Abstract
Bone remodeling requires a precise balance between resorption and formation. It is a complex process that involves numerous factors: hormones, growth factors, vitamins, and cytokines, and notably osteoprotegerin (OPG) and receptor activator for nuclear factor-κB (RANK) ligand. The signaling pathway OPG/RANK/RANKL is key to regulation for maintaining the balance between the activity of osteoblasts and osteoclasts in order to prevent bone loss and ensure a normal bone turnover. In this review, the RANK/RANKL/OPG pathway is described. The multiple interactions of various factors (hormones, cytokines, growth factors, and vitamins) with the OPG/RANK/RANKL pathway are also commented on. Finally, the effects of denosumab, a human monoclonal antibody that binds to RANKL and thereby inhibits the activation of osteoclasts, and of strontium ranelate are also described. Indeed, these two new drugs afford appreciable assistance in daily care practice, helping to prevent bone loss in patients with osteoporosis.
Introduction
Bone remodeling is a phenomenon that first allows growth and then participates in the mineral homeostasis of calcium and phosphorus. Bone remodeling is a complex process involving numerous cells and cytokines. Recently, a major signaling pathway has been discovered that gives a new orientation for osteoporosis treatment. Bone remodeling is a balance between formation and resorption through controlling the activity of osteoblasts and osteoclasts. In numerous bone diseases, this becomes unbalanced in favor of resorption, creating bone loss. Throughout this review, the variation of this balance according to the patient’s gender or age will be described, as well as the new treatment options based on the newly discovered signaling pathway OPG/RANK/RANKL. For this review, we conducted a PubMed search from 1995 to 2010 using the key words “osteoprotegerin (OPG)”, “receptor activator of nuclear factor-κB ligand (RANKL)”, “RANK”, “aging”, “postmenopausal”, “denosumab”, and “strontium ranelate”.
The OPG/RANKL/RANK signaling pathway
Normal bone turnover
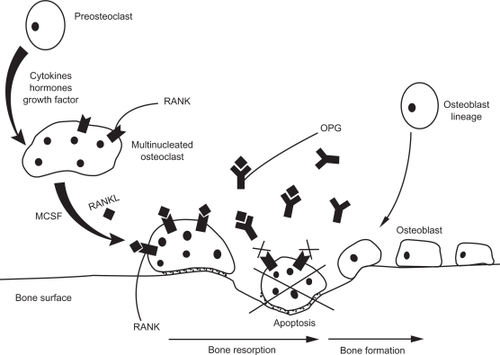
The cells most involved in bone turnover are osteoclasts and osteoblasts. These cells have counter effects on bone. The former are the resorption cells; the latter are the formation cells. The balance between activation and apoptosis of cells is the key to maintaining bone mass. Formation of new bone goes through four steps: osteoclast activation, bone resorption, reversal with osteoclast inhibition and osteoblast activation, and finally bone formation. Therefore, everything starts by osteoclastogenesis. Osteoclasts are derived from the hematopoietic lineage and differentiate in order to degrade bone.Citation1 The osteoclast precursor matures into a multinucleated cell and attaches itself to the bone surface, where it is attracted by different factors such as cytokines, hormones, and growth factor, and differentiates into an activated osteoclast. Once activated, the osteoclast starts degrading the bone surface, forming a lacuna. The third phase of the cycle is apoptosis of the osteoclast once the resorption phase is achieved, to allow the formation of new bone by the preosteoblasts that have matured in order to constitute new bone and regulate its mineralization. Once bone formation is achieved, osteoblasts apoptosis leads them either to osteocytes or to transform to bone surface lining cells.
Throughout aging, bone turnover unbalances in favor of bone resorption. Osteoporosis is a chronic bone disease characterized by a decreased bone mass leading to fragile bone and an increased risk for fractures, notably hip, vertebral, and forearm fractures, which are the source of a loss of autonomy and increased mortality in the elderly.Citation2
Suppression or control of bone resorption is therefore a major therapeutic strategy to prevent or diminish bone loss. A critical signaling pathway with three major proteins, OPG, RANK, and RANKL, was discovered, thereby enlightening the cellular regulation of bone formation. OPG was the first protein of the pathway to be identified in 1997.Citation3 It was discovered in mice by sequencing random clones, and the full-length gene was proven to encode for a new tumor necrosis factor (TNF) receptor. OPG knockout mice revealed severe osteoporosis, whereas OPG overexpressing mice showed osteopetrosis.Citation4 OPG is a member of the TNF receptor family but is atypical, as it is a secreted protein with no transmembrane domain. It contains four homologous domains for binding its target, RANKL. OPG is produced by many types of tissue, including osteoblasts, endothelial cells, vascular smooth muscle, and lymphoid cells, and other cell types, raising the question of the specificity of this protein in the bone mass regulation.Citation5
It has been established that osteoclastogenesis requires osteoclast activation by two newly identified molecules: macrophage colony-stimulating factor (M-CSF) and RANKL. Both of them are necessary to activate gene transcription allowing osteoclast differentiation.Citation6 RANKL is another new member of the TNF ligand family. It is produced by osteoblast lineage cells and activated T-cells. M-CSF and RANKL have complementary activities. M-CSF increases the pool of osteoclast precursors, whereas RANKL binds to its receptor RANK expressed on osteoclast precursors and mature osteoclasts, enhances osteoclast differentiation, and promotes its activation while inhibiting its apoptosis.Citation7,Citation8
RANK, the receptor of RANKL, as with OPG, is a receptor from the TNF family. Its essential role for the transduction of the RANKL signal was established in the late 1990s.Citation9 In transgenic mice, knockout of either RANK or RANKL led to the same phenotypes, concluding that RANK and RANKL had very few roles apart from their interactions.Citation4
The bone turnover pathway is a triad: RANKL binds to its receptor RANK in order to induce osteoclast differentiation, activation, and survival, whereas OPG acts as a decoy receptor to RANKL and therefore inhibits osteoclast activation and bone resorption.Citation10 The balance of RANKL/RANK and OPG is thus essential to modulate osteoclastogenesis and bone remodeling, and the regulators involved in this tight control are numerous ().
