Abstract
Background
Aging of skin is associated with environmental factors such as ultraviolet rays, air pollution, gravity, and genetic factors, all of which can lead to wrinkling of skin. Previous reports suggest that the wound repair is impaired by the aging process and strategies to manipulate the age-related wound healing are necessary in order to stimulate repair.
Objective
Several traditional plant extracts are well-known for their properties of skin protection and care. Piper cambodianum P. Fourn. (PPF), a member of Piperacecae, is a plant found in Vietnam that might have therapeutic properties. Therefore, the effects of PPF stem and leaf extract on aging process were investigated in vitro and in vivo.
Methods
PPF extract dissolved in methanol was investigated using Western blotting, real-time quantitative reverse transcription-polymerase chain reaction, flow cytometry, and cell wound-healing assays. We assessed the anti-aging effect of PPF in mouse using the wound-healing assay. The results were analyzed by Student’s unpaired t-test; *P<0.05 and **P<0.01 were considered to indicate significant and highly significant values, respectively, compared with corresponding controls.
Results
PPF treatment demonstrated in vitro and in vivo anti-aging activity. Western blot analysis of PPF-treated normal human dermal fibroblast cells showed a dose-dependent increase in the expression of extracellular matrix genes such as collagen and elastin, but decreased expression of the aging gene matrix metalloproteinase-3. Quantitative polymerase chain reaction showed that PPF-treated cells displayed dose-dependent increase in messenger RNA expression levels of collagen, elastin, and hyaluronan synthase-2 and decreased expression levels of matrix metalloproteinase-1 aging gene. PPF treatment led to decreased production of reactive oxygen species in cells subjected to ultraviolet irradiation. Furthermore, PPF extract showed positive wound-healing effects in mice.
Conclusion
This study demonstrated the anti-aging and wound-healing effects of PPF extract. Therefore, PPF extract represents a promising new therapeutic agent for anti-aging and wound-healing treatments.
Introduction
Human skin consists of epidermal, dermal, and subcutaneous tissues. Epidermis is negatively affected by abiotic factors,Citation1 and aging involves structural, functional, and biochemical changes.Citation2 Aging of skin is associated with environmental factors such as ultraviolet (UV) rays, air pollution, gravity, and genetic factors,Citation3 all of which can lead to wrinkling of skin.Citation4,Citation5 Reactive oxygen species (ROS), including superoxide anion radical (·O2−), hydrogen peroxide (H2O2), hydroxyl radical (·OH), singlet oxygen (1O2), lipid peroxides (LOOH), and their radicals (LOO·) are formed in skin exposed to UVA (320–400 nm) and UVB (290–320 nm). These factors induce skin aging, phototoxicity, inflammation, formation of malignant tumors, and breakdown of cell membranes.Citation6–Citation8 Several traditional plant extracts have well-known effects for skin protection and care. Piper cambodianum P. Fourn. (PPF), a member of Piperacecae, is a plant found in Vietnam that might have therapeutic properties, since Piper betle Linn., which belongs to same family, is used in variety of decoctions for curing wounds, burns, lymphangitis, and eczema. In addition, juice from the leaf of Kammaru, which is a variety of Piper betle, has the ability to heal pharyngitis, abdominal pain, and swelling. Its roots and fruits are well known for use in the treatment of malaria and asthma.Citation9,Citation10 The mechanistic action and pharmaceutical function of PPF in skin protection has yet to be investigated in detail. This study investigated the potential anti-aging and wound-healing effects of PPF stem and leaf extract in normal human dermal fibroblast (NHDF) cells and a mouse model of wound healing.
Materials and methods
Preparation of PFF extract
PFF extract was provided by the Korea Research Institute of Bioscience & Biotechnology (KRIBB). Dried powdered material was extracted in methylethanol 99.9% for 3 days with SD-Ultrasonic Cleaner (Seoul, South Korea) at 45°C for 72 hours. The extract was filtered and concentrated at 45°C (Rotary Evaporator, N-1000SWD-EYELA, Tokyo Rikakikai Co., Ltd., Bohemya, NY, USA), and dried at 70°C for 24 hours with Modul spin 40 (Biotron Corporation, Alberta, Canada). Extracted PPF was diluted with pure water with different dose for each experiment.
