Abstract
Fine-needle aspiration biopsy of the abdominal fat pad is considered to be a minimally invasive procedure for diagnosing systemic amyloidosis. However, this procedure is sometimes difficult and can be dangerous for elderly patients whose abdominal fat layer is thin because of malnutrition. In such cases, alternative diagnostic methods are required. We report three elderly patients with heart failure complicated by malnutrition. In all cases, electrocardiogram showed low voltage in the limb leads and a pseudoinfarct pattern in the chest leads, and echocardiography showed left ventricular wall thickening with granular sparkling appearance. These patients were suspected of having amyloid cardiomyopathy but could not undergo myocardial biopsies because of their poor conditions. After failed attempts at biopsy of the abdominal fat pad or the other organs, subcutaneous fat tissue biopsy over the hip led to the diagnosis of systemic amyloidosis with cardiomyopathy. The resultant diagnosis guided us to choose the appropriate treatment for the patients. This article illustrates that subcutaneous fat tissue biopsy of the hip could be a useful procedure for diagnosing systemic amyloidosis in elderly patients, particularly when a fat tissue biopsy of the abdomen is associated with a high risk of complications because of malnutrition.
Introduction
The diagnosis of systemic amyloidosis (SA) requires tissue biopsies from affected organs, owing to a lack of specific disease markers. Fine-needle aspiration biopsy (FNAB) of the abdominal fat pad (AFP) is reported to be a minimally invasive, highly reliable screening method for the diagnosis of SA.Citation1–Citation10 However, this procedure has not been standardized and requires skill to ensure precise sampling.Citation4 Moreover, this procedure may be less successful than previously reported, because of variation in practice setting, experience level, or diagnostic technique.Citation11 Additionally, abdominal wall centesis can be dangerous in elderly patients; these patients may present with a thin AFP owing to malnutrition or may have difficulties remaining at rest because of cognitive impairment. Although new devices and techniques have been developed to increase the certainty of revealing amyloid deposits,Citation12,Citation13 adipocytes are often difficult to aspirate by FNAB-AFP in malnourished, elderly patients, leading to an inability to make a diagnosis. In this study, we present three cases of SA that were diagnosed by subcutaneous fat tissue biopsy over the hip; all of these patients were ineligible for deep organ biopsy because of their poor condition and because they had failed to be diagnosed by conventional FNAB of the AFP. Written informed consent was obtained from the patients or the next of kin for publication of this report and any accompanying images.
Case report 1
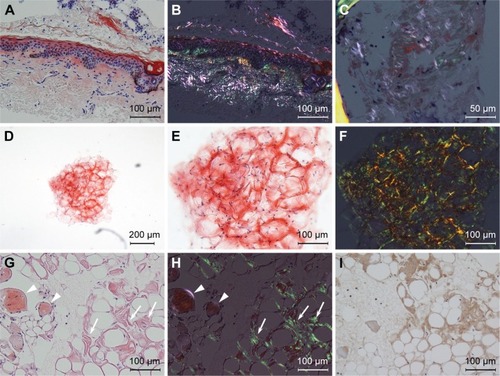
Case 1, a 76-year-old female with decompensated chronic heart failure (CHF), was admitted to our hospital in October 2011. Her CHF did not respond to medical therapy and her general condition continued to deteriorate. An electrocardiogram showed low voltage in the limb leads and low R-wave progression, compatible with pseudoinfarct pattern, in the chest leads V1–V3 (). Echocardiography showed increased left ventricular wall thickness () with granular sparkling appearance (). Blood chemistry examination revealed M proteins derived from immunoglobulin (Ig) G lambda light chain, but no abnormalities in IgG levels (). Based on these findings, amyloid cardiomyopathy due to amyloid light chain amyloidosis was strongly suspected as a cause of her CHF. To obtain histological evidence of amyloid light chain amyloidosis, bone marrow and gastric mucosa were biopsied, but both specimens failed to show amyloid deposition. However, amyloid deposits were revealed in the subcutaneous fat tissue () that was biopsied incidentally with the bone marrow tissue from her posterior iliac crest (). The percentage of plasma cells in the bone marrow (14.8% of all nucleated cells) was slightly increased but did not fulfill the criteria for symptomatic multiple myeloma. These findings allowed us to make a diagnosis of SA with cardiomyopathy, but we could not determine the type of SA because there was no biased staining of lambda light chain in the fat tissue. The patient was treated with oral melphalan and dexamethasone to ameliorate M proteinemia without apparent effects, and she died of CHF 2 months after the diagnosis.