Figure 1 Bone turnover through the OPG/RANK/RANKL pathway. The osteoclast precursor matures into a multinucleated cell under the influence of numerous factors such as cytokines, hormones, and growth factors. The multinucleated cell differentiates into an activated osteoclast in the presence of MCSF and RANKL. Once activated, the osteoclast starts degrading the bone surface, forming a lacuna. OPG, the decoy receptor of RANKL, inhibits the continuous binding of RANKL on RANK, therefore leading the osteoclast to its apoptosis. Thereafter, bone formation starts with preosteoblasts that have matured into osteoblasts in order to constitute new bone.
Expression of the OPG/RANKL pathway
Multiple factors (hormones, cytokines, growth factors, and vitamins) have been described as interacting with the OPG/RANKL pathway. The first of these regulation factors, estrogen, is one of the most important known hormones to participate in bone turnover. The results of the different work concerning estrogen and its interaction with the pathway are not conflicting because estrogen has been shown to stimulate OPG secretionCitation11,Citation12 and also to downregulate the expression of RANKL.Citation13
Studies on the effects of androgens have yielded conflicting results on their effects on OPG. Androgens upregulated OPG expression in cultured mice osteoblasts,Citation14 whereas in human osteoblastic cells it was shown that OPG mRNA levels and protein serum levels were significantly decreased by 5-α-dihydrotestosterone.Citation15 RANKL expression is not affected by androgens in both studies.Citation14,Citation15 However, Proell et al in 2009 studied orchiectomized (ORX) rats and measured free serum RANKL in bone marrow supernatants. In ORX rats, this level was threefold higher than controls; once treated with testosterone, RANKL returned to control levels.Citation16 These results were in the same trend as in another study in which an inverse correlation was found between RANKL in bone marrow cell extracts and testosterone in ORX rats.Citation17
Parathyroid hormone (PTH) is a proresorptive hormone as it has been described from the bone stimulation observed in patients with hyperparathyroidism. PTH treatment on cultured bone marrow cells and osteoblasts in mice showed a decreased expression of mRNA levels of OPG and a reciprocal enhanced regulation of RANKL.Citation18 The same results were found in a study on parathyroidectomized infusion with continuous PTH. A dose-dependent OPG decrease with a reciprocal RANKL increase was shown.Citation19 Seck et al found conflicting results while analyzing the expression of OPG and RANKL in human bone tissue in vivo in women having surgery for breast cancer. Their conclusion stated that menopausal status failed to show any change in the expression of OPG and RANKL. However, higher levels of PTH were correlated to low levels of both OPG and RANKL mRNA transcript.Citation20 A cohort study of women of all ages also showed that serum OPG negatively correlated to PTH.Citation21
Among other regulators of the bone turnover signaling pathway, numerous cytokines can promote or, on the contrary, decrease bone resorption. IL-1 is a proresorptive cytokine that upregulates RANKL and RANK and has yielded conflicting results on its action among OPG. Interleukin 7 (IL-7),Citation22 IL-17, and TNF-α upregulate RANKL and are therefore considered proresorptive agents, whereas IL-4, IL-13, and interferon-γ are considered antiresorptive agents by suppressing osteoclastogenesis.Citation4
Growth factors are another category of the OPG/RANKL pathway regulators. Insulin-like growth factor (IGF)-1 is already known as an important growth factor for bone turnover because bone responsiveness to IGF-1 decreases with age.Citation23 Zhao et al studied the interaction between IGF-1 and the OPG/RANKL pathway in healthy Chinese women. Their results showed a negative correlation between IGF-1 and OPG as well as a negative correlation with the OPG/RANKL ratio. However, IGF-1 positively correlated to RANKL. The authors’ conclusion was that the effects of IGF-1 on bone may be mediated by the OPG/RANKL pathway.Citation24 The major limitations of this study were the use of serum levels that did not reflect the exact bone microenvironment RANKL levels and also the use of serum levels of RANKL, which are very hard to define given that the assays are not very sensitive.
