nosocomial pneumonia show similar overall costs for linezolid ($45,004) and vancomycin ($44,897) treated patients despite linezolid’s higher drug acquisition price ().Citation50 The higher drug cost for linezolid was offset by lower non-significant cost differences in reduced bed days and dialysis utilization for linezolid compared to vancomycin therapy. Results from this cost-effectiveness analysis show that linezolid treatment provides better efficacy with lower or only slightly higher costs than vancomycin treatment for the majority (73%) of bootstrap samples evaluated; linezolid treatment dominated vancomycin treatment in 24% of bootstrap samples having both better efficacy and lower costs. In the ZEPHyR trial, total costs were higher for patients who developed renal failure compared to those who did not ().Citation50 This increase in costs for patients with renal failure was driven by significantly higher mechanical ventilation (12 vs 7.8 days) and intensive care unit (13.5 vs 10 days) days. Although the number of patients who developed renal failure in the ZEPHyR trial was low overall (n=43), fewer linezolid (n=9) than vancomycin (n=34) treated patients developed renal failure (P<0.001).
Figure 1 Mean cost by treatment for patients enrolled in the ZEPHyR trial (modified intent-to-treat population).
Abbreviation: MV, mechanical ventilation.
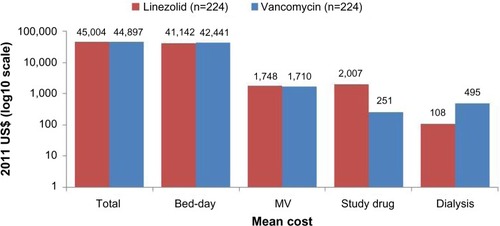
Figure 2 Mean cost by renal failure status for patients enrolled in the ZEPHyR trial (modified intent-to-treat population).
Abbreviation: MV, mechanical ventilation.
Post hoc ZEPHyR analysis: Chinese perspective
Data from four cities representing geographical areas in the North (Beijing), South (Guangzhou), East (Nanjing), and West (Xi’an) were utilized to capture economic and clinical differences in treating patients with nosocomial pneumonia in the People’s Republic of China.Citation9 Total treatment costs were highest in Guangzhou and lowest in Xi’an; total treatment costs for linezolid and vancomycin therapy were similar when compared within each city (). Results from this analysis show a tradeoff between costs and effectiveness for tier 3A hospitals located in Beijing, Guangzhou, Nanjing, and Xi’an.
Figure 3 Mean cost by treatment for linezolid and vancomycin therapy in Beijing, Guangzhou, Nanjing, and Xi’an, the People’s Republic of China.
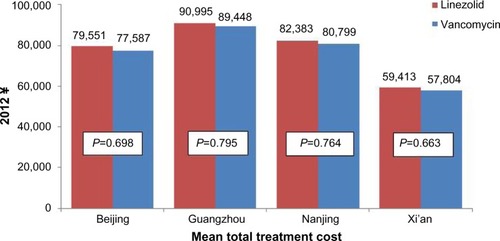
Total inpatient costs were similar between linezolid and vancomycin therapy in all four cities evaluated. Total inpatient costs for linezolid ranged from ¥58,835 to ¥86,894 and for vancomycin ¥58,390 to ¥87,033. Analysis by city shows that linezolid compared to vancomycin therapy was dominant in Guangzhou, meaning linezolid compared to vancomycin therapy had a higher probability of treatment success with lower total treatment costs. Linezolid therapy was associated with an incremental cost-effectiveness ratio of ¥1,861 in Beijing, ¥163 in Nanjing, and ¥16,509 in Xi’an. Results for sensitivity analyses suggested by Chinese clinical experts show that linezolid dominated vancomycin therapy for many of the scenarios evaluated in all four cities.
Retrospective analysis
Results from a retrospective analysis conducted in Shanghai, People’s Republic of China, were similar to those previously reported.Citation11 Although drug costs were higher for linezolid compared to vancomycin therapy, median total hospital costs for linezolid (¥133,825) and vancomycin (¥113,160; P=0.076) therapy for patients with HAP did not significantly differ.
Conclusion
Hospitalized patients with MRSA nosocomial pneumonia have few treatment options. Historically, this patient population was treated with intravenous vancomycin therapy. Published evidence from both clinical studies and meta-analyses suggest similar if not better outcomes for patients with MRSA nosocomial pneumonia treated with linezolid compared to vancomycin therapy. Evidence from economic studies, including those conducted from a Chinese perspective, suggests that linezolid is a cost-effective therapy for hospitalized patients with MRSA nosocomial pneumonia. Both clinical and economic evidence supports the use of linezolid for the treatment of patients with MRSA nosocomial pneumonia.
Disclosure
Pfizer Investment Co. Ltd provided financial support to Pharmerit International for manuscript development. Pharmerit International retained independent control not only of studies included and reviewed in this manuscript but also analysis of data obtained from those studies. B Lesher and X Gao are employees of Pharmerit International. Y Chen is an employee of Pfizer Investment Co. Ltd, Beijing, People’s Republic of China. Z Liu has served as a speaker for Pfizer Investment Co. Ltd. The authors report no other conflicts of interest in this work.