Antibodies and reagents
The following antibodies were used: anti-elastin (Santa Cruz Biotechnology Inc., Dallas, TX, USA), anti-matrix metalloproteinase (MMP)-3 (Santa Cruz), anti-extracellular signal-regulated kinase (ERK) (Santa Cruz), anti-collagen (Abcam, Cambridge, UK), anti-actin (Sigma-Aldrich Co., St Louis, MO, USA), anti-tumor necrosis factor receptor (TNFR)-1 (Thermo Fisher Scientific, Waltham, MA, USA), anti-epidermal growth factor receptor (EGFR) (Thermo Fisher Scientific), anti-pp38 (Thr180/Tyr182; Cell Signaling Tech, Danvers, MA, USA), anti-c-Jun (Santa Cruz), anti-p53 (Cell Signaling Tech), and secondary antibodies (anti-mouse or anti-rabbit) from Komabiotech (Seoul, South Korea).
Cell culture
Normal Adult Human Primary Dermal Fibroblasts (NHDF) cells were purchased from ATCC (PCS-201-012, Manassas, VA, USA). NHDF cells were maintained in cultures in Dulbecco’s Modified Eagle’s Media (1:1) containing 10% fetal bovine serum and 1% antibiotic. NHDF cells were grown at 37°C in humidified 5% CO2.
Analysis for cell viability
NHDF cells were plated at a density of 1.0×104 cells/well in 96-well culture plates for complete attachment at 37°C with 5% CO2 for 24 hours. The cells were then treated with PPF at doses of 1, 10, and 50 μg/mL for 24 hours. The culture medium was then removed, followed by incubation with 90 mL of EXCyto (Lucigen Corporation, Middleton, WI, USA) 10 μL at 37°C for 3 hours. The absorbency was measured at 450 nm (referenced 659 nm) with an enzyme-linked immunosorbent assay reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). The results were expressed as the relative cell viability of treated cells against those of the controls.
Cell wound-healing assay (cell migration assay)
For the cell migration assay, the monolayers were carefully scratched using a 10 μL pipette tip. The cells were then treated with the PPF at doses of 1, 10, and 50 mg/mL for 24 hours and then the wounded area was photographed.
Analysis by real-time quantitative reverse transcription polymerase chain reaction
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis was performed as described previously.Citation11,Citation12 Total RNA was extracted from NHDF cell samples using the RNeasy kit (Qiagen NV, Venlo, the Netherlands). Complementary DNA was synthesized from total RNA with the SuperScript First-Strand Synthesis System (Thermo Fisher Scientific) and random hexamer primers. The real-time PCR measurement of individual complementary DNAs was performed using SYBR green dye to measure duplex DNA formation with the Thermo Fisher Scientific system (StepOne Plus) and normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase. The primers and probes used in the real-time qRT-PCR are explained in .
Table 1 Primer set for real-time PCR
Western blot analysis
The Western blot analysis was performed as described previously.Citation13–Citation15 NHDF cells were treated with PPF extract for 24 hours, washed twice in ice-cold phosphate-buffered saline, and homogenized at 4°C in the lysis buffer containing 50 mM Tris–HCl, pH 7.5, 1% Triton X-100, 300 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM sodium vanadate, 20 mM β-glycerol phosphate, 2 mM dithiothreitol, 1 mM leupeptin, and 10 mM p-nitrophenyl phosphate. Homogenates were centrifuged at 15,000× g for 10 minutes and supernatants were used for Western blot analysis. The protein concentration of lysates was measured by bovine serum albumin protein assay, and 50 μg of the cell lysates was fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel and blotted onto polyvinylidene difluoride membrane. After blocking with 5% skim milk tris-buffered saline containing 0.02% Tween 20, the membrane was probed with the relevant antibodies and visualized by enhanced chemiluminescence, according to the manufacturer’s instructions (GE Healthcare, Pittsburgh, PA, USA). Actin was used for a loading control.
ROS analysis
NHDF cells were plated and incubated at a density of 1.0×106 cells/well in six-well culture plates for 24 hours. The next day, the cells were UV irradiated at 40 J for 180 seconds with UV Crosslinker (Vilber Loirmat, Eberhardzell, Germany), nontreated and treated of PPF extracts for 24 hours. Cells were then washed and stained with 5 μM CM-H2DCFDA (Thermo fisher Scientific) in Hank’s balanced salt solution, incubated at 37°C for 30 minutes. FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA) was used for measuring H2O2 activity, using emission filter of 532 nm. At least 10,000 cells were analyzed in each of the three independent experiments.