Another study on miceCitation23 showed that IGF-1 can stimulate the expression of RANKL and M-CSF in young but not old animals; therefore, the regulatory role of this growth factor is lost with aging. The authors also determined that the alteration of the IGF-1 role was due to impaired receptor activation even though it did not account for the entire alterations of the IGF-1 role in bone turnover.
Another growth factor was looked into. Pigment epithelium-derived factor (PEDF) is a potent growth factor for inhibiting angiogenesis. PEDF expression in bone has been demonstrated, as well as its positive action on differentiation of osteoblastic cells. Akiyama et al explored the link between bone turnover, and particularly osteoclast function, and PEDF.Citation25 PEDF was shown to stimulate OPG expression in osteoblasts and in osteoclast precursor cells; on the other hand, RANKL expression was suppressed by PEDF. Moreover, PEDF was shown to inhibit osteoclast differentiation. The authors’ conclusion was that PEDF could be a bone resorption inhibitor via the upregulation of OPG.
Genetic variations and mutations have been identified in the OPG/RANKL pathway, and the contribution of their polymorphism to bone mass density (BMD) has been studied. In a large cohort of Chinese patients with either an extremely high hip BMD or an extremely low hip BMD, men with TC/CC genotypes of the polymorphism rs9594782 of the RANKL gene (TNFRSF11 A) had a significantly higher risk of extremely low hip BMD and lower whole-body BMD. In the same cohort, the A163G polymorphism of the promoter region of the OPG gene (TNFRSF11B) was also linked to hip BMD with a significant association between the GG genotype and a reduced risk of low hip BMD.Citation26 In a recent and large European cohort of men, Roshandel et al identified multiple single nucleotide polymorphisms in OPG, RANKL, and RANK genes associated with bone turnover markers and BMD.Citation27 Genetic variations of this essential pathway seemed to influence bone turnover, but these results have still to be confirmed in other populations.
The OPG/RANK/RANKL pathway through aging
Although rodent studies in aged mice had shown that RANKL mRNA levels increased with age, OPG mRNA levels decreased in aged mice.Citation28,Citation29 Studies in humans have drawn the consensus that OPG increases with age in both men and women.Citation21,Citation30–Citation32 This increase has been shown in a healthy aged population as well as in patients who have osteoporosis, but it can also be seen in other diseases, such as Paget’s disease of the bone and rheumatoid arthritis.Citation4 On the other hand, RANKL evolution through aging has yielded conflicting results in several studies regarding analysis in women and in men of the RANKL/OPG ratio.
In women
Menopause is a crucial aging step for women, characterized by an estrogen deficiency leading to bone loss. In vitro studies of human osteoblast revealed that estrogen induces OPG production.Citation11,Citation33,Citation34 Studies involving postmenopausal women to determine a relationship between the menopause and an increase in OPG put forth different conclusions. Oh et al in a study on healthy Korean women of all ages, found that OPG levels were significantly higher in postmenopausal women compared with premenopausal women,Citation35 and in a large cohort of Austrian women aged from 19 to 96 years, OPG serum level was found to be negatively correlated with serum estradiol.Citation21 However, it has also been shown that OPG serum levels were positively correlated to age but not significantly to the menopausal status.Citation20,Citation36
In women treated for menopause, Han et al showed a significant decrease in OPG serum levels after 1 year of hormone replacement therapy for estrogen alone or combined with progesterone.Citation37 Dehydroepiandrosterone (DHEA) is another hormone linked to aging, and it is known to decrease with aging. An in vitro study of osteoblasts cultured with DHEA showed that the expression of the ratio of OPG/RANKL mRNA was increased, leading the authors to conclude that DHEA could inhibit bone resorption through the OPG/RANKL pathway.Citation38
As the results of the relationship between OPG and menopause are conflicting, the link between bone resorption markers and OPG is also conflicting. One study reported a weak negative correlation between OPG serum level and bone turnover markers in a cohort of postmenopausal women.Citation39 In several other cohorts, no correlation was found between OPG and bone resorption markers in women whatever their menopause status was.Citation36,Citation40 In contrast, a positive relationship between bone turnover markers and OPG was found in postmenopausal womenCitation41 and in both men and women in a study by Indridason et al.Citation42
Finally, the link between OPG levels, BMD, and vertebral fracture has also yielded conflicting results. In some studies, no association was found between BMD and OPG,Citation21,Citation39,Citation42,Citation43 whereas other works demonstrated a significant inverse correlation between OPG and BMD,Citation44 notably in postmenopausal women without any hormone replacement therapy.Citation45 A positive correlation was found in two small studies of postmenopausal womenCitation36,Citation46 and corroborated by a larger study with a follow-up of 5–10 years.Citation47
In addition to the conflicting relationship between BMD and OPG, the link between the prevalence of vertebral fractures and OPG serum levels has still not reached a consensus. Indeed, lowCitation36,Citation41,Citation46 and highCitation48 OPG levels have been associated with vertebral fractures ().
