Abstract
Background
The aim of this study was to evaluate the clinically significant predictors of hepatocellular carcinoma (HCC) development among hepatitis C virus (HCV) cirrhotic patients receiving combination therapy.
Patients and methods
One hundred and five compensated cirrhosis patients who received pegylated interferon plus ribavirin between January 2005 and December 2011 were enrolled. All the patients were examined with abdominal sonography and liver biochemistry at baseline, end of treatment, and every 3–6 months posttreatment. The occurrence of HCC was evaluated every 3–6 months posttreatment.
Results
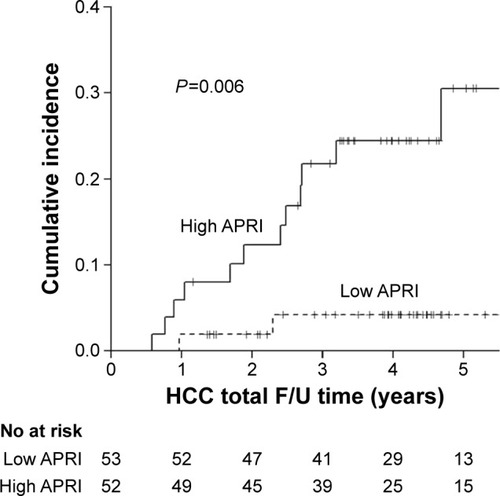
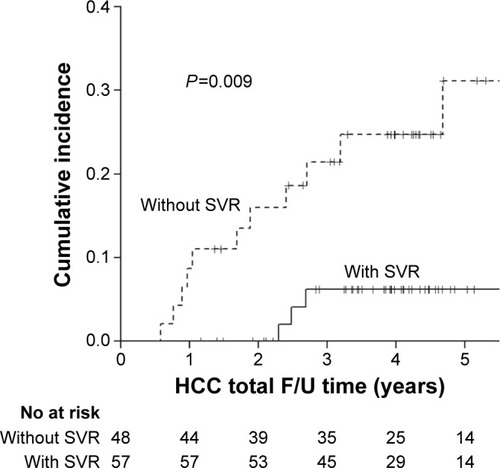
A total of 105 patients were enrolled (mean age 58.3±10.4 years). The average follow-up time for each patient was 4.38 years (standard deviation 1.73 years; range 1.13–9.27 years). Fifteen (14.3%) patients developed HCC during follow-up period. Thirteen of them had high baseline aspartate aminotransferase to platelet ratio index (APRI) (ie, an APRI >2.0). Multivariate analysis showed that those without sustained virologic response (SVR) (hazard ratio [HR] 5.795; 95% confidence interval [CI] 1.370–24.5; P=0.017) and high APRI (HR 5.548; 95% CI 1.191–25.86; P=0.029) had a significantly higher risk of HCC occurrence. The cumulative incidence of HCC was significantly higher (P=0.009) in patients without SVR (3-year cumulative incidence 21.4%; 95% CI 7.4%–35.5%; 5-year cumulative incidence 31.1%; 95% CI 11.2%–51.1%) compared to those with SVR (3- and 5-year cumulative incidence 6.2%; 95% CI 0%–1.3%). Further, the cumulative incidence of HCC was significantly higher (P=0.006) in patients with high APRI (3-year cumulative incidence 21.8%; 95% CI 8.2%–35.3%; 5-year cumulative incidence 30.5%, 95% CI 11.8%–49.3%) compared to those with low APRI (3- and 5-year cumulative incidence 4.2%, 95% CI 0%–1.0%).
Conclusion
In HCV-infected cirrhotic patients who received combination therapy, APRI and SVR are the two major predictors of HCC development.
