Abstract
Most colon tumors develop via a multistep process involving a series of histological, morphological, and genetic changes that accumulate over time. This has allowed for screening and detection of early-stage precancerous polyps before they become cancerous in individuals at average risk for colorectal cancer (CRC), which may lead to substantial decreases in the incidence of CRC. Despite the known benefits of early screening, CRC remains the second leading cause of cancer-related deaths in the United States. Hence, it is important for health care providers to have an understanding of the risk factors for CRC and various stages of disease development in order to recommend appropriate screening strategies. This article provides an overview of the histological/molecular changes that characterize the development of CRC. It describes the available CRC screening methods and their advantages and limitations and highlights the stages of CRC development in which each screening method is most effective.
Keywords:
Introduction
Screening for colorectal cancer (CRC) has substantially contributed to the downward trends in CRC incidence and mortality over the last 2 decades.Citation1,Citation2 The clinical value of screening is reflected in its ability to prevent cancer morbidity, mortality, and excess treatment cost by detecting significant lesions before they become cancerous and early-stage cancer before it spreads beyond the bowel wall. The 5-year survival rates for those with early-stage, localized disease (Stages I and II) approach 90%.Citation2 The survival rate for those diagnosed with late-stage CRC, which is associated with spread to distant organs, is 13.1%. At this stage, treatment often becomes palliative and treatment-related financial burdens are the greatest.Citation1,Citation3
Despite the well-known benefits of screening and recommendations for average-risk adults aged 50 years and older to be screened, CRC is the second leading cause of cancer-related death among both men and women and contributes ~$14 billion to annual health care costs in the US, an expenditure that continues to increase.Citation4,Citation5 Screening may help control the costs of CRC treatment, as most screening strategies have become cost saving relative to increasing costs associated with chemotherapy for advanced CRC.Citation6 At present, only 65% of US adults are in compliance with current screening recommendations.Citation7 The National Colorectal Cancer Roundtable has a goal of increasing screening rates to 80% by 2018. It is estimated that achieving this goal will avert 280,000 new cases of CRC and save 200,000 lives in the US by 2030.Citation8,Citation9 Discussions of CRC screening with patients that lead to successful screenings will be of vital importance to achieving this goal.Citation7 This review provides a concise overview of the risk factors for CRC, the histological and molecular changes that characterize the development of CRC, and a discussion of screening methods.
Risk factors for CRC
Although anyone can develop CRC, several factors are associated with an increased risk for the disease. Some risk factors are modifiable, such as diet, obesity, lack of physical activity, tobacco use, and moderate-to-heavy alcohol use. Conversely, higher intakes of dietary fiber, green leafy vegetables, folate, and calcium have been reported to be protective against the development of CRC.Citation2 Although modification of these factors may result in a decreased risk for CRC, no effect of modification is sufficient to reduce the need for screening.Citation1 Other risk factors are nonmodifiable, such as a personal or familial history of colorectal polyps or CRC, hereditary conditions such as Lynch syndrome, a personal history of inflammatory bowel disease, racial and ethnic backgrounds, and the presence of type 2 diabetes.Citation1,Citation2,Citation4 Although CRC can occur in early- to mid-adulthood, particularly in those with certain inherited predispositions, most cancer occurs in persons considered as average risk, and age is the most significant risk factor in this group.Citation4 The chance of developing CRC increases markedly after age 50 years, with 90% of new cases and 94% of CRC-associated deaths occurring in those ≥50 years of age.Citation1 Screening persons of average risk who are ≥50 years of age and closely monitoring those individuals at higher risk provide the best opportunity for prevention and early detection of CRC. The high-risk designation includes individuals with personal or familial history of CRC, adenomatous polyps, inflammatory bowel disease, and hereditary CRC syndromes; high-risk individuals should consult with their health care provider to determine when to begin screening and how often to repeat the screening.Citation2
Development of CRC
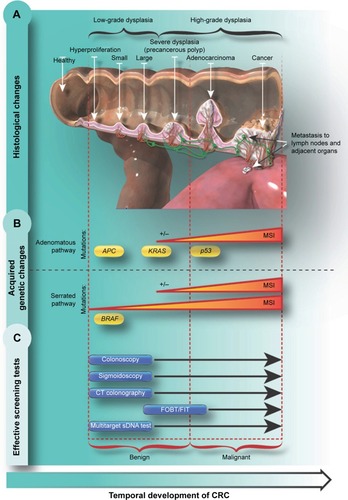
Underscoring the need for screening, CRC often grows slowly, and generally does not produce symptoms until reaching a considerable size of several centimeters, which may block the passage of feces and lead to cramping, pain, or bleeding that can present as visible bleeding with bowel movements or, rarely, dark “tarry” stools. Most colon tumors develop via a multistep process involving a series of histological, morphological, and genetic changes that accumulate over time.Citation10 The various stages of CRC progression, along with their accompanying histological, morphological, and genetic changes, are outlined later and in .