Table 1 Correlation between osteoprotegerin and different markers in women
The study of RANKL in postmenopausal women is even more complicated than OPG because the assay that measures RANKL serum levels is not as sensitive as the one used for OPG. In an animal study, serum RANKL of OPG knockout mice was increased by ovariectomy.Citation49 In a few studies that were conducted to determine the relationship between aging and RANKL in postmenopausal women, an increase of RANKL expression was described in post-menopausal women compared with premenopausal women and women using estrogen treatment.Citation13 Conflicting results can again be found. Some studies did not demonstrate any significant difference in RANKL and menopausal status,Citation20 whereas some others found a decrease of RANKL in elderly subjects.Citation31 Similar to OPG, the relationship between RANKL and BMD does not reach any consensus. Some works found no association;Citation36,Citation43 others found an inverse correlation.Citation45,Citation50
In men
All studies performed in men found that the OPG serum level increases with age.Citation21,Citation30,Citation35,Citation40,Citation42,Citation44,Citation45,Citation51–Citation53 As in women, the correlation between OPG and sex steroids in men is very conflicting in the different publications. Some articles showed a positive correlation between free testosteroneCitation21 and free estradiol levels and OPG.Citation52 In contrast, some studies concluded that there was a negative correlation between OPG and sex steroids in men: bioavailable testosterone and estradiol.Citation40 Khosla et al went further and compared the role of testosterone with estrogen in elderly males. The results showed that in hypogonadal conditions a testosterone supplementation treatment decreased OPG serum levels.Citation12 Furthermore, a negative association between OPG and PTH was found in men.Citation21,Citation52
Regarding the possible association between OPG levels and BMD, numerous studies observed very different results. Stern et al found that higher OPG levels were associated with higher BMD of the lumbar spine.Citation47 In the same trend, another study found a borderline positive correlation between OPG and whole-body BMD.Citation42 Mostly, it seems to be either a negative correlation or no correlation at all that is found. Khosla et al and Oh et al both concluded that there was a negative correlation between OPG serum levels and BMD.Citation40,Citation44 Furthermore, in a recent article, Jorgensen et al also found a negative association between OPG and BMD according to a multiple linear regression. In the same study, however, they did not find any correlation between OPG and bone loss after a 6-year follow-up.Citation45 This absence of correlation between OPG serum levels and BMD was also the conclusion of Szulc et al in a study of men aged 19–85 years.Citation52
When looking at the correlation between OPG and bone turnover markers, the results once again are very conflicting in men as well as in women. Some authors report a positive association between OPG and osteocalcin, whereas a negative correlation to bone resorption markers was observed.Citation42 This negative correlation with bone resorption is also noticed by Szulc et al.Citation52 On the other hand, some studies showed a negative association to osteocaclinCitation44 and a positive one between OPG and bone resorption markersCitation40 ().
Table 2 Correlation between osteoprotegerin and different markers in men
Conflicting results are also found in men regarding the evolution of serum RANKL. A study that was conducted to determine the relationship between RANKL serum levels and mRNA in the bone microenvironment showed that serum RANKL was negatively correlated to age, whereas mRNA was positively correlated to the age of the subjects. Levels of mRNA were also positively associated to bone turnover markers such as osteocalcin, alkaline phosphatase, and urinary deoxypyridinoline.Citation51 When looking at the ratio RANKL/OPG, a positive correlation was found with osteocalcin and estradiol levels, whereas no correlation was found with serum total testosterone. A trend for a significant positive correlation between RANKL and osteocalcin was noticed.Citation44 In a large recent study, Jorgensen et al found no correlation between RANKL and age, and no correlation either to BMD or bone loss after 6 years of follow-up. Moreover, the ratio RANKL/OPG was not correlated to bone loss.Citation45 On the other hand, Stern et al in the Rancho Bernardo Study, found that the serum RANKL concentrations were negatively correlated to BMD in men.Citation47
Limits and difficulties of the studies
As previously shown, studying OPG and RANKL in humans is very difficult, as it appears that the results are conflicting from one publication to another. All these data have to be balanced by some major problems highlighted in a few publications. First of all, the assays used to quantify RANKL serum level are not very sensitive. Indeed, in a recent study, only 63% of the patients had a detectable RANKL.Citation45 Secondly, only a few studies looked at the specific bone environment regarding the expression of OPG and RANKL, mostly due to the difficulty of obtaining bone samples. Moreover, OPG and RANKL are locally active molecules, and tested serum is removed from the site of disease; therefore, serum levels might only partially reflect the actual local mechanism. Finally, no reference ranges have been established regarding OPG and RANKL serum level. They vary from one study to another depending on the assays used. Currently, standard levels for OPG and RANKL are not well established.
Preventing bone loss: treatment through the RANKL/OPG pathway
As shown in the rodent studies, inhibiting RANKL or RANK led to the concept of inhibiting their pathway as a treatment for osteoporosis. After testing recombinant molecules of either OPG or RANK and finding all exhibiting the ability to bind RANKL and suppress bone resorption, all these molecules were abandoned. Recently, two new drugs emerged: 1) denosumab, directly from translation of the basic science of RANKL inhibition, and 2) strontium ranelate, which has proven effects on the RANKL/OPG pathway that emerged secondarily.