Wound-healing treatments with mice
C57Bl/6 male mice (7 weeks-old, n=3) from the Jungang Animal Company (Seoul, Korea) were first anesthetized, and the area assigned for wounding was shaved. Four 0.25 cm2 (0.5×0.5 cm) excisional wounds were created on the dorsal skin (negative control and 1, 10, 50 μg/mL PPF extract). The treatment of wounded skin with PPF extracts was done with a cotton swab for 4 days (once a day). Mice were housed in standard mouse cages with a 12-hour light/dark cycle. The animal protocol in our study has been approved by the animal care and ethics committee of Chungnam National University. All animal-handling procedures were performed according to the Guide for the Care and Use of Laboratory Animals of the Korea National Institutes of Health and followed the guidelines of the Animal Welfare Act.
Statistical analysis
Quantification of Western blot analysis was carried out using the ImageJ program (NIH, Bethesda, MD, USA). Data are presented as mean ± SD. The results were analyzed by Student’s unpaired t-test (SPSS version 12.0 software, SPSS Inc., Chicago, IL, USA). *P<0.05 was considered significant, and **P<0.01 was highly significant compared with corresponding control values.
Results
Cell viability and wound-healing effects of PPF extract in NHDF cells
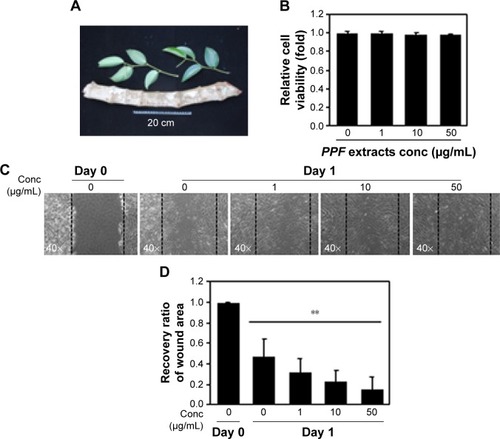
PPF was extracted from the leaf of Kammaru (). NHDF cells were treated with PPF extract at doses of 1, 10, and 50 μg/mL for 24 hours. Cells treated with PPF extract doses of 0, 10, and 50 μg/mL exhibited no effects on cell viability compared to untreated control cells (). In a cell wound-healing assay, confluent cell monolayers were injured by inflicting a scratch and then treated with PPF extracts at concentrations from 1 to 50 μg/mL for 24 hours. The PPF-treated cells showed enhanced repair of the scratched area in a dose-dependent manner ().
Figure 1 Cell viability and wound-healing assays.
Abbreviations: Conc, concentration; NHDF, normal human dermal fibroblast; PPF, Piper cambodianum P. Fourn.
Enhanced extracellular matrix gene expression levels in PPF-treated NHDF cells
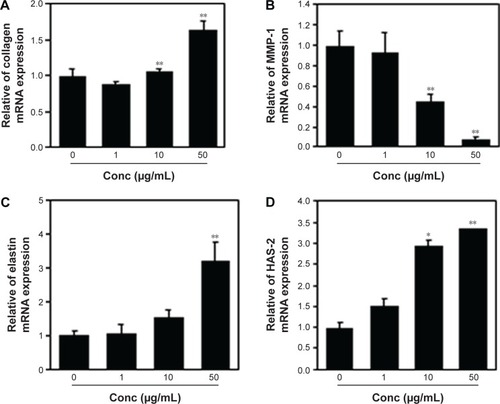
Collagen is the major structural protein present in the extracellular space of various connective tissues in animals.Citation16 Elastin is an elastic connective tissue protein that helps to maintain tissue shape after stretching or contracting by ensuring that tissues return to their original position. Hyaluronan or hyaluronic acid (HA) is a component of the extracellular matrix (ECM) involved in lubricating joints and filling space through which cells can migrate. During wound healing and tissue repair, HA provides a framework for fibroblasts. HAS-2 is a newly identified hyaluronan synthase (HAS). After treatment with various doses of PPF extract for 24 hours, ECM genes such as collagen, elastin, and HAS-2 expression levels were increased significantly in a dose-dependent manner. MMP-1 encoded by the MMP1 gene in humans is known as an interstitial and fibroblast collagenase.Citation16–Citation18 MMPs are involved in the breakdown of ECM in normal physiological processes and tissue remodeling, and MMP-1 specifically breaks down the interstitial collagen types I, II, and III. We demonstrated that the messenger RNA level of MMP-1 was significantly decreased in PPF-treated cells in a dose-dependent manner ().