Introduction
Hepatocellular carcinoma (HCC) is one of the most deadly cancers and is the third leading cause of cancer-related death among males and the sixth among females worldwide.Citation1 According to a recent national analysis in Taiwan, HCC remains the second leading cause of cancer-related death among both males and females during the past 10 years.Citation2 Chronic hepatitis is one of the most important causes of chronic liver diseases, including cirrhosis that can lead to subsequent decompensation and development of HCC.Citation3
The estimated risk of HCC is 15–20 times higher among persons infected with hepatitis C virus (HCV) compared to those without infection, and the greatest excess risk occurs in those with advanced hepatic fibrosis or cirrhosis.Citation4,Citation5 In chronic HCV antiviral therapy, a sustained viral response (SVR) had been a clinically meaningful end point at which viral clearance contributes to reduced inflammation and histologically identified fibrosis, fewer hepatic complications, lower liver-related mortality, and a reduced incidence of HCC.Citation6–Citation8 Even in patients with advanced liver disease, SVR has been found to be associated with HCC risk reduction.Citation9
Risk factors for HCV-related HCC include older age, male sex, coinfection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), obesity, hepatic fibrosis, alcohol abuse, and sex hormone dysregulation.Citation4,Citation10,Citation11 The liver fibrosis stage provides important prognostic information about the development of HCC. The aspartate aminotransferase to platelet ratio index (APRI) is a noninvasive marker that has been validated for the diagnosis of both significant fibrosis and cirrhosis.Citation12,Citation13 APRI is a useful marker for the prognosis in chronic hepatitis C (CHC) patients.Citation14 Some recent studies report that the APRI score could be a predictor of HCC in chronic hepatitis patients.Citation15,Citation16 APRI could be a useful marker to classify HCC risk in CHC patients who achieved SVR and predict HCC recurrence after radiofrequency ablation.Citation17–Citation19 However, the prognostic value of APRI in cirrhotic patients for predicting the occurrence of HCC is uncertain.
Hence, the aim of this study was to evaluate the clinically significant predictors of HCC development among HCV cirrhotic patients. These factors may affect physicians’ clinical decisions.
Patients and methods
Selection of patients
HCV patients with Child A cirrhosis without decompensation and who underwent treatment at the Dalin Tzu Chi General Hospital with either pegylated interferon (PEG-IFN)-α-2a or PEG-IFN-α-2b plus ribavirin (RBV) between January 2005 and December 2011 were enrolled in this retrospective study. All the patients were positive for antihepatitis C antibody for more than 6 months, had an alanine aminotransferase (ALT) level higher than the upper limit of normal, and had detectable serum HCV RNA. We excluded patients with posttreatment follow-up <1 year, HCC history, incomplete medical records, autoimmune diseases, HIV infection, neutropenia (<1,500 neutrophils/mL), thrombocytopenia (<50,000 platelets/mL), anemia (<12 g of hemoglobin/dL in females and <13 g/dL in males), or poorly controlled psychiatric disease.
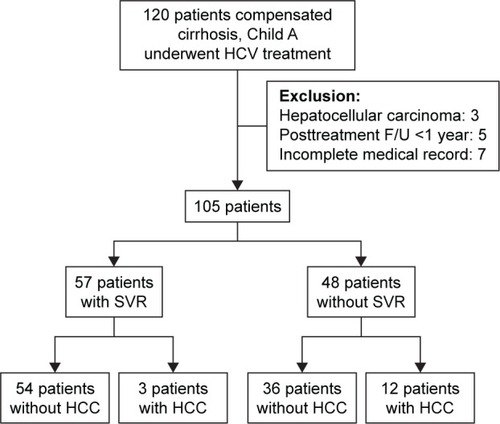
One hundred and twenty compensated cirrhosis patients who received PEG-IFN plus RBV were initially enrolled (). Five patients had posttreatment follow-up time <1 year, seven did not have complete medical records, and three previously had HCC, and all these patients were excluded from the study. The remaining 105 patients were finally included. The average follow-up time for each patient was 4.38 years (standard deviation [SD] 1.73 years; range 1.13–9.27 years). The study was approved by the Research Ethics Committee of Dalin Tzu Chi Hospital (B099010124). Written informed consent was obtained from each patient, and the Research Ethics Committee of Dalin Tzu Chi Hospital approved the written consent process.