Denosumab
Denosumab is a human monoclonal IgG2 antibody with a very high affinity and specificity to RANKL. By binding to RANKL, as OPG does in physiological conditions, denosumab blocks its binding to RANK and thereby inhibits the osteoclast activation. In preclinical studies, denosumab did not suppress bone resorption in normal mice because of its specificity to human RANKL. In knockin mice expressing chimeric (human/murine) RANKL, denosumab reduced bone resorption and increased cortical and cancellous bone mass.Citation54
In a phase I study comparing denosumab with placebo in healthy postmenopausal women, a single subcutaneous injection resulted in a rapid, profound, and sustained decrease in bone resorption marker.Citation55 In a phase II study, McClung et al compared denosumab with placebo and alendronate in postmenopausal women with low BMD. Denosumab was administrated at a dose of 6, 14, or 30 mg every 3 months or 14, 60, 100, 210 mg every 6 months over a 12-month period. Results showed an increase in BMD at lumbar spine, total hip, and distal third of the radius. The results on BMD were superior to placebo and similar to or better than for weekly alendronate. Again, a decrease of bone resorption marker was observed rapidly after denosumab injection, and the suppression of bone turnover appeared to be dose dependent.Citation56 In a trial extension, these results were sustained over 24 months.Citation57 In phase I and II studies, the dose of 60 mg every 6 months seemed to be the more effective dose and was thus investigated in further phase III studies.
Bone et al conducted a randomized controlled versus placebo study on denosumab over 2 years in postmenopausal osteopenic women. They observed a significant increase in BMD at all sites (P <0.0001 versus placebo) after 24 months of treatment. At the same time, bone turnover markers (serum C-telopeptide [CTX1], tartrate-resistant acid phosphatase 5b, and intact N-terminal propeptide of type 1 procollagen [P1 NP]) were significantly suppressed.Citation58 A large phase III study, the FREEDOM trial, was conducted by Cummings et al in a population of 7868 postmenopausal osteoporotic women. They received either 60 mg of denosumab every 6 months or a placebo over a period of 36 months. The occurrence of any new vertebral, nonvertebral, and hip fractures was studied, and the authors observed that denosumab significantly reduced the risk of new fracture with a relative decrease of 68%, 40%, and 20% of vertebral, hip, and nonvertebral fractures, respectively.Citation59
The unique effect of denosumab as a RANKL inhibitor creates a new category of antiresorptive agent that is very different from bisphosphonates. Under denosumab, the bone turnover markers decreased quicker and in a more pronounced way than in the alendronate group.Citation56 Moreover, once the denosumab treatment is stopped, its effects on bone remodeling seem to be completely reversible.Citation60 Furthermore, compared with alendronate and placebo, denosumab was shown to be the more efficient treatment in improving bone mechanical properties of the femoral neck.Citation61
A noninferiority trial was conducted in a head-to-head comparison of denosumab and alendronate whereby 1189 postmenopausal women with a T-score of −2.0 or less at lumbar spine or total hip were included and randomized. They received either 60 mg subcutaneous injection of denosumab every 6 months and weekly oral placebo or 70 mg oral alendronate and subcutaneous placebo injections every 6 months. The change from baseline BMD (in the total hip, femoral neck, trochanter, lumbar spine, and one-third radius) at 6 and 12 months was studied, as well as the bone turnover markers at months 1, 3, 6, 9, and 12. At 12 months, 94% of the patients had completed the study. The results showed a significant increase in BMD in the denosumab treatment group compared with alendronate. Increase in BMD at the total hip in denosumab group was 3.5% at 12 months versus 2.6% in the alendronate group (P < 0.0001). A significant increase was also observed at all measured sites. The bone turnover markers studied were serum CTX1 and intact N-terminal P1 NP. They were significantly decreased in the denosumab group compared with alendronate. A maximal reduction of P1 NP was observed at 3 months in the denosumab group compared with a maximal decrease at 9 months in the alendronate group. Nevertheless, the median decreases in CTX1 were similar for both treatment groups at 12 months.Citation62
In terms of safety and tolerability, phase I and II studies reported similar rates of all adverse and severe adverse events in placebo, denosumab, and alendronate groups. However, a small, transient, non-dose-dependent and asymptomatic increase of serum PTH associated with a decrease of serum calcium was observedCitation56 under denosumab. In phase III studies, the same security profile was observed with no significant difference between placebo and denosumab. Although patients undergoing treatment with bisphosphonates, notably those receiving IV bisphosphonate therapy with chemo-, radiation-, or corticosteroid therapy, may develop jaw osteonecrosis, no such disabling adverse event was reported under denosumab.Citation63
Strontium ranelate
Strontium ranelate is an oral daily drug formed of two atoms of stable strontium and an organic moiety (ranelic acid). Strontium ranelate combines the antiresorptive effect on bone with an additional anabolic action. In vitro studies have shown different effects of strontium ranelate on osteoblasts and osteoclasts. When studying in vitro osteoblasts, strontium ranelate induced a concentration-dependent increase in OPG mRNA expression as well as OPG secretion. On the other hand, RANKL mRNA and concentration were markedly decreased. The proposed mediator of these effects by Brennan et al was the calcium-sensing receptor. Indeed, knocking down the receptor abolished the effects of strontium ranelate on OPG and RANKL.Citation64 These findings were corroborated in another in vitro study in which increasing concentrations of strontium ranelate downregulated osteoclastic differentiation. Moreover, the authors showed, as in the previous publication, that this downregulation was mediated by calcium-sensing receptor and by the inhibition of the RANKL-induced nuclear translocation.Citation65 Atkins et al observed the same increase of the OPG/RANKL by strontium ranelate in osteoblasts.Citation66 The increase in BMD is not obligatorily correlated to a reduction of the risk of fracture, as the ‘quality’ of bone formation, and notably of the network of trabeculae, is as important as its ‘quantity’. To date, no studies have demonstrated that the in vitro effects of strontium ranelate on OPG/RANKL are fully relevant to its risk reduction of fracture.