Figure 2 ECM gene expression level by q-PCR.
Abbreviations: Conc, concentration; ECM, extracellular matrix; HAS, hyaluronan synthase; MMP, matrix metalloproteinase; mRNA, messenger RNA; NHDF, normal human dermal fibroblast; PPF, Piper cambodianum P. Fourn.; q-PCR, quantitative polymerase chain reaction; RNA, ribonucleic acid; SD, standard deviation.
Enhanced ECM protein expression in PPF-treated NHDF cells
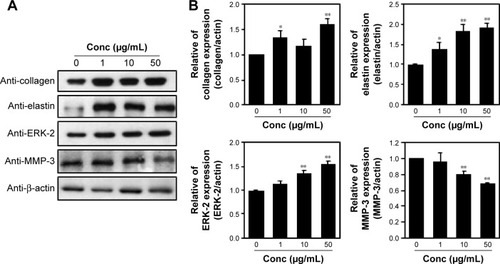
Protein extracts of untreated or PPF-treated cells were analyzed by Western blot analysis. PPF-treated cells showed increased collagen and elastin protein levels compared with untreated controls (). The ERK signaling pathway regulates type I collagen gene expression in human fibroblasts.Citation17 Our data showed that protein levels of ERK were enhanced significantly, and the interstitial collagenase enzyme MMP-3 was downregulated significantly by PPF extract in a dose-dependent manner (). The messenger RNA levels of collagen and elastin were correlated with the changes in their protein levels. These results suggested that the anti-aging effects of PPF on NHDF cells are related to the translational and transcriptional regulation of molecules involved in ECM.
Figure 3 Regulation of ECM proteins in PPF-treated cells.
Abbreviations: Conc, concentration; ECM, extracellular matrix; ERK, extracellular signal-regulated kinase; MMP, matrix metalloproteinase; NHDF, normal human dermal fibroblast; PPF, Piper cambodianum P. Fourn.; SDs, standard deviations.
PPF extract protected NHDF cells from the effects of UV irradiation
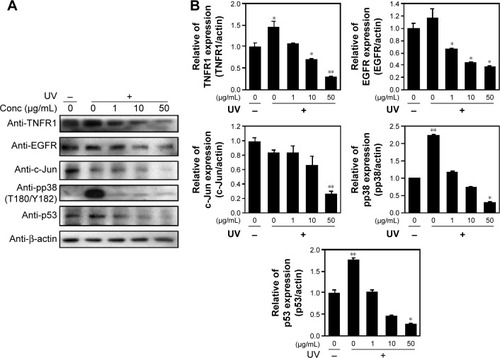
Skin cells respond quickly when exposed to UV or cytokines such as interleukin (IL)-4 and IL-13.Citation19 Upon UV exposure, various factors are stimulated, including EGFR,Citation20,Citation21 TNFR,Citation22 mitogen-activated protein (MAP) kinases, ERK, and c-Jun amino terminal kinase (JNK).Citation23 Both JNK and p38 phosphorylate and activate the transcription factor c-Jun, which, in turn, elevates c-Fos and increases levels of transcription factor activator protein-1,Citation24 which is required for transcription of MMPs. These receptors are activated by phosphorylation of MAP kinase and other downstream proteins.Citation25 MAP kinases are activated by phosphorylation of specific threonine and tyrosine residues.Citation24,Citation26 UV exposure leads to high expression of c-Jun and c-Fos heterodimers, numerous cytokines, and growth factors, which are phosphorylated by JNK and p38.Citation27,Citation28 Similarly, p53 protein levels were elevated, starting from 2.5 hours to 24 hours following UV-exposure.Citation29 We demonstrated that cells exposed to UV irradiation for 24 hours expressed TNFR1 and EGFR protein levels, which decreased in a dose-dependent manner when cells were treated with PPF. We examined the effect of UV radiation on both the phosphorylation and activity of p38 (Thr180/Tyr182). Protein levels of c-Jun and p53 were downregulated dramatically in PPF-treated cells ().
Figure 4 PPF extract inhibition of ROS production in UV-irradiated cells.