Table 1 Laboratory data of the cases at diagnosis
Figure 1 Electrocardiogram of the three presented cases.
Notes: In all cases, electrocardiogram showed low voltage in the limb leads. (A) Case 1: low R-wave progression was seen in the chest leads V1 to V3. (B) Case 2: the lack of R-wave was seen in the chest leads V1 to V5. (C) Case 3: low R-wave progression was seen in the chest leads V1 to V4, similar to Case 1. These abnormal R-waves were considered a pseudoinfarct pattern.
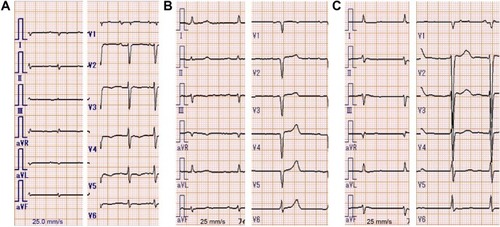
Figure 2 Echocardiography of the three presented cases.
Notes: Parasternal long-axis views (A, C, and E) and apical four-chamber views (B, D, and F) are presented. (A and B) Case 1: granular sparkling appearance was seen in the interventricular septum (IVS), which was 11.5 mm in thickness. (C and D) Case 2: granular sparkling appearance was seen in the IVS and the lateral wall. The IVS of Case 2 was less thickened (9.8 mm in thickness), but the left ventricular wall exhibited diffuse hypertrophy with diastolic dysfunction (E/e′ 15.55) without valvular dysfunction. (E and F) Case 3: granular sparkling appearance was shown in the IVS and lateral wall, and the IVS was 13.0 mm in thickness.
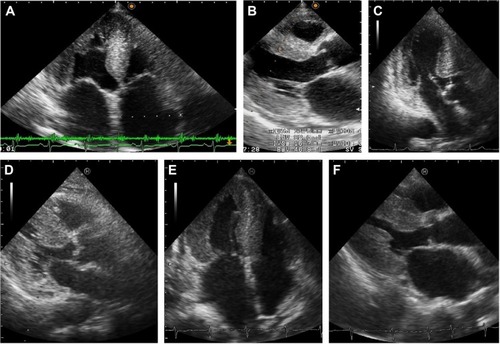
Figure 3 Light micrograph of the subcutaneous fat tissue.
Case report 2
Case 2, a 91-year-old female with a 6-month history of progressive chronic kidney disease and CHF, presented with anorexia and malnutrition in January 2015. Her serum was positive for IgG-lambda M proteins, with a decrease in the free light chain kappa/lambda ratio (). Blood tests, electrocardiogram, and echocardiography showed characteristics of amyloid cardiomyopathy, similar to those seen in Case 1 (; , , and ). The patient also had comorbidities such as anemia, proteinuria associated with chronic kidney disease, sclerotic skin lesions, dysautonomia, and progressive muscle weakness. She was suspected to have SA and underwent the FNAB-AFP procedure, but no adipocytes could be aspirated because of malnutrition. Based on the experience gained with Case 1, we suspected that subcutaneous fat tissue aspiration from the hip might be useful for diagnosing SA. A specimen aspirated from the left posterior iliac crest contained adipocytes and amyloid deposits were evident in the subcutaneous fat tissue (). However, the presence of ALs could not be confirmed because the specimen was too small to analyze with immunohistochemical staining. The patient was eventually diagnosed with SA complicated by cardiomyopathy. Bone marrow biopsy was not performed because no specific treatment was indicated even if she had been found to have multiple myeloma. Her anorexia improved with supportive care, including treatment for CHF.