In rodents,Citation67 in vivo studies showed that strontium ranelate decreased bone resorption and increased bone formation. In a phase II study, strontium ranelate was compared with placebo in postmenopausal osteoporotic women with at least one vertebral fracture. An increase in lumbar spine BMD was significantly higher in the strontium ranelate group than in the placebo group. Moreover, urinary excretion of cross-linked N-telopeptide significantly decreased in the treatment group compared with placebo, showing a decrease of bone resorption. In this study, the dose of 2 g/day was considered to offer the best balance between efficacy and safety.Citation68 In the phase III study, a dose of 2 g/day of strontium ranelate was compared with placebo over 3 years. After 3 years of treatment, the increase in BMD at all measured sites was significant in the strontium group compared with the placebo group. The increase in BMD at lumbar spine was notably of 12.7% in the strontium group. However, it is well known that this BMD increase is not fully correlated to a risk reduction of new fracture, as strontium ranelate produces a BMD measurement artifact; therefore, its action is hardly comparable with denosumab. After 3 months of therapy, bone formation markers were significantly higher in the strontium ranelate group than in the placebo group. Moreover, the authors observed a risk reduction of 41% of new vertebral fractures during the 3-year study period in the treated group compared with placebo. No significant difference in the incidence of serious adverse events was observed between the groups. However, diarrhea was the most frequent adverse event in the strontium ranelate group, with a small but significantly higher rate compared with the placebo group (P + 0.02). This effect disappeared after the first 3 months of treatment.Citation69
Conclusion
Normal bone turnover and stable bone mass depend on the balance between OPG and RANKL. The catabolic effects of RANKL are controlled by OPG, which prevents activation of RANKL and therefore limits the formation, activity, and survival of osteoclasts. The OPG/RANK/RANKL signaling pathway is complex and requires numerous factors that interact together. Clinical trials of denosumab and strontium ranelate demonstrate evidence of their ability to prevent bone loss in patients with osteoporosis. These treatments may also play a significant role in future therapeutic strategies by preventing structural damage in rheumatoid arthritis and by reducing bone resorption in metastatic bone diseases, notably in breast and prostate cancer.
Acknowledgements
The authors thank Dr Claire Wenham (Unit of Musculoskeletal Disease, Leeds Teaching Hospital, Leeds, UK) for her comments and second reading of the manuscript.
Disclosure
The authors disclose no conflict of interest.
References
- BoyleWJSimonetWSLaceyDLOsteoclast differentiation and activationNature2003423693733734212748652
- CompstonJOsteoporosis: social and economic impactRadiol Clin North Am201048347748220609886
- RogersAEastellRCirculating osteoprotegerin and receptor activator for nuclear factor kappaB ligand: clinical utility in metabolic bone disease assessmentJ Clin Endocrinol Metab200590116323633116105967
- KearnsAEKhoslaSKostenuikPJReceptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and diseaseEndocr Rev200829215519218057140
- KhoslaSMinireview: the OPG/RANKL/RANK systemEndocrinology2001142125050505511713196
- LaceyDLTimmsETanHLOsteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activationCell19989321651769568710
- SchoppetMPreissnerKTHofbauerLCRANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular functionArterioscler Thromb Vasc Biol200222454955311950689
- KongYYYoshidaHSarosiIOPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesisNature199939767173153239950424
- NakagawaNKinosakiMYamaguchiKRANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesisBiochem Biophys Res Commun199825323954009878548
- LiuCWalterTSHuangPStructural and functional insights of RANKL-RANK interaction and signalingJ Immunol2010184126910691920483727
- BordSIrelandDCBeavanSRCompstonJEThe effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblastsBone200332213614112633785
- KhoslaSAtkinsonEJDunstanCRO’FallonWMEffect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly menJ Clin Endocrinol Metab20028741550155411932280
- Eghbali-FatourechiGKhoslaSSanyalABoyleWJLaceyDLRiggsBLRole of RANK ligand in mediating increased bone resorption in early postmenopausal womenJ Clin Invest200311181221123012697741
- ChenQKajiHKanataniMSugimotoTChiharaKTestosterone increases osteoprotegerin mRNA expression in mouse osteoblast cellsHorm Metab Res2004361067467815523591