Regimen of PEG-IFN plus RBV
PEG-IFN-α-2a (Pegasys; Hoffman-La Roche Ltd., Basel, Switzerland) or PEG-IFN-α-2b (PegIntron; Schering-Plough Corp., Kenilworth, NJ, USA) plus RBV were prescribed to eligible patients for 6 months. PEG-IFN-α-2a (180 μg/kg) or PEG-IFN-α-2b (1.5 μg/kg) was administered once per week by subcutaneous injection. The fixed duration (6 months) without consideration of HCV genotypes is due to restrictions imposed by the reimbursement policy of the Bureau of National Health Insurance in Taiwan. RBV was prescribed orally at a dose of 800 mg/day for patients <55 kg, 1,000 mg/day for patients between 55 and 75 kg, and 1,200 mg/day for patients >75 kg. Adjustments to the dose of PEG-IFN and RBV and treatment with either erythropoietin or blood transfusion were determined according to published practice guidelines.Citation3,Citation20–Citation22
Clinical monitoring
The primary outcome was time to develop HCC. All patients were examined at baseline, end of treatment, and every 3–6 months posttreatment with a liver function test and abdominal sonography at the hospital’s gastrointestinal outpatient clinic. The serum aspartate aminotransferase (AST), ALT, total bilirubin, creatinine, hemoglobin, white blood cell count, and platelet count were measured at each time point. HCV RNA was quantified at baseline and 24 weeks posttreatment. The diagnosis of liver cirrhosis was made by liver biopsy or by clinical diagnostic criteria including twice documented ultrasonographic evidence of a coarse and nodular parenchyma, irregular surface, and dull margin with splenomegaly, ascites, hepatic encephalopathy, or varices.Citation23 Liver biopsy with optional procedure was performed at baseline with patients’ consent. Liver biopsy was obtained from 62 patients (59.6%) in this study. A diagnosis of fatty liver was based on the results of biopsy and/or abdominal ultrasound. Other clinical factors, including diabetes mellitus, chronic hepatitis B (CHB), and alcoholism, were also evaluated by chart review. The diagnosis of CHB was based on seropositive status for hepatitis B surface antigen for at least 6 months. HCC was diagnosed either by biopsy or by imaging in the setting of liver cirrhosis. The specific imaging pattern was defined by increased contrast uptake in the arterial phase followed by contrast washout in the venous/delayed phase as seen via computer tomography or magnetic resonance.Citation24,Citation25 In this study, we calculated APRI using the formula of ([AST/upper limit of normal]/platelet count [109/L]) ×100, and further used it as a noninvasive marker validated for the diagnosis of both significant fibrosis and cirrhosis. The cutoff value of APRI >2.0 defined high APRI and APRI <2.0 defined low APRI.Citation3,Citation12,Citation13
HCV quantification and genotyping
Serum HCV RNA was quantified at baseline and 24 weeks posttreatment using real-time polymerase chain reaction with a detection limitation of 15 IU/mL.Citation26 The threshold for discriminating low- and high-baseline HCV RNA was 400,000 IU/mL.Citation22 HCV genotyping was performed using melting curve analysis (Roche LightCycler; Biotronics Tech Corp., Lowell, MA, USA).Citation27
Sustained virologic response
SVR was defined as an undetectable HCV RNA for at least 24 weeks after the patient completed the combined treatment of PEG-IFN plus RBV. Patients who were positive for HCV RNA at week 24 posttreatment were considered non-SVR.
Statistical analysis
SPSS 19.0 for Windows (IBM Corporation, Armonk, NY, USA) was used for all statistical analyses. The chi-square test or the Fisher’s exact test was used for nominal variables. Student’s t-test was used to compare continuous variables with normal distributions, and the Mann–Whitney U-test was used for continuous variables with nonnormal distributions. In competing risk data ratios, we conducted calculations and comparisons of cumulative incidences using a modified Kaplan–Meier method. We tested differences in the full time-to-event distributions between the study groups using a log-rank test. To determine the independent risk factors for HCC occurrence, we carried out multivariate analyses and stratified analyses using HRs with Cox proportional hazards regression models in the presence of a competing risk event after adjusting for age, sex, high APRI, SVR, genotype, and cohepatitis (including hepatitis B coinfection, alcoholism, or fatty liver). Results were shown as HRs with 95% CIs. Significances in all analyses were set as P<0.05.