Case report 3
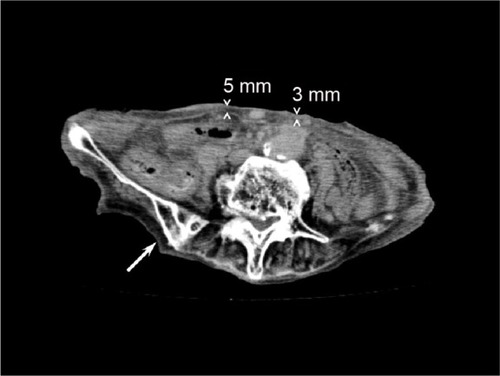
Case 3, a 93-year-old female with a 10-year history of hypertension, presented with left hemiparalysis and was admitted to our hospital in September 2015. Diffusion-weighted magnetic resonance imaging revealed a widespread high-intensity lesion in the right middle cerebral artery area and electrocardiogram was compatible with paroxysmal atrial fibrillation. The patient was diagnosed with cardiogenic cerebral embolism and was treated with edaravone and heparin for 2 weeks. However, she gradually developed signs of heart failure, including whole body edema, pulmonary edema, massive pleural effusion, and anorexia. Based on echocardiogram findings similar to those seen in Cases 1 and 2, the patient was suspected to have SA (, , , and ). We could not perform FNAB-AFP because her abdominal wall was too thin to apply centesis safely () and she could not remain still during the FNAB procedure because of stroke-induced cognitive impairment. Thus, we performed FNAB of subcutaneous fat tissue from the hip. Amyloid deposits in the fat tissue were revealed by Congo red staining with polarizing light exposure, as well as with transthyretin immunostaining (). The patient was diagnosed with senile SA and amyloid cardiomyopathy. The irreversible condition of her disease led us to provide supportive care instead of intensive treatment. The patient died of multiple organ failure, as a result of circulatory insufficiency, 7 days after the diagnosis.
Figure 4 The abdominal computerized tomography of Case 3.
Abbreviations: AFP, abdominal fat pad; FNAB, fine-needle aspiration biopsy.
Discussion
This report illustrates three cases of SA diagnosed by subcutaneous fat tissue biopsy of the hip. Two of these were diagnosed as SA by an FNAB from the posterior iliac crest. Although a previous study has suggested that a diagnosis of SA may not change the recommended treatment in many cases, a definitive diagnosis can be helpful in ruling out other underlying conditions as the cause of patients’ symptoms.Citation6 In Cases 2 and 3, the diagnosis of SA offered a poor prognosis, allowing us to select supportive care rather than intensive treatment. Making a definitive diagnosis of SA in Case 2 also helped us rule out other causes of CHF and choose appropriate care for the patient, which significantly relieved the patient’s symptoms. The prognosis of Case 3 was considered to be more miserable because her heart failure was developed subsequent to many irreversible impairments such as stroke-induced dementia, hemiparalysis, aphasia, and severe dysphagia, which led to malnutrition. Owing to advanced dementia and malnutrition, her prognosis was estimated to be <6 months according to a guideline.Citation14 In addition, the diagnosis of SA indicated that her heart failure needed maintenance therapies, but these impairments were thought to disturb continuous oral medication. Therefore, after diagnosis of SA, the patient was thought to be in the end-of-life and was provided palliative care for SA.Citation15
According to current guidelines or experts’ reviews, a diagnosis of SA requires the detection of amyloid deposits, which are then confirmed by Congo red staining with classical apple green birefringence under polarized light.Citation16–Citation20 FNAB-AFP is considered to be an excellent screening procedure for diagnosing SA,Citation21 with a specificity of 92%–100%Citation1–Citation8 and is introduced as the simplest and least invasive procedure in many tissue biopsies.Citation20 This diagnostic procedure has been established by dermatologists, surgeons, and pathologists.Citation22 The choice of the anterior abdominal skin for the aspiration biopsy was based on a systematic study of the occurrence of amyloid A in the skin of different parts of the body.Citation23 However, this study did not examine about the detection rate of amyloid deposits in the subcutaneous tissue of the hips.Citation20 Most primary care physicians are not skilled in this procedure because the prevalence of SA is relatively low and physicians have few chances to carry out FNAB. On the other hand, centesis of the posterior iliac crest is often performed by physicians and hematologists to aspirate bone marrow and is recognized as a safe procedure that does not risk damaging deep organs. In this respect, FNAB of the subcutaneous tissue from the posterior iliac crest has some advantages over FNAB-AFP, especially in patients for whom FNAB-AFP is associated with considerable risks. To our knowledge, however, there has been no study that evaluated the usefulness of the subcutaneous fat tissue biopsy of the hip in diagnosing SA.
The sensitivity of detecting amyloid deposits in subcutaneous fat tissue through the AFP biopsy varies from one type of amyloidosis to another. It is higher in patients with amyloid light chain and amyloid A amyloidosis than in patients with senile SA. This is probably true for the subcutaneous fat tissue biopsy of the hip, but we do not have exact data regarding different types of amyloidosis. Further studies are required to clarify this issue.