Abbreviations: Conc, concentration; EGFR, epidermal growth factor receptor; JNK, c-Jun amino terminal kinase; JNK, c-Jun N-terminal kinase; NHDF, normal human dermal fibroblast; PPF, Piper cambodianum P. Fourn.; ROS, reactive oxygen species; SD, standard deviation; TNFR, tumor necrosis factor receptor; UV, ultraviolet.
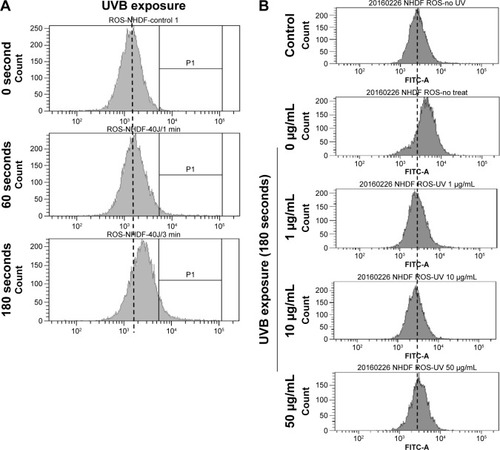
PPF extract protected UV-irradiated NHDF cells from ROS
UVB light stimulates the production of ROS,Citation30,Citation31 and UV exposure on the skin produces ROS such as O2−, H2O2, and 1O2.Citation32 Untreated and PPF-treated cells were subjected to UV irradiation for 24 hours, and the generation of ROS was measured by fluorescence-activated cell sorting (FACS). UV irradiation increased ROS production, while PPF extract decreased ROS production in NHDF cells. The ROS levels of H2O2 were measured using the cell permeants 2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) (). UV irradiation increased the ROS levels of H2O2,whereas ROS levels were reduced by PPF extract in a dose-dependent manner (). These results indicated that PPF extract protects dermal fibroblasts from UV irradiation by reducing ROS generation.
Figure 5 PPF-mediated inhibition of ROS production in UV-irradiated cells.
Abbreviations: FACS, fluorescence-activated cell sorting; FITC, fluorescein isothiocyanate; NHDF, normal human dermal fibroblast; PPF, Piper cambodianum P. Fourn.; ROS, reactive oxygen species; UV, ultraviolet; UVB, ultraviolet B.
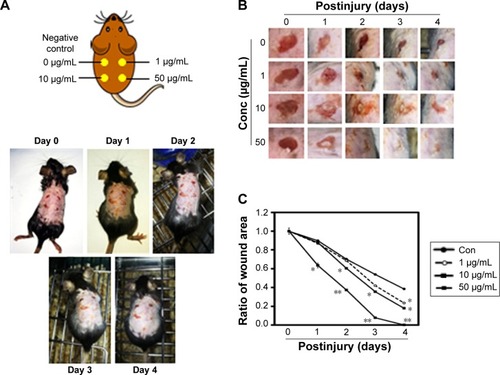
Macroscopic observation of wound healing in mice
Wounds were created on the backs of mice and then treated with PPF extract (0, 1, 10, and 50 μg/mL); the wound areas were measured each day for 4 days. On day 4, the areas surrounding the wounds in PPF-treated mice showed decreased redness in a dose-dependent manner compared to untreated wound areas (). The extract-treated wound areas were smaller than the untreated wound area, showing a dose-dependent pattern. On day 4, the wound areas treated with 50 μg/mL of PPF were completely covered by new epithelium. No wound areas were infected (). The ratios of wound areas on days 0 and 4 were calculated ().
Figure 6 Wound healing effects on impaired mouse skin.
Abbreviations: PPF, Piper cambodianum P. Fourn; Conc, concentration.