Figure 1 CRC development and screening methods.
Abbreviations: CRC, colorectal cancer; CIMP, CpG island methylator phenotype; CT, computed tomography; FOBT, fecal occult blood test; FIT, fecal immunochemical test; MSI, microsatellite instability; sDNA, stool DNA.
Polyps
CRC typically develops from focal changes within benign, precancerous polyps. These polyps are localized growths or aggregations of abnormal cells within the intestinal mucosa that protrude into the intestinal lumen.Citation1 Polyps can be sessile ( and ) or pedunculated ( and ).Citation4 With time, the dividing cells in these polyps may accumulate sufficient genetic changes by which they acquire the ability to invade the bowel wall, the hallmark of CRC, and eventually may become more altered and spread to local lymph nodes and finally to distant metastatic sites.Citation1 Fortunately, only a small percentage of polyps acquire malignant features, and even for the ones that do, the complete progression from polyps to cancer generally takes several years or even a decade.Citation11
Figure 2 Polyps.
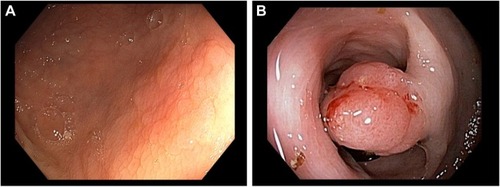
There are two main types of polyps with malignant potential, adenomas and sessile serrated polyps (SSPs), each of which are associated with different risks for developing into CRC. In general, most adenomas have a tubular histology with small, roundish, atypical glands but often develop areas of long filamentous architecture as they grow, which is described on pathology reports as villous or tubulovillous. By definition, adenomas are characterized by dysplasia (low degree of cellular and structural atypia). Tubulovillous and villous adenomas, especially those with ≥25% villous content, are typically larger in size and have a greater potential for harboring cancerous cells. In contrast, SSPs are flat and carpet like, with serrated or saw-toothed glands. SSPs include sessile serrated adenomas, traditional serrated adenomas, and mixed polyps, which have all been associated with CRC development.Citation12,Citation13
The risk for adenomas to develop into CRC increases as the size of the polyp increases.Citation12 Although only ~10% of even the most advanced adenomas (adenomas ≥1 cm in size or that have ≥25% villous component or high-grade dysplasia of any size) become cancerous, 60%–70% of CRCs develop from adenomas. The remaining 25%–35% of CRCs develop from SSPs.Citation13–Citation15
Polyps can develop along the entire length of the colon and rectum; however, polyps that develop within the proximal (right) side of the colon, which includes the cecum through the transverse colon up to and including the splenic flexure, account for 42% of all CRCs in the US.Citation16 Proximal polyps are often the SSP type and can be difficult to detect as they are often flattened or depressed and may not bleed or ulcerate.Citation12
Progression from polyp to cancer: histological and morphological changes
The histological changes that occur during the development of CRC are outlined in . As the cells within the polyp proliferate, the size of the polyp increases, and genetic mutations and epigenetic changes may begin to accumulate; this is reflected by cytologic and histologic dysplasia.Citation10,Citation17 As damage to cellular DNA increases over time, the features of high-grade dysplasia may develop, which connotes a very high degree of risk for progression to invasive carcinoma.Citation10 If not removed, these polyps may develop the ability to invade nearby tissue and grow into and beyond the wall of the colon and rectum. This localized, malignant growth may become neovascularized, thus providing easy access to the lymph and circulatory systems and thereby promoting the spread of cancerous cells to distant organs.Citation10,Citation18 Early detection and resection of precancerous polyps are critical to interrupt the adenoma-carcinoma sequence and prevent the development and spread of CRC.