Results
General characteristics of the patients
A total of 105 patients with HCV-associated Child A cirrhosis without decompensation were enrolled, consisting of 57 patients (54.3%) with SVR and 48 patients without SVR (45.7%) by intention-to-treat analysis. Of the 105 patients, 66 (62.9%) were infected with HCV genotype 1, and the remaining 39 patients (37.1%) were infected with other genotypes of HCV. There were 47 males and 58 females, and the mean patient age was 58.3±10.4 years. Thirteen patients (12.4%) did not complete the full treatment regimen (mean treatment duration, 61 days). One patient (1/13) stopped treatment due to major trauma with renal hemorrhage. Twelve patients (12/13, 92.3%) did not accept complete treatment due to treatment side effects. Among those patients, decompensation with jaundice (4/13) was the most common reason. An intention-to-treat analysis was performed in this study. No deaths or severe treatment-related complications were found during the course of treatment. The average follow-up time for each patient was 4.38 years (SD 1.73 years; range 1.13–9.27 years) (). Fifteen (14.3%) patients developed HCC during follow-up period; 13 in this group had high baseline APRI (>2.0) and two had low APRI (<2.0).
Table 1 Baseline characteristics and treatment outcomes between patients with and without progression to HCC
Demographics and clinical features that predispose to HCC
shows the baseline characteristics and treatment outcomes of the patients with and without progression to HCC. We performed univariate analyses on the two groups and found significant differences in age, SVR, baseline APRI, platelet count, AST, and ALT. No significant differences in sex, diabetes, cohepatitis (alcoholism, HBV coinfection, or fatty liver), HCV genotype, HCV RNA load, alpha-fetoprotein, albumin, international normalized ratio of prothrombin time, and total bilirubin were found between the two groups.
Multivariate stratified analysis
shows the results of the Cox regression analysis. After adjustments for age, sex, high APRI, SVR, genotype, and cohepatitis (including hepatitis B coinfection, alcoholism, or fatty liver), those without SVR (HR 5.795; 95% CI 1.370–24.5; P=0.017) and those with a high APRI level (HR 5.548; 95% CI 1.191–25.86; P=0.029) were associated with a significantly higher risk of HCC occurrence.
Table 2 Adjusted HRs for HCC progression with Cox regression analysis*
Cumulative incidences of HCC occurrence
The cumulative incidences of HCC among patients with and without SVR after treatment are shown in . The cumulative incidence of HCC was significantly higher (P=0.009) in patients without SVR (3-year cumulative incidence 21.4%; 95% CI 7.4%–35.5%; 5-year cumulative incidence 31.1%; 95% CI 11.2%–51.1%) compared to those with SVR (3- and 5-year cumulative incidence 6.2%; 95% CI 0%–1.3%). The difference in 5-year cumulative incidence was 24.9%. The unadjusted number needed to treat associated with 1 less HCC within 5 years was 4, suggesting that four patients who achieved SVR were associated with 1 less HCC within 5 years of treatment.
Figure 2 Cumulative risk of hepatocellular carcinoma with and without SVR.
shows the cumulative incidence among patients with high and low APRI values, based on a cutoff value of 2.0. The cumulative incidence of HCC was significantly higher (P=0.006) in patients with a high APRI value (3-year cumulative incidence 21.8%; 95% CI 8.2%–35.3%; 5-year cumulative incidence 30.5%; 95% CI 11.8%–49.3%) compared to those with low APRI values (3- and 5-year cumulative incidence 4.2%; 95% CI 0%–1.0%). The difference in 5-year cumulative incidences was 26.3%.