In our three cases, SA could only be diagnosed by subcutaneous fat tissue of the hip because the poor conditions of the patients did not allow biopsies from other organs. These patients had no complication associated with the diagnostic procedures. Moreover, the resultant diagnosis of SA guided us to choose appropriate treatments for the patients.
Conclusion
Subcutaneous fat tissue biopsy of the hip was useful for diagnosing SA in elderly patients for whom FNAB-AFP failed to obtain sufficient material and biopsy of other organs was associated with a high risk of complications. These promising results warrant further clinical studies to clarify the usefulness of FNAB of the hip in the diagnosis of SA.
Acknowledgments
The authors wish to thank Hiroe Niwa and Kazumi Mukai, the cytotechnologists at the Department of Pathology Laboratory of Nanto Municipal Hospital, for their special works and efforts.
Disclosure
The authors report no conflicts of interest in this work.
References
- DustonMASkinnerMShirahamaTCohenASDiagnosis of amyloi-dosis by abdominal fat aspiration. Analysis of four years’ experienceAm J Med19878234124142435149
- GertzMALiCYShirahamaTKyleRAUtility of subcutaneous fat aspiration for the diagnosis of systemic amyloidosis (immunoglobulin light chain)Arch Intern Med198814849299332451487
- DustonMASkinnerMMeenanRFCohenASSensitivity, specificity, and predictive value of abdominal fat aspiration for the diagnosis of amyloidosisArthritis Rheum198932182852912466
- MasouyeIDiagnostic screening of systemic amyloidosis by abdominal fat aspiration: an analysis of 100 casesAm J Dermatopathol199719141459056653
- GuyCDJonesCKAbdominal fat pad aspiration biopsy for tissue confirmation of systemic amyloidosis: specificity, positive predictive value, and diagnostic pitfallsDiagn Cytopathol200124318118511241901
- Ansari-LariMAAliSZFine-needle aspiration of abdominal fat pad for amyloid detection: a clinically useful test?Diagn Cytopathol200430317818114986298
- van GamerenIIHazenbergBPBijzetJvan RijswijkMHDiagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practiceArthritis Rheum20065462015202116732553
- DhingraSKrishnaniNKumariNPandeyREvaluation of abdominal fat pad aspiration cytology and grading for detection in systemic amy-loidosisActa Cytol200751686086418077977
- BogovBLubomirovaMKiperovaBBiopsy of subcutaneus fatty tissue for diagnosis of systemic amyloidosisHippokratia200812423623919158968
- MiyazakiKKawaiSSuzukiKAbdominal subcutaneous fat pad aspiration and bone marrow examination for the diagnosis of AL amy-loidosis: the reliability of immunohistochemistryInt J Hematol2015102328929526115876
- HalloushRALavrovskayaEModyDRLagerDTruongLDiagnosis and typing of systemic amyloidosis: the role of abdominal fat pad fine needle aspiration biopsyCytojournal200962420165547
- KettwichLGSibbittWLJrEmilNSNew device technologies for subcutaneous fat biopsyAmyloid2012192667322452536
- ShidhamVBHuntBJardehSSBarboiACDevataSHariPPerforming and processing FNA of anterior fat pad for amyloidJ Vis Exp201044e1747
- The National Hospice OrganizationMedical guidelines for determining prognosis in selected non-cancer diseasesHosp J199611247638949013
- MitchellSLAdvanced dementiaN Engl J Med2015372262533254026107053
- WechalekarADGillmoreJDHawkinsPNSystemic amyloidosisLancet2015 Epub 2015 Dec 21
- GillmoreJDWechalekarABirdJGuidelines on the diagnosis and investigation of AL amyloidosisBr J Haematol2015168220721825312307
- RajkumarSVDimopoulosMAPalumboAInternational Myeloma Working Group updated criteria for the diagnosis of multiple myelomaLancet Oncol20141512e538e54825439696
- AndoYCoelhoTBerkJLGuideline of transthyretin-related hereditary amyloidosis for cliniciansOrphanet J Rare Dis201383123425518
- PalladiniGMerliniGSystemic amyloidosis: what an internist should knowEur J Intern Med201324872973924262289
- WestermarkPSubcutaneous adipose tissue biopsy for amyloid protein studiesMethods Mol Biol201284936337122528102
- WestermarkPAmyloid diagnosis, subcutaneous adipose tissue, immunohistochemistry and mass spectrometryAmyloid201118417517622080760
- WestermarkPOccurrence of amyloid deposits in the skin in secondary systemic amyloidosisActa Pathol Microbiol Scand A19728067187204654794