Progression from polyp to cancer: genetic changes
The histological progression from polyp to cancer is the result of a series and/or accumulation of genetic and epigenetic changes. DNA mutations can be acquired or inherited. True inherited mutations associated with CRC, such as the MLH1, MSH2, PMS2, and the APC gene mutations, are uncommon and account for ~5% of CRCs. However, studying these inherited mutations, in addition to sporadically occurring APC and DNA mismatch repair mutations, has provided key insights into the stepwise genetic progression from premalignant polyps to cancer.Citation18–Citation20
There are two main genetic pathways that lead to the development of CRC ().Citation21 These generally correspond with the two types of polyps from which CRC develops: adenomas and SSPs. The chromosomal instability pathway, generally associated with traditional adenomas, is observed in 65%–70% of all sporadic cancers and is characterized by a cascade of accumulating mutations. Typically, the first mutations that develop are within the APC gene, which affects chromosome segregation during cell division. Subsequent mutations then develop in the KRAS oncogene, which has downstream effects on cell growth, differentiation, motility, and survival. Over time, these mutations can cause a loss of function of the p53 gene, which is a master regulator of transcription and apoptosis, thus impacting a wide range of cellular functions that ultimately results in carcinogenesis.Citation22
In contrast, development of SSPs tends to begin with mutations in the BRAF gene, which results in altered growth signaling and loss of apoptosis.Citation13,Citation21,Citation23,Citation24 KRAS mutations can also occur in SSPs, but they are much less frequently associated with SSPs than adenomatous polyps.Citation21,Citation23 Another common epigenetic alteration seen in serrated lesion-based CRC is aberrant gene promoter region hypermethylation. Promoter region methylation inhibits gene transcription, functionally turning affected genes “off ”. This gene deactivation impacts many genes including those regulating other growth-promoting genes.Citation23 Aberrantly methylated genes associated with CpG island methylator phenotype include, among others, the bone morphogenic protein 3 (BMP3)Citation25 and N-Myc downstream-regulated gene 4 (NDRG4).Citation25,Citation26
Another mechanism that leads to genetic diversity in CRC is microsatellite instability (MSI), which is caused by the disruption of DNA repair genes. MSI can result in uneven replication of repetitive DNA sequences in short, noncoding regions (microsatellites) and increased susceptibility to additional genetic mutations.Citation13,Citation21,Citation23,Citation27,Citation28 MSI can occur in both adenomatous and serrated polyps and is associated with germline mutations in DNA mismatch repair genes (eg, in hereditary nonpolyposis colorectal cancer) as well as sporadic mutations due to aberrant methylation of the MLH1 promoter regions (associated with CpG island methylator phenotype).Citation13,Citation23,Citation27,Citation29
Current screening options for CRC
There are several different screening tests for the detection of CRC, each of which has advantages and limitations. The individual features of each test also affect patient and physician perceptions and preferences, which in turn can affect the informed decision-making process in choosing the appropriate screening test. The most important characteristic of a screening test is its sensitivity (the true-positive rate), which is the percentage of patients with the disease who get a positive result with the test. Also important, but less so than sensitivity, is reasonably high specificity, which is the percentage of patients without disease who receive a negative result (also known as the true-negative rate). Together, sensitivity and specificity define the accuracy of a test, and they are generally traded off against each other depending on the clinical situation. When missing a lesion or disease state has the potential for severe or grave consequences, sensitivity is favored over specificity. When the chance of harm from overtreatment is of greatest concern, specificity is favored over sensitivity. In many screening applications, a more specific test may follow a more sensitive test, but a single test with both high sensitivity and high specificity is preferred.Citation30 High accuracy for detecting CRC is critical, as false-positive results would cause unnecessary anxiety and follow-up, whereas false-negative results would result in missed detection of CRC. In addition, the test should be precise, providing consistent results when repeated. Individuals who need to be screened are often asymptomatic, and achieving a high rate of cooperation from such individuals is necessary for an effective screening program; therefore, the test should be acceptable from the individual’s perspective. The test should be easy to administer and use, be easily accessed, have minimal out-of-pocket expenses, and be safe to encourage high participation in screening efforts.Citation30 Here, we provide a brief description of the common CRC screening methods, their advantages and limitations, and where in the process of CRC development they are most effective ( and ).