Discussion
HCV is a single-stranded RNA virus, and its genome is never integrated into the genome of hepatocytes. Although no known oncogenic properties have been reported with respect to HCV, the multiple functions of HCV proteins and their effects on the modulation of intracellular signaling transduction processes may facilitate carcinogenesis via interactions of viral proteins with host cell proteins.Citation28 HCV core proteins lead to the promotion of cellular transformation, and HCV-related chronic inflammation has been responsible for promoting mutations during hepatocyte regeneration, thereby contributing to HCC development.Citation29,Citation30 Hepatocyte regeneration and cycling that occur after antiviral therapy may activate cellular pathways involved in the development of dysplasia, increasing the risk of hepatocarcinogenesis.Citation31 Interferon-based therapy was found to reduce the risk of HCC development, particularly in virologic or biochemical responders.Citation32–Citation34 Despite the larger magnitude of risk reduction in patients with SVR, there remains a definite risk of HCC in SVR patients.Citation11,Citation35 The risk factors for HCV-related HCC include older age, male, coinfection with HIV or HBV, obesity, hepatic fibrosis, alcohol abuse, and sex hormone dysregulation.Citation4,Citation10,Citation11
A meta-analysis summarized the evidence from 30 observational studies that examined the risk of HCC among HCV-infected patients and found that SVR was associated with a reduction in the relative risk of HCC for patients at all stages of liver disease (HR 0.24; 95% CI 0.18%–0.31%; P<0.001) and a higher absolute benefit (HCC prevention) in patients with advanced liver disease who achieved SVR.Citation9 Similarly, in our study, HCC development in cirrhotic HCV patients was significantly lower in the SVR group. Those patients without SVR (HR 5.795; 95% CI 1.370–24.5; P=0.017) were associated with a significantly higher risk of HCC occurrence. A large cohort study conducted in Taiwan showed similar results in HCC risk reduction among cirrhotic HCV patients.Citation10
Notably, our study results found that high APRI (>2) is an independent risk factor for HCC development (HR 5.548; 95% CI 1.191–25.86; P=0.029). APRI is a noninvasive measurement for diagnosing hepatic fibrosis and cirrhosis and was recommended by the World Health Organization (WHO) guidelines for HCV infection published in 2014.Citation3 Based on the WHO recommendation, patients with values above the high APRI cutoff value (>2) should be treated with higher priority because they have a high probability of developing F4 cirrhosis.Citation3 A recent study showed that a higher APRI significantly increased the risk of HCC in cirrhotic HBV-infected patients, but this finding was attenuated after multivariate adjustment.Citation16 More recently, in a cohort of 642 SVR Taiwan patients (13% cirrhotics), HCC was strongly associated with cirrhosis (HR 4.98; 95% CI 2.32–10.71; P<0.001) and less strongly with age (HR 1.06; 95% CI 1.02–1.11; P=0.005) and Gamma-glutamyl transpeptidase (γGT) (HR 1.01; 95% CI 1.00–1.013; P<0.001).Citation36 This study supports the importance of cirrhosis as the predictor of HCC in CHC patients and potential role of APRI. As far as we know, few studies have evaluated the possible relationship between APRI and HCC development in HCV-infected patients after SVR.Citation15,Citation17,Citation18 Our study included pure cirrhotic cohort and showed that high APRI (>2.0) is significantly related to HCC development even after multivariate adjustment. The data from recent cohort support the recommendation that a higher treatment priority should be given to those patients who present with a high APRI.Citation3
Although a high proportion of genotype 1 (62%) and advanced liver disease with short treatment duration (6 months) cause lower SVR rate (54.3%), a recent study showed the achievement of SVR could slow down the rapid progression of HCC among cirrhotic patients.Citation9 In the era of new, interferon-sparing, antiviral strategies, the SVR rate for similar patients is more than 90%. The results of our study support the significant benefit of SVR achievement in lowering the progression of HCC, and the APRI score could help us identify patients at higher risk of HCC development. The direct antiviral agent treatment should be provided for the cirrhotic patients even though it is expensive.
Our study found no difference between 3- and 5-year cumulative incidences of HCC in patients with SVR or with low APRI, as shown in and . The stabilization of cumulative incidences may indicate that HCC development is more closely related to developments during the first few years after SVR achievement in low APRI patients. The interaction between SVR and APRI appears to be complicated. A higher fibrosis stage is associated with lower SVR rate, and typically the fibrosis stage improves after SVR is achieved. This may be due to high SVR durability with a low relapse rate, which has been confirmed in HCV-infected liver transplant recipients, hemophiliacs, cirrhotics, those coinfected with HIV, and children. A durable SVR may improve the fibrosis stage and decrease the incidence of HCC 3 years later. Hence, HCV cirrhotic patients (especially those with a high APRI) should be treated as early as possible because of potential beneficial effects in HCC prevention.