- HofbauerLCHicokKCChenDKhoslaSRegulation of osteoprotegerin production by androgens and anti-androgens in human osteoblastic lineage cellsEur J Endocrinol2002147226927312153751
- ProellVXuHSchulerCWeberKHofbauerLCErbenRGOrchiectomy upregulates free soluble RANKL in bone marrow of aged ratsBone200945467768119501680
- LiXOminskyMSStolinaMIncreased RANK ligand in bone marrow of orchiectomized rats and prevention of their bone loss by the RANK ligand inhibitor osteoprotegerinBone200945466967619539794
- LeeSKLorenzoJAParathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formationEndocrinology199914083552356110433211
- MaYLCainRLHalladayDLCatabolic effects of continuous human PTH 1–38 in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formationEndocrinology200114294047405411517184
- SeckTDielIBismarHZieglerRPfeilschifterJSerum parathyroid hormone, but not menopausal status, is associated with the expression of osteoprotegerin and RANKL mRNA in human bone samplesEur J Endocrinol2001145219920511454517
- KudlacekSSchneiderBWoloszczukWPietschmannPWillvonsederRAustrian Study Group on Normative Values of Bone MetabolismSerum levels of osteoprotegerin increase with age in a healthy adult populationBone200332668168612810175
- PonchelFCuthbertRJGoëbVIL-7 and lymphopeniaClin Chim Acta2010915 [Epub ahead of print]
- CaoJJKurimotoPBoudignonBRosenCLimaFHalloranBPAging impairs IGF-I receptor activation and induces skeletal resistance to IGF-IJ Bone Miner Res20072281271127917488198
- ZhaoHYLiuJMNingGRelationships between insulin-like growth factor-I (IGF-I) and OPG, RANKL, bone mineral density in healthy Chinese womenOsteoporos Int200819222122617703270
- AkiyamaTDassCRShinodaYKawanoHTanakaSChoongPFPEDF regulates osteoclasts via osteoprotegerin and RANKLBiochem Biophys Res Commun2010391178979419945427
- HsuYHNiuTTerwedowHAVariation in genes involved in the RANKL/RANK/OPG bone remodeling pathway are associated with bone mineral density at different skeletal sites in menHum Genet2006118556857716249885
- RoshandelDHollidayKLPyeSRGenetic variation in the RANKL/RANK/OPG signaling pathway is associated with bone turnover and bone mineral density in menJ Bone Miner Res20102581830183820205168
- CaoJVentonLSakataTHalloranBPExpression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 miceJ Bone Miner Res200318227027712568404
- CaoJJWronskiTJIwaniecUAging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouseJ Bone Miner Res20052091659166816059637
- MazziottiGAmatoGSorvilloFIncreased serum osteoprotegerin values in long-lived subjects: different effects of inflammation and bone metabolismEur J Endocrinol2006154337337716498049
- PulsatelliLDolzaniPSilvestriTSoluble receptor activator of nuclear factor-kappaB ligand (sRANKL)/osteoprotegerin balance in ageing and age-associated diseasesBiogerontology20045211912715105586
- NabipourILarijaniBVahdatKRelationships among serum receptor of nuclear factor-kappaB ligand, osteoprotegerin, high-sensitivity C-reactive protein, and bone mineral density in postmenopausal women: osteoimmunity versus osteoinflammatoryMenopause200916595095519387415
- HofbauerLCKhoslaSDunstanCRLaceyDLSpelsbergTCRiggsBLEstrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cellsEndocrinology199914094367437010465311
- MichaelHHarkonenPLVaananenHKHentunenTAEstrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorptionJ Bone Miner Res200520122224223216294275
- OhESRheeEJOhKWCirculating osteoprotegerin levels are associated with age, waist-to-hip ratio, serum total cholesterol, and low-density lipoprotein cholesterol levels in healthy Korean womenMetabolism2005541495415562379
- Mezquita-RayaPde la HigueraMGarciaDFThe contribution of serum osteoprotegerin to bone mass and vertebral fractures in postmenopausal womenOsteoporos Int200516111368137415711777
- HanKOChoiJTChoiHAThe changes in circulating osteoprotegerin after hormone therapy in postmenopausal women and their relationship with oestrogen responsiveness on boneClin Endocrinol (Oxf)200562334935315730418
- WangYDWangLLiDJWangWJDehydroepiandrosterone inhibited the bone resorption through the upregulation of OPG/RANKLCell Mol Immunol200631414516549048
- RogersASalehGHannonRAGreenfieldDEastellRCirculating estradiol and osteoprotegerin as determinants of bone turnover and bone density in postmenopausal womenJ Clin Endocrinol Metab200287104470447512364420
- KhoslaSArrighiHMMeltonLJ3rdCorrelates of osteoprotegerin levels in women and menOsteoporos Int200213539439912086350
- Fahrleitner-PammerADobnigHPiswanger-SoelknerCOsteoprotegerin serum levels in women: correlation with age, bone mass, bone turnover and fracture statusWien Klin Wochenschr2003115929129712793029