Table 1 CRC screening tests
Colonoscopy
Colonoscopy is the current reference method for CRC screening and is recommended every 10 years in average-risk patients aged 50 years or older.Citation31 The ability of colonoscopy to detect both cancerous and precancerous lesions via direct visualization has been demonstrated in several large cohort studies.Citation32–Citation35 The sensitivity of colonoscopy for detecting CRC is >95%, while its sensitivity for detecting advanced adenomas (≥10 mm in diameter) is 88%–98%.Citation33–Citation39 Case-control studies have shown a 53%–72% reduction in the incidence of CRC and a 31% reduction in CRC-related mortality with colonoscopy.Citation40–Citation42 Reductions in CRC incidence and mortality with colonoscopy have not been demonstrated in randomized controlled trials, nor are there trials directly comparing outcomes of colonoscopy with other screening tests. The recommended 10-year interval after a normal colonoscopy is largely based on case-control studies.Citation42 One of the greatest advantages of colonoscopy is the ability to remove precancerous and small cancerous lesions at the time of detection. It can also assess both the proximal and distal portions of the colon. Limitations associated with colonoscopy include the invasiveness of the procedure, the required bowel preparation, the use of sedation or anesthesia, time off of work or other activities, and assistance to get home postprocedure.Citation43 Bowel preparation is often unpleasant and time-consuming, necessitating a temporary change in medications and diet, and entails use of a cleansing agent.Citation44 There is a risk of bowel perforation during colonoscopy and a risk of postcolonoscopy bleeding, especially in postpolypectomy patients.Citation45 These limitations contribute to low compliance with colonoscopy screening.Citation46 Additionally, there is a high reliance on the technical expertise of endoscopic examiners to visualize and remove lesions, especially those in the proximal colon, which can be more difficult to detect.Citation11 Flat or sessile polyps may be especially difficult to detect and may require special techniques.Citation47 Another limitation is the potential for “interval cancers”, which are cases of CRC that occur in the long interval between normally scheduled screening colonoscopies and have been reported to account for 6%–9% of all CRC cases.Citation48–Citation50
Sigmoidoscopy
Flexible sigmoidoscopy, which is less frequently used in the US for screening, is similar to colonoscopy except that only the distal half of the colon is examined, sedation is not necessary, and the bowel preparation consists of an enema on the day of the examination.Citation43 It has a >95% sensitivity for the detection of CRC in the portion of the colon that is examined and a 70% sensitivity for advanced adenomas (≥10 mm in diameter). If lesions are identified in the distal colon, a follow-up colonoscopy is required, at which time proximal lesions may be found.Citation34,Citation51 Case-control studies of sigmoidoscopy have demonstrated a 60% reduction in mortality from CRCs of the distal colon, but there is little effect on reducing proximal CRC morbidity and mortality due to lack of screening in this portion of the colon.Citation33,Citation34,Citation52 When used, current guidelines suggest that sigmoidoscopy should be paired with high-sensitivity fecal occult blood testing (FOBT; described later) and should be repeated every 5 years in asymptomatic individuals with no previous history of colon polyps.Citation31
Similar to colonoscopy, the advantages of sigmoidoscopy include its ability to identify both cancerous and precancerous lesions (but only in the distal colon) and its ability to remove lesions at the time of detection. Sigmoidoscopy also shares many of the limitations of colonoscopy, including the need for bowel preparation, access to health care facilities, long waits for appointments, and safety concerns. Because the procedure is limited to the distal portion of the colon and sedation is not typically offered, sigmoidoscopy may cause considerable discomfort compared with colonoscopy under anesthesia, while leaving a large portion of the colon unscreened.Citation43,Citation53
Computed tomography colonography
Computed tomography (CT) colonography is a structural radiologic examination of the colon sometimes referred to as “virtual colonoscopy”. It uses CT and special software to create a three-dimensional image of the colon to identify colonic lesions, which may be found on subsequent optical colonoscopy to be cancer and precancerous polyps.Citation11 Although the sensitivity of CT colonography for detection of CRC and advanced adenomas in daily clinical practice is somewhat uncertain, clinical studies suggest that rates are >90% for detection of CRC, 90% for detection of polyps ≥10 mm, and 78% for detection of polyps ≥6 mm.Citation54 In the US, the reception of CT colonography as a recommended CRC screening tool is mixed,Citation43,Citation55,Citation56 and its use is limited mainly to patients who are not good candidates for colonoscopy due to other comorbidities or structural issues that preclude adequate optical examination of the colon.Citation43,Citation55–Citation57 However, two organizational screening studies conducted in Europe demonstrate that participation rates for CT colonography are superior to those of colonoscopy.Citation58,Citation59 Notably, there is no empirical evidence to demonstrate that CT colonography can reduce CRC incidence or related mortality. Although the screening interval for negative CT colonography results is uncertain, studies suggest that for average-risk, asymptomatic patients, the test should be repeated every 5 years.Citation43 The Centers for Medicare and Medicaid Services currently does not pay for CT colonography as a screening test.Citation60
Like colonoscopy, CT colonography carries the advantage of allowing visualization of the entire colon but is only semi-invasive. To facilitate better visualization, the colon is inflated with air; although this is uncomfortable for many patients, it does not require sedation.Citation43 Limitations of CT colonography include unpleasant bowel preparation, similar to that used with colonoscopy, and the use of ionizing radiation, which imparts additional safety concerns and costs and requires access to specific health care facilities. This screening method is highly dependent on the technical expertise of the radiologist interpreting the results. CT colonography requires a follow-up colonoscopy if lesions are detected.Citation61 Due to similar limitations, the use of double-contrast barium enema as an alternative screening method for radiologic evaluation of the colon has been largely discontinued.Citation62