References
- World Health OrganizationAntimicrobial Resistance: Global Report on Surveillance2014 Available from: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1Accessed February 10, 2015
- PletzMWBurkhardtOWelteTNosocomial methicillin-resistant Staphylococcus aureus (MRSA) pneumonia: linezolid or vancomycin? – Comparison of pharmacology and clinical efficacyEur J Med Res2010151250751321163725
- ChungDRSongJHKimSHHigh prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in AsiaAm J Respir Crit Care Med2011184121409141721920919
- LiuYNCaoBWangHAdult hospital acquired pneumonia: a multicenter study on microbiology and clinical characteristics of patients from 9 Chinese citiesZhonghua Jie He He Hu Xi Za Zhi2012351073974623289990
- ZhangYYaoZZhanSDisease burden of intensive care unit-acquired pneumonia in China: a systematic review and meta-analysisInt J Infect Dis201429849025449241
- ChenHBZhaoCJWangHAn analysis of resistance of nosocomial infection pathogens isolated from 13 teaching hospitals in 2011Zhonghua Nei Ke Za Zhi201352320321223856111
- ZhaoCSunHWangHAntimicrobial resistance trends among 5608 clinical gram-positive isolates in China: results from the gram-positive cocci resistance surveillance program (2005–2010)Diagn Microbiol Infect Dis201273217418122521693
- WunderinkRGNiedermanMSKollefMHLinezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled studyClin Infect Dis201254562162922247123
- WanYLiQChenYHaiderSLiuSGaoXEconomic analysis from a Chinese Payer’s perspective within a randomized clinical trial of linezolid-versus vancomycin-treated patients with nosocomial pneumonia caused by methicillin resistant Staphylococcus aureus (MRSA-NP)Paper presented at: Society for Medical Decision Making Asia-Pacific ConferenceJanuary 7, 2014Singapore
- PeyraniPWiemkenTLKelleyRHigher clinical success in patients with ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus treated with linezolid compared with vancomycin: results from the IMPACT-HAP studyCrit Care2014183R11824916853
- SongYYangYChenWClinical response and hospital costs associated with the empirical use of vancomycin and lin-ezolid for hospital-acquired pneumonia in a Chinese tertiary care hospital: a retrospective cohort studyClinicoecon Outcomes Res2014645146125378939
- ChenXWangWKHanLZEpidemiological and genetic diversity of Staphylococcus aureus causing bloodstream infection in Shanghai, 2009–2011PLoS One201389e7281124039803
- JonesRNCastanheiraMHuBUpdate of contemporary antimicrobial resistance rates across China: reference testing results for 12 medical centers (2011)Diagn Microbiol Infect Dis201377325826624055218
- LuYLiYXueFAnalysis of resistance tendency of bloodstream-infecting pathogens in ChinaZhonghua Jie He He Hu Xi Za Zhi201336641141924103203
- ReinertRRLowDERossiFZhangXWattalCDowzickyMJAntimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecyclineJ Antimicrob Chemother20076051018102917855724
- SunHLWangHChenMJAn antimicrobial resistance surveillance of gram-positive cocci isolated from 12 teaching hospitals in China in 2009Zhonghua Nei Ke Za Zhi201049973574021092441
- WangHLiuYSunHXuYXieXChenMIn vitro activity of ceftobiprole, linezolid, tigecycline, and 23 other antimicrobial agents against Staphylococcus aureus isolates in ChinaDiagn Microbiol Infect Dis200862222622918653301
- WeiLWuRZouFAntibiotic resistance pattern of methicillin-resistance and coagulase-negative Staphylococcus isolates among hospitalized patients at a tertiary hospital in Gansu, North-western ChinaAfr J Microbiol Res201481105108
- ZouMXZhouRRWuWJAntimicrobial resistance and molecular epidemiological characteristics of clinical isolates of Staphylococcus aureus in Changsha areaChin Med J (Engl)2012125132289229422882850
- HuFPZhuDWangF2011 CHINET surveillance of bacterial resistance in ChinaChin J Infect Chemother2012125321329
- ZhengBLVYMinistry of Health National Antimicrobial Resistance Investigation Net Annual Report of 2011: antimicrobial resistance surveillance of gram-positive cocciChin J Clin Pharmacol20122812888892
- SongJHAsian Hospital Acquired Pneumonia WorkingGTreatment recommendations of hospital-acquired pneumonia in Asian countries: first consensus report by the Asian HAP Working GroupAm J Infect Control2008364 SupplS83S9218468550
- CaoBTanTTPoonEConsensus statement on the management of methicillin-resistant Staphylococcus aureus nosocomial pneumonia in AsiaClin Respir J20159212914224725393
- American Thoracic Society, Infectious Diseases Society of AmericaGuidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumoniaAm J Respir Crit Care Med2005171438841615699079
- LiuCBayerACosgroveSEClinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summaryClin Infect Dis201152328529221217178