Discussion
The use of herbal materials and different plant parts has been increasing with concomitant advances in phytotherapy. Herbal materials have been used in various forms such as mono- or polyherbal drugs, dietary supplements, and dietary ingredients, and many have become well-known and safe commercial commodities.Citation33 Various plants, including PPF, have been investigated for their potential pharmacological properties, but no comparative study was performed previously with PPF extract. This study was performed to determine the effects of PPF extract on wound-healing activities using incision wound models in mice. Wound healing involves the re-establishment of tissue integrity through processes associated with inflammation, proliferation, and remodeling stages.Citation34 The skin remodeling stage is characterized by the reformulation and reformation of collagen fiber components that improve tensile strength.Citation35 The healing process is regulated mostly by the biosynthesis and deposition of new collagen and subsequent maturation.Citation36 In our wound study, PPF extract showed increased wound-healing effects compared with the control ( and ). Such healing might be due to the increased collagen concentration and stabilization of the fibers. In the tissue repair process, inflammatory cells enhance the migration and proliferation of endothelial cells, leading to neovascularization of connective tissue cells that synthesize extracellular matrices, including collagen and keratinocytes, resulting in the reepithelialization of wounded tissue.Citation37 The faster wound contraction rate may increase the gap junctional intracellular communication in fibroblasts and enhance faster maturation of granulation tissue.Citation38 In the incision wound model, mice treated with PPF extract showed faster wound healing compared with controls (). Numerous studies have revealed that downregulation of the p38 kinase pathway protects various wild-type p53-expressing cell lines from genotoxic stress.Citation39,Citation40 Our data showed that p38 expression in UV-irradiated cells was downregulated by PPF treatment. Therefore, PPF extract obtained from leaves and stems demonstrated effective treatment in wound healing. PPF extract decreased ROS production from UV irradiation by scavenging ROS in NHDF cells (). Moreover, PPF extract accelerated wound healing after skin irradiation ((). These results suggested that PPF extract protects skin from UV irradiation damage, and might be useful as a novel raw material in cosmetic application.
Conclusion
This study investigated the potential anti-aging and wound-healing effects of PPF stem and leaf extract in NHDF cells and a mouse model of wound healing. The treatment of NHDF cells with PPF extracts promote the recovery of the scratched area in a dose-dependent manner as well as the enhancement of ECM gene expression. In addition, PPF extracts were able to protect the cell from UV exposure by reducing ROS production. Finally these observation are further evaluated and confirmed in the model mice for in-vivo wound-healing. Therefore, PPF extract represent a promising new therapeutic agent for anti-aging and wound-healing treatment.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2014R1A1A3050752). The authors would like to thank Korea Research Institute of Bioscience & Biotechnology (KRIBB) for providing to PPF extracts.
Disclosure
The authors report no conflicts of interest in this work.
References
- SlominskiATZmijewskiMASemakICytochromes p450 and skin cancer: role of local endocrine pathwaysAnticancer Agents Med Chem2014141779623869782
- GilchrestBASkin aging and photoagingDermatol Nurs19902279822141531
- HaTYDevelopment of functional food materials for healthy lifeKorean J Crop Sci2006512639
- MakrantonakiEZouboulisCCMolecular mechanisms of skin aging: state of the artAnn N Y Acad Sci20071119405018056953
- SeoMYChungSYChoiWKAnti-aging effect of rice wine in cultured human fibroblasts and keratinocytesJ Biosci Bioeng2009107326627119269590
- KligmanAMEarly destructive effect of sunlight on human skinJAMA196921013237723805395389
- OikarinenAKarvonenJUittoJHannukselaMConnective tissue alterations in skin exposed to natural and therapeutic UV-radiationPhotodermatol19852115263885186
- Bech-ThomsenNWulfHCCarcinogenic and melanogenic effects of a filtered metal halide UVA source and a tubular fluorescent UVA tanning source with or without additional solar-simulated UV radiation in hairless micePhotochem Photobiol19956247737797480154
- DeMSLahiriSCStudies on leaves of Piper betleBull Calcutta Sch Trop Med197119369725170641
- FerreresFOliveiraAPGil-IzquierdoAValentãoPAndradePBPiper betle leaves: profiling phenolic compounds by HPLC/DAD-ESI/MS(n) and anti-cholinesterase activityPhytochem Anal201425545346024733630