FOBT and fecal immunochemical tests
FOBT and fecal immunochemical tests (FITs) are designed to detect hemoglobin as a marker of occult blood in the stool.Citation43 FOBT detects the peroxidase activity of heme and, as such, is not specific for human blood, whereas FIT uses human globin-specific antibodies to detect hemoglobin, so it is more specific for human blood than FOBT.Citation62 The performance of FIT and FOBT varies due to the multiplicity of commercially available tests, different number of samples used at each testing event, and whether the studies were observational in nature or used colonoscopy to confirm the findings. Many FOBT/FIT studies are observational, which may overestimate sensitivity.Citation63 In studies that used colonoscopy as a reference standard, the sensitivity of FIT for detecting CRC and precancerous lesions, 71%–75% and 27%–29%, respectively, was higher than FOBT, 33%–75% and 11%–25%, respectively.Citation56,Citation64–Citation66 The specificity of FOBT (98%–99%) was greater than FIT (94%–95%).Citation56,Citation64–Citation66 FOBT has been shown to reduce CRC mortality by 15%–33%.Citation7,Citation69–Citation71 When used as the sole screening method, FOBT and FIT should be repeated annually.Citation43
FOBT and FIT can be performed at home, are noninvasive, and do not require extensive bowel preparation. Limitations including sporadic bleeding from lesions can lead to false-negative test results, and lesions in early development bleed less frequently, if at all, so there is a greater tendency for these tests to detect more advanced lesions and miss precancerous lesions.Citation43,Citation53 In addition, dietary intake of vitamin C can lead to false-negative results as it can inhibit the peroxidase activity of FOBT, and dietary hemoglobin from the ingestion of red meat can generate false-positive results on FOBT.Citation11,Citation43 Although FIT kits only require that the test be performed once, some FOBT tests require that the test be performed in duplicate or triplicate.Citation70 These limitations, in addition to increased sensitivity of FIT, have resulted in more common use of FIT over FOBT.Citation72 Finally, adherence to annually recommended FOBT/FIT screening is low in the US where most screening is opportunistic.Citation62,Citation73–Citation75 In a study of more than a million participants of screening age, adherence to an annual FOBT was found to be 42.1% for men and 42.9% for women.Citation73 Likewise, the rate of follow-up colonoscopy screening for those who receive positive test results is low, thus negating the value of FOBT/FIT screening in those patients.Citation62 However, organizational screening using FIT/FOBT shows an improvement in the annual adherence rate and uptake.Citation76,Citation77
Multitarget stool DNA testing
Multitarget stool DNA (mt-sDNA) testing is a noninvasive screening method designed to detect abnormal DNA and occult blood in stool samples.Citation43,Citation53 At present, there is only one commercially available mt-sDNA test (Cologuard®; Exact Sciences Corporation, Madison, WI, USA). The test is designed to detect ten DNA-based markers and one human hemoglobin biomarker in stool. Results of the assays used to detect DNA and hemoglobin biomarkers are then combined in a diagnostic algorithm that yields the single composite negative or positive result. If a composite level of abnormal DNA and/or blood from a potential cancerous or precancerous lesion is detected, the test will yield a positive result and the patient should be referred for a diagnostic colonoscopy and removal of lesions if necessary. Alternatively, a negative test result is assigned if the normal internal control DNA is detected and the combined levels of the test biomarkers are below the threshold for detection. In this case, patients should continue with recommended, routine screening.Citation65
The clinical sensitivity of the mt-sDNA test was demonstrated in a blinded, cross-sectional, screening study conducted at 90 sites. The study included >10,000 asymptomatic, average-risk participants aged ≥50 years and established the performance of this test as compared with FIT and colonoscopy.Citation65 In this study, the mt-sDNA test had a sensitivity of 92.3%, detecting 60 of 65 cancers compared to the FIT test sensitivity of 73.8% that detected 48 of 65 cancers (OC FIT-CHEK; Polymedco, Cortlandt Manor, NY, USA). mt-sDNA test demonstrated superior sensitivity for detecting advanced precancerous lesions, detecting 42.4% of the subjects with advanced adenomas, 69.2% of the subjects with precancerous lesions with high-grade dysplasia, and 42.4% of the subjects with SSPs. By contrast, FIT identified 23.8%, 46.2%, and 5.1%, respectively.Citation65 The specificity (true-negative rate) of mt-sDNA among subjects with findings other than CRC or advanced precancerous lesions was 86.6%, compared to 94.9% for FIT. Among subjects with totally negative results on colonoscopy, mt-sDNA had a specificity of 89.8%, compared to 96.4% for FIT. In patients with a negative test result, the negative predictive value of the mt-sDNA test was extremely high (99.94%); a negative test result suggests that the likelihood of having a missed CRC was only 0.06% and the chance of having an advanced adenoma was 5.2%.Citation63 A second study assessed the accuracy of mt-sDNA compared to FIT, and the sensitivity and specificity results were similar.Citation78
The mt-sDNA test requires a stool sample that is collected by the patient at home and does not require any bowel preparation or changes to medications and diet.Citation65 A screening interval of 3 years has been recommended by American Cancer Society guidelinesCitation79 and has been subsequently supported via data modeling, and the test is reimbursed by Medicare every 3 years.Citation80–Citation82 The cost of the test is US $649, is covered by many major insurers, and includes all shipping costs and a systematic compliance program that offers patient reminders for screening, handles patient questions, manages patient billing and reimbursement, and provides compliance and tracking records for physicians, all of which may help ease the burden on primary care offices.Citation80 Limitations include the relative newness of the test with limited evidence and while it has a high sensitivity that is offset with somewhat less specificity.Citation65 However, when evaluating screening tests, it is important to remember that sensitivity is the most important characteristic.