- National Institute for Health and Care ExcellencePneumonia: Diagnosis and Management of Community- and Hospital-acquired Pneumonia in Adults2014 Available from: http://www.nice.org.uk/guidance/cg191/documents/pneumonia-guideline-consultation-full-guideline2Accessed February 12, 2015
- ZhuoCXuYCXiaoSNZhangGYZhongNSGlycopeptide minimum inhibitory concentration creep among meticillin-resistant Staphylococcus aureus from 2006–2011 in ChinaInt J Antimicrob Agents201341657858123562222
- RybakMJLomaestroBMRotschaferJCVancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases PharmacistsClin Infect Dis200949332532719569969
- MendesREHoganPAStreitJMJonesRNFlammRKZyvox® Annual Appraisal of Potency and Spectrum (ZAAPS) program: report of linezolid activity over 9 years (2004–2012)J Antimicrob Chemother20146961582158824468866
- MendesREHoganPAStreitJMJonesRNFlammRKUpdate on linezolid in vitro activity through the Zyvox Annual Appraisal of Potency and Spectrum Program, 2013Antimicrob Agents Chemother20155942454245725645839
- RubinsteinECammarataSOliphantTWunderinkRLinezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter studyClin Infect Dis200132340241211170948
- WunderinkRGCammarataSKOliphantTHKollefMHContinuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumoniaClin Ther200325398099212852712
- WunderinkRGRelloJCammarataSKCroos-DabreraRVKollefMHLinezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumoniaChest200312451789179714605050
- KollefMHRelloJCammarataSKCroos-DabreraRVWunderinkRGClinical cure and survival in gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycinIntensive Care Med200430338839414714107
- LinDFZhangYYWuJFLinezolid for the treatment of infections caused by gram-positive pathogens in ChinaInt J Antimicrob Agents200832324124918635341
- StevensDLHerrDLampirisHHuntJLBattsDHHafkinBLinezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infectionsClin Infect Dis200234111481149012015695
- WunderinkRGMendelsonMHSomeroMSEarly microbiological response to linezolid vs vancomycin in ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureusChest200813461200120718719064
- CaffreyARMorrillHJPuzniakLALaplanteKLComparative effectiveness of linezolid and vancomycin among a national veterans affairs cohort with methicillin-resistant Staphylococcus aureus pneumoniaPharmacotherapy201434547348024420846
- ChanJDPhamTNWongJClinical outcomes of linezolid vs vancomycin in methicillin-resistant Staphylococcus aureus ventilator-associated pneumonia: retrospective analysisJ Intensive Care Med201126638539121606058
- BallyMDendukuriNSinclairAAhernSPPoissonMBrophyJA network meta-analysis of antibiotics for treatment of hospitalised patients with suspected or proven meticillin-resistant Staphylococcus aureus infectionInt J Antimicrob Agents201240647949523102749
- BeibeiLYunCMengliCNanBXuhongYRuiWLinezolid versus vancomycin for the treatment of gram-positive bacterial infections: meta-analysis of randomised controlled trialsInt J Antimicrob Agents201035131219900794
- FalagasMESiemposIIVardakasKZLinezolid versus glycopeptide or beta-lactam for treatment of gram-positive bacterial infections: meta-analysis of randomised controlled trialsLancet Infect Dis200881536618156089
- FuJYeXChenCChenSThe efficacy and safety of linezolid and glycopeptides in the treatment of Staphylococcus aureus infectionsPLoS One201383e5824023484002
- JiangHTangRNWangJLinezolid versus vancomycin or teicoplanin for nosocomial pneumonia: meta-analysis of randomised controlled trialsEur J Clin Microbiol Infect Dis20133291121112823568605
- KalilACKlompasMHaynatzkiGRuppMETreatment of hospital-acquired pneumonia with linezolid or vancomycin: a systematic review and meta-analysisBMJ Open2013310e003912
- KalilACMurthyMHHermsenEDNetoFKSunJRuppMELinezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysisCrit Care Med20103891802180820639754
- LinZQHuangYLHuangPChenQLinezolid versus vancomycin in the treatment of pneumonia caused by gram-positive cocci: meta-analysis of randomised controlled trialsZhonghua Jie He He Hu Xi Za Zhi2010331290090621211409
- WalkeyAJO’DonnellMRWienerRSLinezolid vs glycopeptide antibiotics for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a meta-analysis of randomized controlled trialsChest201113951148115520864609
- WangYZouYXieJLinezolid versus vancomycin for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a systematic review employing meta-analysisEur J Clin Pharmacol201571110711525355172
- NiedermanMSChastreJSolemCTHealth economic evaluation of patients treated for nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus: secondary analysis of a multicenter randomized clinical trial of vancomycin and linezolidClin Ther20143691233124325066668
- TanSCWangXWuBCost-effectiveness of linezolid versus vancomycin among patients with methicillin-resistant Staphylococcus aureus confirmed nosocomial pneumonia in ChinaValue Health Reg Issue2014394100