- NaCHHongJHKimWSIdentification of protein markers specific for papillary renal cell carcinoma using imaging mass spectrometryMol Cells201538762462926062552
- CheonJMKimDIKimKSInsulin sensitivity improvement of fermented Korean Red Ginseng (Panax ginseng) mediated by insulin resistance hallmarks in old-aged ob/ob miceJ Ginseng Res201539433133726869825
- KimAYKwakJHJeNKLeeYHJungYSEpithelial-mesenchymal transition is associated with acquired resistance to 5-fluorocuracil in HT-29 colon cancer cellsToxicol Res201531215115626191381
- LiYParkJPiaoLPKB-mediated PHF20 phosphorylation on Ser291 is required for p53 function in DNA damageCell Signal2013251748422975685
- KimISYangSYHanJHJungSHParkHSMyungCSDifferential gene expression in GPR40-overexpressing pancreatic beta-cells treated with linoleic acidKorean J Physiol Pharmacol201519214114925729276
- Di LulloGASweeneySMKorkkoJAla-KokkoLSan AntonioJDMapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagenJ Biol Chem200227764223423111704682
- BrinckerhoffCERubyPLAustinSDFiniMEWhiteHDMolecular cloning of human synovial cell collagenase and selection of a single gene from genomic DNAJ Clin Invest19877925425463027129
- PendasAMSantamaríaIAlvarezMVPritchardMLópez-OtínCFine physical mapping of the human matrix metalloproteinase genes clustered on chromosome 11q22.3Genomics19963722662688921407
- BhogalRKBonaCARegulatory effect of extracellular signal-regulated kinases (ERK) on type I collagen synthesis in human dermal fibroblasts stimulated by IL-4 and IL-13Int Rev Immunol200827647249619065352
- SachsenmaierCRadler-PohlAZinckRNordheimAHerrlichPRahmsdorfHJInvolvement of growth factor receptors in the mammalian UVC responseCell19947869639727923365
- WarmuthIHarthYMatsuiMSWangNDeLeoVAUltraviolet radiation induces phosphorylation of the epidermal growth factor receptorCancer Res19945423743768275472
- DyLCPeiYTraversJBAugmentation of ultraviolet B radiation-induced tumor necrosis factor production by the epidermal platelet-activating factor receptorJ Biol Chem199927438269172692110480902
- RosetteCKarinMUltraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptorsScience19962745290119411978895468
- CofferPJBurgeringBMPeppelenboschMPBosJLKruijerWUV activation of receptor tyrosine kinase activityOncogene19951135615697543196
- PawsonTScottJDSignaling through scaffold, anchoring, and adaptor proteinsScience19972785346207520809405336
- ZhengCFGuanKLProperties of MEKs, the kinases that phosphorylate and activate the extracellular signal-regulated kinasesJ Biol Chem19932683223933239398226933
- AngelPKarinMThe role of jun, fos and the AP-1 complex in cell-proliferation and transformationBiochim Biophys Acta199110722–31291571751545
- ChenCCMoFELauLFThe angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblastsJ Biol Chem200127650473294733711584015
- WuLLevineAJDifferential Regulation of the p21/WAF-1 and mdm2 Genes after High-Dose UV Irradiation: p53-Dependent and p53-Independent Regulation of the mdm2 GeneMol Med1997374414519260156
- HeckDEVetranoAMMarianoTMLaskinJDUVB light stimulates production of reactive oxygen species: unexpected role for catalaseJ Biol Chem200327825224322243612730222
- HansonKMCleggRMObservation and quantification of ultraviolet-induced reactive oxygen species in ex vivo human skinPhotochem Photobiol2002761576312126308
- SakuraiHYasuiHYamadaYNishimuraHShigemotoMDetection of reactive oxygen species in the skin of live mice and rats exposed to UVA light: a research review on chemiluminescence and trials for UVA protectionPhotochem Photobiol Sci20054971572016121282
- Ya’uJChindoBAYaroAHOkhaleSEAnukaJAHussainiIMSafety assessment of the standardized extract of Carissa edulis root bark in ratsJ Ethnopharmacol2013147365366123567035
- KokaneDDMoreRYKaleMBNeheteMNMehendalePCGadgoliCHEvaluation of wound healing activity of root of Mimosa pudicaJ Ethnopharmacol2009124231131519397984
- BitirenMKarakilcikAZZerinMProtective effects of selenium and vitamin E combination on experimental colitis in blood plasma and colon of ratsBiol Trace Elem Res20101361879519774348
- GautamMKPurohitVAgarwalMSinghAGoelRKIn vivo healing potential of Aegle marmelos in excision, incision, and dead space wound modelsScientificWorldJournal2014201474010724737990
- MidwoodKSOrendGThe role of tenascin-C in tissue injury and tumorigenesisJ Cell Commun Signal200933–428731019838819
- MoyerKESaggersGCAllisonGMMackayDREhrlichHPEffects of interleukin-8 on granulation tissue maturationJ Cell Physiol2002193217317912384994
- HuangCMaWYMaxinerASunYDongZp38 kinase mediates UV-induced phosphorylation of p53 protein at serine 389J Biol Chem199927418122291223510212189
- BulavinDVSaitoSHollanderMCPhosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiationEMBO J199918236845685410581258