Conclusion
Precursor lesions of different types typically precede the development of CRC by many years, providing an ample window for early detection via various screening methods. Screening for CRC is a critical part of primary care practice. Primary care practitioners are well positioned to initiate a dialog with patients to discuss the benefits of CRC screening and to provide a comprehensive view of current screening options including the advantages and limitations of each. Offering a variety of screening options, particularly in the context of each patient’s wishes and limitations, may increase compliance with screening.Citation46 Increased compliance with CRC screening recommendations has the potential to improve patients’ health and well-being and to ultimately reduce CRC morbidity and mortality rates. Regardless of all other factors, the best CRC screening test is the one that is completed in a timely manner.
Acknowledgments
The author would like to acknowledge Exact Sciences for critical review of the manuscript.
Disclosure
Dr Simon is a trial investigator and a speaker for Exact Sciences, the manufacturer of Cologuard®, and has received honoraria from Exact Sciences for her speaking events. The author reports no other conflicts of interest in this work.
References
- American Cancer SocietyColorectal Cancer Facts & Figures 2011–2013Atlanta, GAAmerican Cancer Society2011
- American Cancer SocietyCancer Facts & Figures 2015Atlanta, GAAmerican Cancer Society2015
- National Cancer Institute [webpage on the Internet]Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Colon and Rectum Cancer Available from: http://seer.cancer.gov/statfacts/html/colorect.htmlAccessed March 3, 2016
- ACS [webpage on the Internet]What are the survival rates for colorectal cancer by stage?2015 Available from: http://www.cancer.org/cancer/colonandrectumcancer/detailedguide/colorectal-cancer-survival-rates; http://tinyurl.com/grroztxAccessed March 3, 2016
- MariottoABYabroffKRShaoYFeuerEJBrownMLProjections of the cost of cancer care in the United States: 2010–2020J Natl Cancer Inst2011103211712821228314
- Lansdorp-VogelaarIvan BallegooijenMZauberAGHabbemaJDKuipersEJEffect of rising chemotherapy costs on the cost savings of colorectal cancer screeningJ Natl Cancer Inst2009101301412142219779203
- Centers for Disease Control and PreventionVital signs: colorectal cancer screening test use-United States, 2012MMWR Morb Mortal Wkly Rep2013624488188824196665
- National Colorectal Cancer Roundtable [webpage on the Internet]. 80% by 2018 Available from: http://nccrt.org/tools/80-percent-by-2018/80-by-2018-press-kit/Accessed March 3, 2016
- MeesterRGDoubeniCAZauberAGPublic health impact of achieving 80% colorectal cancer screening rates in the United States by 2018Cancer2015121132281228525763558
- FrankSADynamics of Cancer: Incidence, Inheritance, and EvolutionPrinceton (NJ)Princeton University Press2007 Chapter 3, Multistage Progression. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1562/Accessed June 15, 2016
- StracciFZorziMGrazziniGColorectal cancer screening: tests, strategies, and perspectivesFront Public Health2014221025386553
- ConteducaVSansonnoDRussiSDammaccoFPrecancerous colorectal lesions (review)Int J Oncol201343497398423900573
- YamaneLScapulatempo-NetoCReisRMGuimarãesDPSerrated pathway in colorectal carcinogenesisWorld J Gastroenterol201420102634264024627599
- EastJEViethMRexDKSerrated lesions in colorectal cancer screening: detection, resection, pathology and surveillanceGut2015646991100025748647
- SnoverDCUpdate on the serrated pathway to colorectal carcinomaHum Pathol201142111020869746
- SiegelRLWardEMJemalATrends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992–2008Cancer Epidemiol Biomarkers Prev201221341141622219318
- LochheadPChanATGiovannucciEProgress and opportunities in molecular pathological epidemiology of colorectal premalignant lesionsAm J Gastroenterol201410981205121424935274
- NagyJAChangSHDvorakAMDvorakHFWhy are tumour blood vessels abnormal and why is it important to know?Br J Cancer2009100686586919240721
- HeinenCDSchmutteCFishelRDNA repair and tumorigenesis: lessons from hereditary cancer syndromesCancer Biol Ther20021547748512496472
- KinzlerKWVogelsteinBLessons from hereditary colorectal cancerCell19968721591708861899
- BatemanACPathology of serrated colorectal lesionsJ Clin Pathol2014671086587424561317
- PinoMSChungDCThe chromosomal instability pathway in colon cancerGastroenterology201013862059207220420946