- IndridasonOSFranzsonLSigurdssonGSerum osteoprotegerin and its relationship with bone mineral density and markers of bone turnoverOsteoporos Int200516441742315776220
- LiuJMZhaoHYNingGRelationships between the changes of serum levels of OPG and RANKL with age, menopause, bone biochemical markers and bone mineral density in Chinese women aged 20–75Calcif Tissue Int20057611615455183
- OhKWRheeEJLeeWYCirculating osteoprotegerin and receptor activator of NF-kappaB ligand system are associated with bone metabolism in middle-aged malesClin Endocrinol (Oxf)2005621929815638876
- JorgensenLVikAEmausNBone loss in relation to serum levels of osteoprotegerin and nuclear factor-kappaB ligand: the Tromso studyOsteoporos Int201021693193819701599
- ChibaYOnouchiTIkedaTAdachiJTamuraYHoriuchiTImplications of measuring soluble receptor activators of nuclear factor-kappaB ligand and osteoprotegerin in bone metabolism of elderly womenGerontology200955327528019158438
- SternALaughlinGABergstromJBarrett-ConnorEThe sex-specific association of serum osteoprotegerin and receptor activator of nuclear factor kappaB legend with bone mineral density in older adults: the Rancho Bernardo studyEur J Endocrinol2007156555556217468191
- JorgensenHLKuskPMadsenBFengerMLauritzenJBSerum osteoprotegerin (OPG) and the A163G polymorphism in the OPG promoter region are related to peripheral measures of bone mass and fracture odds ratiosJ Bone Miner Metab200422213213814999524
- NakamichiYUdagawaNKobayashiYOsteoprotegerin reduces the serum level of receptor activator of NF-kappaB ligand derived from osteoblastsJ Immunol2007178119220017182555
- UemuraHYasuiTMiyataniYCirculating profiles of osteoprotegerin and soluble receptor activator of nuclear factor kappaB ligand in post-menopausal womenJ Endocrinol Invest200831216316818362509
- FindlayDChehadeMTsangariHCirculating RANKL is inversely related to RANKL mRNA levels in bone in osteoarthritic malesArthritis Res Ther2008101R218182105
- SzulcPHofbauerLCHeufelderAERothSDelmasPDOsteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone statusJ Clin Endocrinol Metab20018673162316511443182
- Gannage-YaredMHFaresFSemaanMKhalifeSJambartSCirculating osteoprotegerin is correlated with lipid profile, insulin sensitivity, adiponectin and sex steroids in an ageing male populationClin Endocrinol (Oxf)200664665265816712667
- KostenuikPJNguyenHQMcCabeJDenosumab, a fully human monoclonal antibody to RANKL inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKLJ Bone Miner Res200924218219519016581
- BekkerPJHollowayDLRasmussenASA single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL in postmenopausal womenJ Bone Miner Res20041971059106615176987
- McClungMRLewieckiEMCohenSBDenosumab in postmenopausal women with low bone mineral densityN Engl J Med2006354882183116495394
- LewieckiEMMillerPDMcClungMRTwo-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMDJ Bone Miner Res200722121832184117708711
- BoneHGBologneseMAYuenCKEffects of denosumab on bone mineral density and bone turnover in postmenopausal womenJ Clin Endocrinol Metab20089362149215718381571
- CummingsSRSan MartinJMcClungMRDenosumab for prevention of fractures in postmenopausal women with osteoporosisN Engl J Med2009361875676519671655
- GeusensPEmerging treatments for postmenopausal osteoporosis – focus on denosumabClin Interv Aging2009424125019554095
- BeckTJLewieckiEMMillerPDEffects of denosumab on the geometry of the proximal femur in postmenopausal women in comparison with alendronateJ Clin Densitom200811335135918495508
- BrownJPPrinceRLDealCComparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trialJ Bone Miner Res200924115316118767928
- LewieckiEMDenosumab–an emerging treatment for postmenopausal osteoporosisExpert Opin Biol Ther201010346747620095877
- BrennanTCRybchynMSGreenWAtwaSConigraveADMasonRSOsteoblasts play key roles in the mechanisms of action of strontium ranelateBr J Pharmacol200915771291130019563530
- CaudrillierAHurtel-LemaireASWattelAStrontium ranelate decreases receptor activator of nuclear factor-KB ligand-induced osteoclastic differentiation in vitro: involvement of the calcium sensing receptorMol Pharmacol201078456957620584969
- AtkinsGJWelldonKJHalboutPFindlayDMStrontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin responseOsteoporos Int200920465366418763010
- LewieckiEMCurrent and emerging pharmacologic therapies for the management of postmenopausal osteoporosisJ Womens Health (Larchmt)200918101615162619857095
- MeunierPJSlosmanDODelmasPDStrontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis–a 2-year randomized placebo controlled trialJ Clin Endocrinol Metab20028752060206611994341
- MeunierPJRouxCSeemanEThe effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosisN Engl J Med2004350545946814749454