There are some limitations in our study. First, liver biopsy is considered the gold standard for cirrhosis diagnosis. Transient elastography could offer a noninvasive alternative to biopsy to measure the stage of fibrosis, given its comparable diagnostic accuracy. Due to the earlier study period with facility limitation, we could not provide these data. In our study, 59.6% (62/105) of patients underwent biopsy and other patients with cirrhosis were diagnosed using the clinical criteria. However, the diagnosis criteria combined with twice documented ultrasonographic evidence of liver cirrhosis with solid clinical end point (splenomegaly, ascites, hepatic encephalopathy, or varices) could complementally increase the accuracy of cirrhosis diagnosis in the current study.Citation18 Second, this is a retrospective, single-center, observational study, which could lead to selection bias because patients with severe cirrhosis were probably not considered for treatment and therefore were not included in our study.
Conclusion
In cirrhotic HCV-infected patients, SVR is a major predictor of HCC development, whereas APRI may be a potent predictor of HCC risk among these patients. Further studies are warranted to validate our findings and their applicability in clinical practice.
Author contributions
Khai-Jing Ng: drafting of the manuscript; Chih-Wei Tseng: statistical analysis, material support, and drafting of the manuscript; Ting-Tsung Chang and Shu-Fen Wu: critical revision of the manuscript for important intellectual content; Shinn-Jia Tzeng: statistical analysis; Yu-Hsi Hsieh, Tsung-Hsing Hung, and Hsiang-Ting Huang: material support; Kuo-Chih Tseng: material support and critical revision of the manuscript for important intellectual content. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank the nursing departments of Dalin Tzu Chi Hospital for their assistance in procuring records. The study was funded by Dalin Tzu Chi General Hospital (DTCRD99(2)-E-13).
Disclosure
The authors report no conflicts of interest in this work. The abstract of this paper was presented at the Asian Pacific Digestive Week, Taipei, Taiwan, 3–6 December, 2015. The abstract was published in “Poster Abstracts” in the Journal of Gastroenterology and Hepatology, special issue: Volume 30, Issue Supplement S4, December 2015.
References
- HerszényiLTulassayZEpidemiology of gastrointestinal and liver tumorsEur Rev Med Pharmacol Sci201014424925820496531
- Ministry of Health and WelfareCause of Death Statistics2013 Available from: http://www.mohw.gov.tw/EN/Ministry/Statistic.aspx?f_list_no=474&fod_list_no=5044Accessed January 19, 2015
- WHOGuidelines for the Screening, Care and Treatment of Persons with Hepatitis C InfectionGeneva, SwitzerlandWHO2014
- El-SeragHBHepatocellular carcinomaN Engl J Med2011365121118112721992124
- ReinDBWittenbornJSWeinbaumCMSabinMSmithBDLesesneSBForecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United StatesDig Liver Dis2011431667220739252
- PearlmanBLTraubNSustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much moreClin Infect Dis201152788990021427396
- VeldtBJHeathcoteEJWedemeyerHSustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosisAnn Intern Med20071471067768418025443
- BrunoSBattezzatiPMBellatiGLong-term beneficial effects in sustained responders to interferon-alfa therapy for chronic hepatitis CJ Hepatol200134574875511434622
- MorganRLBaackBSmithBDYartelAPitasiMFalck-YtterYEradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studiesAnn Intern Med20131585 Pt 132933723460056
- HungCHLeeCMWangJHImpact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapyInt J Cancer2011128102344235220669224
- Asia-Pacific Working Party on Prevention of Hepatocellular CarcinomaPrevention of hepatocellular carcinoma in the Asia-Pacific region: consensus statementsJ Gastroenterol Hepatol201025465766320492323
- WaiCTGreensonJKFontanaRJA simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis CHepatology200338251852612883497
- Vallet-PichardAMalletVNalpasBFIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotestHepatology2007461323617567829
- VergniolJBoursierJCoutzacCEvolution of noninvasive tests of liver fibrosis is associated with prognosis in patients with chronic hepatitis CHepatology2014601657624519328
- YuMLLinSMLeeCMA simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapyHepatology20064451086109717058238
- HannHWWanSLaiYAspartate aminotransferase to platelet ratio index as a prospective predictor of hepatocellular carcinoma risk in patients with chronic hepatitis B virus infectionJ Gastroenterol Hepatol201530113113824995497
- WuCKChangKCHungCHDynamic alpha-fetoprotein, platelets and AST-to-platelet ratio index predict hepatocellular carcinoma in chronic hepatitis C patients with sustained virological response after antiviral therapyJ Antimicrob Chemother20167171943194727073265
- LeeKSinnDHGwakGYPrediction of the risk of hepatocellular carcinoma in chronic hepatitis C patients after sustained virological response by aspartate aminotransferase to platelet ratio indexGut Liver Epub2016428
- ChungHAKimJHHwangYNoninvasive fibrosis marker can predict recurrence of hepatocellular carcinoma after radiofrequency ablationSaudi J Gastroenterol2016221576326831608
- FriedMWSide effects of therapy of hepatitis C and their managementHepatology2002365 Suppl 1S237S24412407599
- OmataMKandaTYuMLAPASL consensus statements and management algorithms for hepatitis C virus infectionHepatol Int20126240943526201405
- European Association for Study of LiverEASL Clinical Practice Guidelines: management of hepatitis C virus infectionJ Hepatol201460239242024331294
- HuangJFYuMLLeeCMSustained virological response to interferon reduces cirrhosis in chronic hepatitis C: a 1,386-patient study from TaiwanAliment Pharmacol Ther20072591029103717439503
- BruixJShermanMAmerican Association for the Study of Liver DiseasesManagement of hepatocellular carcinoma: an updateHepatology20115331020102221374666
- de LopeCRTremosiniSFornerAReigMBruixJManagement of HCCJ Hepatol201256Suppl 1S75S8722300468
- RatgeDScheiblhuberBNitscheMKnabbeCHigh-speed detection of blood-borne hepatitis C virus RNA by single-tube real-time fluorescence reverse transcription-PCR with the LightCyclerClin Chem200046121987198911106331
- BullockGCBrunsDEHaverstickDMHepatitis C genotype determination by melting curve analysis with a single set of fluorescence resonance energy transfer probesClin Chem200248122147215412446470
- SelimovicDEl-KhattoutiAGhozlanHHaikelYAbdelkaderOHassanMHepatitis C virus-related hepatocellular carcinoma: an insight into molecular mechanisms and therapeutic strategiesWorld J Hepatol201241234235523355912
- FabregatIDysregulation of apoptosis in hepatocellular carcinoma cellsWorld J Gastroenterol200915551352019195051
- ButDYLaiCLYuenMFNatural history of hepatitis-related hepatocellular carcinomaWorld J Gastroenterol200814111652165618350595
- SewellJLStickKMMontoAHepatocellular carcinoma after sustained virologic response in hepatitis C patients without cirrhosis on a pretreatment liver biopsyEur J Gastroenterol Hepatol200921222522919212213
- MasuzakiRYoshidaHOmataMInterferon reduces the risk of hepatocellular carcinoma in hepatitis C virus-related chronic hepatitis/liver cirrhosisOncology201078Suppl 1172320616579
- CammàCGiuntaMAndreonePCraxìAInterferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approachJ Hepatol200134459360211394661
- OkudaHHepatocellular carcinoma development in cirrhosisBest Pract Res Clin Gastroenterol200721116117317223503
- BruixJShermanMPractice Guidelines Committee American Association for the Study of Liver DiseasesManagement of hepatocellular carcinomaHepatology20054251208123616250051
- HuangCFYehMLTsaiPCBaseline gamma-glutamyl transferase levels strongly correlate with hepatocellular carcinoma development in non-cirrhotic patients with successful hepatitis C virus eradicationJ Hepatol2014611677424613362