- KangGHFour molecular subtypes of colorectal cancer and their precursor lesionsArch Pathol Lab Med2011135669870321631262
- KambaraTSimmsLAWhitehallVLBRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectumGut20045381137114415247181
- LohKChiaJAGrecoSBone morphogenic protein 3 inactivation is an early and frequent event in colorectal cancer developmentGenes Chromosomes Cancer200847644946018311777
- MelotteVLentjesMHvan den BoschSMN-Myc downstream-regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancerJ Natl Cancer Inst20091011391692719535783
- BolandCRGoelAMicrosatellite instability in colorectal cancerGastroenterology201013862073.e32087.e320420947
- O’BrienMJYangSMackCComparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end pointsAm J Surg Pathol200630121491150117122504
- KoinumaKShitohKMiyakuraYMutations of BRAF are associated with extensive hMLH1 promoter methylation in sporadic colorectal carcinomasInt J Cancer2004108223724214639609
- LingBSMoskowitzMAWachsDPearsonBSchroyPCAttitudes toward colorectal cancer screening testsJ Gen Intern Med2001161282283011903761
- Agency for Healthcare Research and QualityClinical Summaries of Recommendations for Adults: Colorectal Cancer. Guide to Clinical Preventive ServicesRockville, MDAgency for Healthcare Research and Quality2014
- RegulaJRupinskiMKraszewskaEColonoscopy in colorectal-cancer screening for detection of advanced neoplasiaN Engl J Med2006355181863187217079760
- ImperialeTFWagnerDRLinCYLarkinGNRoggeJDRansohoffDFRisk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findingsN Engl J Med2000343316917410900275
- LiebermanDAWeissDGBondJHAhnenDJGarewalHChejfecGUse of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380N Engl J Med2000343316216810900274
- SchoenfeldPCashBFloodAColonoscopic screening of average-risk women for colorectal neoplasiaN Engl J Med2005352202061206815901859
- CottonPBDurkalskiVLPineauBCComputed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasiaJAMA2004291141713171915082698
- LiebermanDAHolubJEisenGKraemerDMorrisCDPrevalence of polyps greater than 9 mm in a consortium of diverse clinical practice settings in the United StatesClin Gastroenterol Hepatol20053879880516234009
- PickhardtPJChoiJRHwangIComputed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adultsN Engl J Med2003349232191220014657426
- RockeyDCPaulsonENiedzwieckiDAnalysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparisonLancet2005365945630531115664225
- BaxterNNGoldwasserMAPaszatLFSaskinRUrbachDRRabeneckLAssociation of colonoscopy and death from colorectal cancerAnn Intern Med200915011819075198
- MüllerADSonnenbergAPrevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veteransAnn Intern Med1995123129049107486484
- SinghHTurnerDXueLTargownikLEBernsteinCNRisk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopiesJAMA2006295202366237316720822
- LevinBLiebermanDAMcFarlandBScreening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of RadiologyGastroenterology200813451570159518384785
- WexnerSDBeckDEBaronTHA consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES)Gastrointest Endosc200663789490916733101
- CooperGSKouTDRexDKComplications following colonoscopy with anesthesia assistance: a population-based analysisJAMA Intern Med2013173755155623478904
- InadomiJMVijanSJanzNKAdherence to colorectal cancer screening: a randomized clinical trial of competing strategiesArch Intern Med2012172757558222493463
- SoetiknoRMKaltenbachTRouseRVPrevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adultsJAMA200829991027103518319413
- CorleyDAJensenCDMarksARAdenoma detection rate and risk of colorectal cancer and deathN Engl J Med2014370261298130624693890
- PatelSGAhnenDJPrevention of interval colorectal cancers: what every clinician needs to knowClin Gastroenterol Hepatol201412171523639602
- SamadderNJCurtinKTuohyTMCharacteristics of missed or interval colorectal cancer and patient survival: a population-based studyGastroenterology2014146495096024417818
- SelbyJVFriedmanGDQuesenberryCPJrWeissNSA case-control study of screening sigmoidoscopy and mortality from colorectal cancerN Engl J Med1992326106536571736103
- HoffGGrotmolTSkovlundEBretthauerMNorwegian Colorectal Cancer Prevention Study GroupRisk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trialBMJ2009338b184619483252
- BinefaGRodríguez-MorantaFTeuleAMedina-HayasMColorectal cancer: from prevention to personalized medicineWorld J Gastroenterol201420226786680824944469
- JohnsonCDChenMHToledanoAYAccuracy of CT colonography for detection of large adenomas and cancersN Engl J Med2008359121207121718799557
- U.S. Preventive Services Task ForceScreening for colorectal cancer: U.S. Preventive Services Task Force recommendation statementAnn Intern Med2008149962763718838716
- WhitlockEPLinJSLilesEBeilTLFuRScreening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task ForceAnn Intern Med2008149963865818838718
- SaliLMascalchiMFalchiniMSAVE Study InvestigatorsReduced and full-preparation CT colonography, fecal immunochemical test, and colonoscopy for population screening of colorectal cancer: a randomized trialJ Natl Cancer Inst20161082
- StoopEMde HaanMCde WijkersloothTRParticipation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trialLancet Oncol2012131556422088831
- ASGE Technology CommitteeFarrayeFAAdlerDGUpdate on CT colonographyGastrointest Endosc2009693 Pt 139339819231482
- Centers for Medicare and Medicaid ServicesDecision Memo for Screening Computed Tomography Colonography (CTC) for Colorectal Cancer (CAG-00396N)Baltimore, MDCenters for Medicare and Medicaid Services2009
- de HaanMCPickhardtPJStokerJCT colonography: accuracy, acceptance, safety and position in organised population screeningGut201564234235025468258
- LiebermanDAClinical practice. Screening for colorectal cancerN Engl J Med2009361121179118719759380
- Canadian Agency for Drugs and Technologies in HealthFecal immunochemical tests for colorectal cancer screening: a systematic reviewCADTH Technol Overv201013e011722977407
- de WijkersloothTRStoopEMBossuytPMImmunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasiaAm J Gastroenterol2012107101570157822850431
- ImperialeTFRansohoffDFItzkowitzSHMultitarget stool DNA testing for colorectal-cancer screeningN Engl J Med2014370141287129724645800
- LeeJKLilesEGBentSLevinTRCorleyDAAccuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysisAnn Intern Med2014160317124658694
- ImperialeTFRansohoffDFItzkowitzSHTurnbullBARossMEColorectal Cancer Study GroupFecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk populationN Engl J Med2004351262704271415616205
- AhlquistDASargentDJLoprinziCLStool DNA and occult blood testing for screen detection of colorectal neoplasiaAnn Intern Med20081497441450W8118838724
- KronborgOFengerCOlsenJJørgensenODSøndergaardORandomised study of screening for colorectal cancer with faecal-occult-blood testLancet19963489040146714718942774
- MandelJSBondJHChurchTRReducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control StudyN Engl J Med199332819136513718474513
- HardcastleJDChamberlainJORobinsonMHRandomised controlled trial of faecal-occult-blood screening for colorectal cancerLancet19963489040147214778942775
- YoungGPSymondsELAllisonJEAdvances in fecal occult blood tests: the FIT revolutionDig Dis Sci201560360962225492500
- GelladZFStechuchakKMFisherDALongitudinal adherence to fecal occult blood testing impacts colorectal cancer screening qualityAm J Gastroenterol201110661125113421304501
- LiangPSWheatCLAbhatAAdherence to competing strategies for colorectal cancer screening over 3 yearsAm J Gastroenterol2016111110511426526080
- JensenCDCorleyDAQuinnVPFecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort studyAnn Intern Med2016164745646326811150
- SteeleRJMcDonaldPJDigbyJClinical outcomes using a faecal immunochemical test for haemoglobin as a first-line test in a national programme constrained by colonoscopy capacityUnited European Gastroenterol J201313198205
- KapidzicAGrobbeeEJHolLAttendance and yield over three rounds of population-based fecal immunochemical test screeningAm J Gastroenterol201410981257126424980879
- RedwoodDGAsayEDBlakeIDStool DNA testing for screening detection of colorectal Neoplasia in Alaska native peopleMayo Clin Proc2016911617026520415
- American Cancer SocietyColorectal Cancer Prevention and Early DetectionAtlanta, GAAmerican Cancer Society2016
- BergerBMSchroyPC3rdRosenbergJLColorectal cancer screening using stool DNA analysis in clinical practice: early clinical experience with respect to patient acceptance and colonoscopic follow-up of abnormal testsClin Colorectal Cancer20065533834316512992
- BergerBMSchroyPC3rdDinhTScreening for colorectal cancer using a multitarget stool DNA test: modeling the impact of the inter-test interval on clinical effectivenessClin Colorectal Cancer Epub2015121810.1016/j.clcc.2015.12.003
- BergerBMPartonMALevinBUSPSTF CRC screening guidelines: an extended look at multi-year interval testingAm J Manag Care2016222e77e8126881323
